Abstract
Lipoic acid (LA) is an antioxidant with therapeutic properties on several diseases like diabetes and obesity. Apelin is a novel adipokine with potential beneficial actions on glucose metabolism and insulin resistance. The aim of this study was to examine in 3T3-L1 adipocytes the effects of LA on apelin gene expression and secretion, as well as elucidate the signaling pathways involved. We also tested the regulation of adipose apelin gene expression by LA supplementation in a model of high-fat diet-induced obesity. LA increased apelin secretion but not apelin gene expression in 3T3-L1 adipocytes. The AMPK inhibitor Compound C induced an increase in LA-stimulated apelin production, and, on the contrary, the AMPK activator AICAR completely reversed the LA stimulatory effects on apelin secretion, also inducing a significant reduction in apelin mRNA levels in this in vitro model. Apelin mRNA levels were increased in those animals fed with the high-fat diet, while the caloric restriction decreased apelin mRNA to control levels. However, apelin gene expression was not significantly modified in rats treated with LA compared with the obese group. The current data suggest the ability of LA to modulate apelin secretion by adipocytes. However the insulin-sensitizing effect of LA in vivo is not related to changes in apelin gene expression in our model of diet-induced obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apelin is a bioactive peptide identified as the endogenous ligand of the orphan G protein-coupled receptor, APJ. It is secreted by adipocytes as well as by several tissues including heart, brain, lungs, and pancreatic islet cells among others [14]. Apelin has been shown to be involved in the regulation of cardiovascular functions, fluid homeostasis, vessel formation, and cell proliferation [5, 31]. More recently, apelin has been described as an adipocyte-secreted factor (adipokine) with an emerging role in energy metabolism. In fact, several studies have also described that apelin is up-regulated in obesity and insulin resistance [2, 32]. Also, insulin and TNFα have been found to be important up-regulators of apelin gene expression in adipose tissue [8]. Recent studies have demonstrated that apelin, administered in a physiological range, improves in vivo glucose metabolism in normal and insulin-resistant high-fat-fed mice by increasing glucose utilization in insulin-sensitive tissues [10]. A very recent study has also demonstrated that apelin stimulates glucose uptake in human adipose tissue ex vivo [1]. Because of this and other beneficial actions of apelin, it has been suggested that over-production of apelin could be one of the last protective defense before the emergence of obesity-related disorders such as insulin resistance and type 2 diabetes [5, 6, 9, 38].
Lipoic acid (LA) is a short chain fatty acid, described as an important cofactor of several mitochondrial bioenergetic enzymes [36] and with important antioxidant properties due to its ability to chelate free radicals and regenerate other intracellular antioxidants [39]. Several studies have demonstrated the beneficial properties of LA on obesity and its related comorbidities. In this context, previous studies from our group described that ingestion of a high-fat diet supplemented with LA decreased body weight gain by a reduction of food intake, feed efficiency, and inhibition of intestinal sugar absorption [27]. Moreover, Butler et al. [3] showed that LA improves hypertriglyceridemia in rats by down-regulating hepatic GPAT-1 and DGAT-2 gene expression, by inhibiting liver TG secretion as VLDL, and by stimulating clearance of TG-rich lipoproteins. Recent studies in humans also reported that LA has beneficial effects in the treatment of pre-obese and obese subjects by decreasing body weight, blood pressure and abdominal circumference [4]. In addition, LA has also beneficial effects on glucose metabolism and it has been proposed as a potential therapy for type 2 diabetes [15, 23, 30, 34, 37]. Thus, LA treatment improved glucose tolerance and insulin sensitivity of skeletal muscle in obese insulin-resistant Zucker rats by reducing oxidative stress [16, 35]. Furthermore, studies carried out in 3T3-L1 adipocytes described that LA stimulates basal and insulin-stimulated glucose transport [33]. Moreover, LA also provides partial protection against the impaired insulin-stimulated glucose transporter 4 translocation and protein kinase B/Akt activation mediated by oxidative stress [11].
In the present study, we investigated the direct effects of LA on apelin production by 3T3-L1 adipocytes and the signaling pathways involved. Furthermore, we also aimed to investigate the effects of dietary supplementation with LA on apelin mRNA levels in white adipose tissue (WAT) in rats fed a high-fat diet in order to find out if LA effects on apelin could explain, at least in part, its protective properties against the development of insulin resistance.
Materials and methods
Culture and differentiation of 3T3-L1 cells
Mouse 3T3-L1 cells (American Type Culture Collection, Rockville, MD, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/L glucose, 10% (v/v) calf bovine serum (Invitrogen, CA, USA), and 1% (v/v) antibiotics (penicillin/streptomycin) (Gibco, Invitrogen Corporation, CA, USA) and mantained in an incubator set to 37°C and 5% of carbon dioxide [20, 22]. Two days post-confluence pre-adipocytes were induced to differentiate into adipocytes by culturing them for 48 h in DMEM containing 4.5 g/L glucose, 10% fetal bovine serum (FBS) (Invitrogen), antibiotics, and supplemented with dexamethasone (1 mM; Sigma, St. Louis, MO, USA), isobutylmethylxantine (0.5 mM; Sigma) and insulin (10 mg/mL; Sigma). Then, cells were cultured with 10% FBS and insulin for another 48 h. After that, media were replaced with 10% FBS and antibiotics, without insulin and changed every 2 days up to day 8 post-confluence, when cells were considered to be differentiated into mature adipocytes.
Different treatments were added to differentiated 3T3-L1 adipocytes day 8 post-confluence for a period time of 24 h.
Treatments
α-Lipoic acid (Sigma-Aldrich) was dissolved in ethanol. The inhibitors SP600125 (Calbiochem, San Diego, CA, USA), PD98059 (Sigma), LY294002 (Sigma), Compound C (Calbiochem), H89 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), and KT5823 (Calbiochem) were dissolved in DMSO. The AMPK activator AICAR (Sigma) was dissolved in deonized sterile water. All compounds were prepared as 1,000× stock solutions and added to the culture medium. Control cells were treated with the same proportion of the corresponding vehicle.
Prior to the addition of the appropriate treatments, fully differentiated 3T3-L1 adipocytes were serum starved overnight. Then, cells were treated with or without LA (250 μM) during 24 h. For the identification of the signaling pathways involved in LA action on apelin, 3T3-L1 adipocytes were preincubated for 1 h with selective inhibitors or activators, the JNK inhibitor, SP600125 (20 μM), the MAPK inhibitor PD98059 (50 μM), the PI3K inhibitor, LY 294002 (50 μM), the AMPK inhibitor, Compound C (20 μM), and the AMPK activator AICAR (2 mmol/l).
Animals and diets
Six-week-old male Wistar rats were obtained from the Centre of Applied Pharmacology (CIFA, Pamplona, Spain). Animals were housed in polycarbonate cages (3–4 rats per cage) in temperature-controlled rooms (22 ± 2°C) with a 12-h light–dark cycle, fed a pelleted chow diet, and given deionized water ad libitum for an adaptation period of 5 days.
Rats were then assigned into four experimental groups for 8 weeks. The control group (n = 10) was fed with a standard diet (Harlam Tekland Global Diets, Madison, WI, USA) containing 16.7% of energy as protein, 78.6% as carbohydrate, and 4.6% as lipid per dry weight. The other three experimental groups (obese, OLIP, and PF) were fed with a high-fat diet (OpenSource diets Research Diets Inc. New Brunswick, NJ, USA) containing 60% of energy as lipid, 20% as carbohydrate, and 20% as protein per dry weight. The obese group was fed ad libitum with the high-fat diet (n = 10), the OLIP group was fed ad libitum with the high-fat diet supplemented with LA in a proportion of 0.25 g LA/100 g of diet (n = 12), and the pair-fed (PF) group received the same amount of high-fat diet eaten by the group OLIP, but without adding LA (n = 6). At the end of the experimental period, rats were euthanized, and blood and tissue samples were collected, including WAT depots (epididymal, retroperitoneal, mesenteric, and subcutaneous) as previously described [7]. All fat depots were weighed and kept at −80°C for subsequent analysis. Visceral WAT depot was estimated by the sum of epididymal, retroperitoneal, and mesenteric depot weights.
All experimental procedures were approved and performed according to National and Institutional Guidelines for Animal Care and Use at the University of Navarra.
Assays
Apelin concentration in the media was determined after 24 h of culture by ELISA for mouse/rat apelin from Phoenix Peptide (Burlingame, CA, USA).
Serum insulin levels were measured by ELISA for rat/mouse Insulin ELISA kit (Linco, St. Charles, MI, USA).
Serum glucose levels were assayed using a Cobas Mira Autoanalyzer (Roche Diagnostic, Basel, Swiss), as previously described [26].
Analysis of mRNA levels
Total RNA was extracted from 3T3-L1 cells and epididymal fat depots using TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. RNA concentrations and quality were measured by Nanodrop Spectrophotometer ND1000 (Thermo Scientific, Wilmington, DE, USA). RNA was then incubated with an RNAse-free kit DNase (Ambion, Austin, TX, USA) for 30 min at 37°C. RNA (2 μg) was reverse-transcribed to cDNA using MMLV (Moloney Murine Leukaemia Virus) reverse transcriptase (Invitrogen). For the real-time quantitative polymerase chain reaction analyses, 4.5 μl of 1/50 dilution of cDNA per reaction were used in a final reaction volume of 10 μl. Apelin mRNA levels were determined using predesigned TaqMan® Assays-on-Demand (Mm00443562_m1 and Rn00581093_m1) (Applied Biosystems, Foster City, CA, USA). Taqman Universal Master Mix was also provided by Applied Biosystems. The reaction conditions were followed according to manufacturer's instructions. Amplification and detection of specific products were performed using the ABI PRISM 7900HT Fast System Sequence Detection System (Applied Biosystems).
Apelin mRNA levels were normalized by the housekeeping gene beta actin also obtained from Applied Biosytems (Mm02619580 and Rn00667869_m1). All samples were analyzed in duplicate. Ct values (the cycle where the emitted fluorescence signal is significantly above background levels and is inversely proportional to the initial template copy number) were generated by the ABI software. Finally, the relative expression level of each gene was calculated as 2−ΔΔCt.
Data analysis
Data are expressed as mean with standard errors (SE). Differences were set up as statistically significant at p < 0.05. Comparisons between the values for different variables were analyzed by one-way ANOVA, followed by Bonferroni post hoc test or by Student's t test or Mann–Whitney U test after testing the normality with the Kolmogorov–Smirnoff and Shapiro–Wilk tests. GraphPad Prism 4.0 (Graph-Pad Software Inc., San Diego, CA, USA) was used for the statistical analyses.
Results
Effects of LA on apelin secretion and gene expression in 3T3-L1 adipocytes
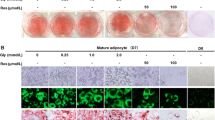
Treatment with LA (250 μM) during 24 h significantly increased basal apelin secretion (p < 0.01) in 3T3-L1 adipocytes (Fig. 1a). A similar pattern of expression was observed in apelin mRNA levels after LA treatment, although it did not reach statistical significance (Fig. 1b).
Signaling pathways involved in the LA stimulatory effects on apelin secretion
To further characterize the stimulatory effects of LA on apelin secretion, 3T3-L1 adipocytes were preincubated with several kinase inhibitors or activators as described in the “Materials and methods” section. Interestingly, the AMPK inhibitor Compound C stimulated (p < 0.05) apelin secretion and induced an additional and significant (p < 0.05) increase in LA-stimulated apelin production (Fig. 2a), but without significantly affecting apelin gene expression (Fig. 2b). On the contrary, the AMPK activator AICAR completely reversed (p < 0.05) the LA stimulatory effects on apelin secretion (Fig. 2a), suggesting that AMPK inhibition could be involved in the regulation of apelin secretion by LA. However, treatment with AICAR alone did not modify basal apelin secretion, while it induced a significant reduction in apelin mRNA levels, which was also observed in the presence of LA (Fig. 2b).
Signaling pathways involved in the regulation of a apelin secretion and b gene expression. 3T3-L1 adipocytes were treated with LA (250 μM) in the presence or absence of JNK inhibitor SP600125, the MAPK inhibitor PD98059, the PI3K inhibitor LY294002, the AMPK inhibitor Compound C, and the AMPK activator AICAR. *p < 0.05 and ***p < 0.001 vs. basal control group; a p < 0.05, b p < 0.01, and c p < 0.001 vs. basal LA-treated group
Treatment with the PI3K inhibitor LY294002 increased (p < 0.05) basal apelin secretion, while a significant down-regulation was observed on apelin mRNA levels (Fig. 2b).
Finally, treatment with the JNK inhibitor SP600125 or the MAPK inhibitor PD98059 did not modify either apelin gene expression (Fig. 2b) or protein secretion (Fig. 2a).
Effects of LA supplementation on body and white adipose tissue weights
As expected, the high-fat diet intake significantly increased (p < 0.001) both body weight (Fig. 3a) and fat mass (Fig. 3b) compared with the control group, which was completely prevented by the dietary supplementation with LA. This anti-obesity effect of LA was secondary in part to reduced food intake (19.39 ± 0.27 and 16.19 ± 0.19 g/day, p < 0.001 for obese vs. OLIP groups). However, the fat mass lowering actions observed in LA-treated animals were higher than those observed in the PF group (p < 0.05) (Fig. 3b).
Effects of LA supplementation on glucose metabolism
As expected, high-fat feeding caused hyperglycemia (p < 0.05) (Fig. 3c) and hyperinsulinemia (p < 0.01) (Fig. 3d) as well as increased HOMA index (p < 0.001) (Fig. 3e), suggesting the development of insulin resistance in these animals.
Interestingly, dietary supplementation with LA dramatically decreased insulin levels and HOMA index (p < 0.001 and p < 0.01, respectively), indicating that LA supplementation improves the insulin resistance caused by the high-fat diet. Moreover, the reduction in insulin levels observed in the OLIP animals was higher than the one observed in the PF group, suggesting a direct effect of LA on insulin homeostasis and not secondary to calorie restriction (Fig. 3d).
Effects of LA on apelin gene expression in high-fat-fed rats
We next evaluated the effects of a high-fat diet and LA supplementation on apelin gene expression. As shown in Fig. 4, apelin mRNA levels were increased in those animals fed with the high-fat diet (p < 0.05), whereas calorie restriction (PF group) decreased apelin mRNA to control levels. However, apelin gene expression was not modified in rats treated with LA in comparison with the obese group while a tendency to increase apelin mRNA levels in comparison with the PF group was observed.
Discussion
Previous studies have demonstrated the ability of LA to regulate the production of several adipokines involved in energy metabolism such as leptin [13, 28, 29].
However, this is the first study where the direct effects of LA on apelin production in adipocytes have been analyzed. Thus, we have demonstrated the ability of LA treatment to increase apelin secretion in 3T3-L1 adipocytes. Previous studies have also reported that other bioactive molecules such as the omega-3 eicosapentaenoic acid (EPA) are able to stimulate apelin production in 3T3-L1 adipocytes [21] as well as in adipose tissue [25].
The lack of significant effects of LA on apelin gene expression suggests that LA stimulates apelin production via post-transcriptional mechanisms. Moreover, our data also suggest that these mechanisms could involve inhibition of AMPK in adipocytes. The role of AMPK pathway in mediating LA actions is complex and seems to be tissue-dependent. In this context, the anti-obesity effects of LA are mediated by suppression of hypothalamic AMPK [15]. However, other studies have reported that AMPK activation is mediating some of the beneficial effects of LA in different disease models. Thus, α-LA acid increases insulin sensitivity by activating AMPK in skeletal muscle in Otsuka Long Evans Tokushima Fatty (OLEFT) rats [18]. Moreover, it has been found that LA decreases hepatic lipogenesis through both AMPK-dependent and independent mechanisms [24]. LA has been also shown to prevent endothelial dysfunction in obese rats via activation of AMPK [17].
Furthermore, we also found that the AMPK activator AICAR caused a down-regulation of basal apelin gene expression, which suggests that this pathway could be also involved in the transcriptional regulation of apelin. To our knowledge, this is the first study reporting the potential role of AMPK in regulating apelin production, although the mechanisms seem to be complex and require further investigation.
Our data also showed that treatment with the PI3K inhibitor LY294002 dramatically inhibited apelin gene expression, as it has been previously reported [2]. However, we found that basal apelin secretion was increased after treatment with this inhibitor, suggesting a complex post-transcriptional regulation of this adipokine, as we have previously described [21].
Many studies have reported an upregulation of apelin production in obesity associated to hyperinsulinemia both in humans and in rodents [2, 6, 19]. However, it has been suggested that the over-production of apelin in obesity could be one of the last protections before the emergence of the obesity-related disorders such as type 2 diabetes [25]. In this context, a previous study from our group has demonstrated that oral supplementation with EPA ethyl ester induced a significant increase in apelin gene expression in adipose tissue of rats fed a high-fat diet. Moreover, HOMA, an index of insulin resistance, was negatively correlated with apelin, suggesting that, to some extent, the insulin-sensitizing effects of EPA could be also related to its stimulatory action on apelin gene expression in visceral fat [25]. Other studies found that dietary supplementation of high-fat diet with vitamin C counteracts the upregulation of apelin mRNA expression induced by high-fat diet in subcutaneous fat, probably as a consequence of the reduction observed on the size of this fat depot [12]. In the present study, we found an over-expression of apelin mRNA levels in epididymal fat from obese and hyperinsulinemic rats, as previously reported [2, 25]. This upregulation of apelin gene expression was reversed by caloric restriction (PF group) in parallel with the reduction of adiposity and hyperinsulinemia. In contrast, rats treated with LA, which also exhibited lower adiposity and insulin levels, did not show a significant decrease in apelin mRNA levels in comparison with the obese group and even a certain stimulatory effect of LA on this adipokine was observed when compared with the PF group. This fact suggests a certain stimulatory action of LA on apelin in vivo in agreement with our in vitro data. However, no significant relationships were observed between these effects of LA on apelin and the HOMA index or insulin levels, suggesting that the previously described insulin-sensitizing effects of LA are not mediated by apelin, at least under our experimental conditions.
In conclusion, our data suggest that LA increased apelin secretion in 3T3-L1 adipocytes. However, we cannot conclude that changes in apelin mRNA levels are involved in the insulin-sensitizing effects of LA observed in an in vivo model of obesity induced by a high-fat diet.
References
Attane C, Daviaud D, Dray C, Dusaulcy R, Masseboeuf M, Prevot D, Carpene C, Castan-Laurell I, Valet P (2011) Apelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivo. J Mol Endocrinol 46:21–28
Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, Audigier Y, Saulnier-Blache JS, Valet P (2005) Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146:1764–1771
Butler JA, Hagen TM, Moreau R (2009) Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. Arch Biochem Biophys 485:63–71
Carbonelli MG, Di Renzo L, Bigioni M, Di Daniele N, De Lorenzo A, Fusco MA (2010) Alpha-lipoic acid supplementation: a tool for obesity therapy? Curr Pharm Des 16:840–846
Carpene C, Dray C, Attane C, Valet P, Portillo MP, Churruca I, Milagro FI, Castan-Laurell I (2007) Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem 63:359–373
Castan-Laurell I, Boucher J, Dray C, Daviaud D, Guigne C, Valet P (2005) Apelin, a novel adipokine over-produced in obesity: friend or foe? Mol Cell Endocrinol 245:7–9
Cinti S (2005) The adipose organ. Prostaglandins Leukot Essent Fatty Acids 73:9–15
Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache JS, Valet P, Castan-Laurell I (2006) TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J 20:1528–1530
Dray C, Debard C, Jager J, Disse E, Daviaud D, Martin P, Attane C, Wanecq E, Guigne C, Bost F, Tanti JF, Laville M, Vidal H, Valet P, Castan-Laurell I (2010) Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab 298:E1161–E1169
Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M, Cani PD, Attane C, Guigne C, Carpene C, Burcelin R, Castan-Laurell I, Valet P (2008) Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab 8:437–445
Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A (1996) Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes 45:1798–1804
Garcia-Diaz D, Campion J, Milagro FI, Martinez JA (2007) Adiposity dependent apelin gene expression: relationships with oxidative and inflammation markers. Mol Cell Biochem 305:87–94
Huong D, Ide T (2008) Dietary lipoic acid-dependent changes in the activity and mRNA levels of hepatic lipogenic enzymes in rats. Br J Nutr 100:79–87
Kapica M, Puzio I, Kato I, Kuwahara A, Zabielski R (2008) Role of feed-regulating peptides on pancreatic exocrine secretion. J Physiol Pharmacol 59(Suppl 2):145–159
Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU (2004) Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10:727–733
Koh EH, Lee WJ, Kim MS, Park JY, Lee IK, Lee KU (2005) Intracellular fatty acid metabolism in skeletal muscle and insulin resistance. Curr Diabetes Rev 1:331–336
Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY (2005) Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol 25:2488–2494
Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY (2005) Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun 332:885–891
Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, Li K (2006) Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes 114:544–548
Lorente-Cebrian S, Bustos M, Marti A, Martinez JA, Moreno-Aliaga MJ (2009) Eicosapentaenoic acid stimulates AMP-activated protein kinase and increases visfatin secretion in cultured murine adipocytes. Clin Sci (Lond) 117:243–249
Lorente-Cebrian S, Bustos M, Marti A, Martinez JA, Moreno-Aliaga MJ (2010) Eicosapentaenoic acid up-regulates apelin secretion and gene expression in 3T3-L1 adipocytes. Mol Nutr Food Res 54(Suppl 1):S104–S111
Moreno-Aliaga MJ, Matsumura F (2002) Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p, p'-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem Pharmacol 63:997–1007
Packer L, Kraemer K, Rimbach G (2001) Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17:888–895
Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, Kim JB, Choi HS, Park JY, Lee IK, Lee KU (2008) Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology 48:1477–1486
Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Martinez JA, Moreno-Aliaga MJ (2009) Effects of eicosapentaenoic acid ethyl ester on visfatin and apelin in lean and overweight (cafeteria diet-fed) rats. Br J Nutr 101:1059–1067
Perez-Matute P, Marti A, Martinez JA, Fernandez-Otero MP, Stanhope KL, Havel PJ, Moreno-Aliaga MJ (2005) Eicosapentaenoic fatty acid increases leptin secretion from primary cultured rat adipocytes: role of glucose metabolism. Am J Physiol Regul Integr Comp Physiol 288:R1682–R1688
Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Barber A, Martinez JA, Moreno-Aliaga MJ (2009) Lipoic acid prevents body weight gain induced by a high fat diet in rats: effects on intestinal sugar transport. J Physiol Biochem 65:43–50
Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Martínez JA, Moreno-Aliaga MJ (2009) Effects of dietary supplementation with lipoic acid on leptin and adiponectin plasma levels in lean and obese Wistar rats. Acta Physiol 195(suppl 667):129
Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Martinez JA, Moreno-Aliaga MJ (2011) Lipoic acid inhibits leptin secretion and Sp1 activity in adipocytes. Mol Nutr Food Res. doi:10.1002/mnfr.201000534
Purnell JQ, Hirsch IB (1997) New oral therapies for type 2 diabetes. Am Fam Physician 56:1835–1842
Quazi R, Palaniswamy C, Frishman WH (2009) The emerging role of apelin in cardiovascular disease and health. Cardiol Rev 17:283–286
Ringstrom C, Nitert MD, Bennet H, Fex M, Valet P, Rehfeld JF, Friis-Hansen L, Wierup N (2010) Apelin is a novel islet peptide. Regul Pept 162:44–51
Rudich A, Tirosh A, Potashnik R, Khamaisi M, Bashan N (1999) Lipoic acid protects against oxidative stress induced impairment in insulin stimulation of protein kinase B and glucose transport in 3T3-L1 adipocytes. Diabetologia 42:949–957
Sena CM, Nunes E, Louro T, Proenca T, Fernandes R, Boarder MR, Seica RM (2008) Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol 153:894–906
Sena CM, Nunes E, Louro T, Proenca T, Seica RM (2007) Endothelial dysfunction in type 2 diabetes: effect of antioxidants. Rev Port Cardiol 26:609–619
Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM (2004) Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem 11:1135–1146
Song KH, Lee WJ, Koh JM, Kim HS, Youn JY, Park HS, Koh EH, Kim MS, Youn JH, Lee KU, Park JY (2005) Alpha-lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem Biophys Res Commun 326:197–202
Wei L, Hou X, Tatemoto K (2005) Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept 132:27–32
Xu DP, Wells WW (1996) Alpha-lipoic acid dependent regeneration of ascorbic acid from dehydroascorbic acid in rat liver mitochondria. J Bioenerg Biomembr 28:77–85
Acknowledgements
This work has been supported, in part, by Ministerio de Ciencia e Innovación of Spain (AGL2006-04716/ALI and AGL2009-10873/ALI) and Línea Especial de Investigación “Nutrición, Obesidad y Salud” (University of Navarra LE/97).
M. Fernández-Galilea was supported by a predoctoral grant by the “Asociación de Amigos de la Universidad de Navarra”. CTP Network is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández-Galilea, M., Pérez-Matute, P., Prieto-Hontoria, P. et al. Effects of lipoic acid on apelin in 3T3-L1 adipocytes and in high-fat fed rats. J Physiol Biochem 67, 479–486 (2011). https://doi.org/10.1007/s13105-011-0087-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0087-1