Abstract
Optical coherence tomography (OCT) is a novel imaging technology based on low-coherence interferometry that use near-infrared light in real-time, and allows cross-sectional in-situ visualization of the vessel wall at the microscopic level. OCT provides 10-fold higher resolution than intravascular ultrasound which is currently the most used modality for intra-coronary imaging. OCT offers the obvious advantages when characterizing precise plaque microstructure and distinguishing various type of plaques. OCT is also being assessed for its potential role in the understanding of neointimal coverage, vascular healing and the progression of atherosclerosis in coronary vasculature after stenting on the micron scale. These unique capabilities could be helpful in guiding coronary management and interventions. Recent improvement in next generation OCT technology, such as frequency-domain OCT, will allow for a simple imaging procedure, providing more useful information and complementing other modalities on both clinical and research applications for the cardiologists.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intravascular imaging devices can offer information that is necessary for intervention therapy by clarifying the structure and tissue characteristics of vessels which cannot be depicted by traditional angiography, and they play an important role both in the elucidation of the pathology of coronary artery disease and guidance of clinical treatment. Optical coherence tomography (OCT) is a novel optical imaging technology that provides cross-sectional tomographic imaging which yields detailed structural information superior to any other currently available modality. In this review, this new imaging modality will be discussed with an emphasis on image interpretation.
Advancements in intravascular OCT systems
1st generation optical coherence tomography
The first commercially available OCT system was the M2 OCT Imaging System (LightLab Imaging, Inc., Westford, MA, USA). The M2 OCT Imaging System has been included in the insurance coverage in Japan since 2008. The M2 OCT system uses a broadband light source that emits multiple wavelengths across a spectrum, and also is called time-domain OCT (TD-OCT). The image is formed by the backscattering of light from different sites of the tissue with a 15–20 μm axial resolution and 25–30 μm lateral resolution. The resolution of TD-OCT is about ten times that of intravascular ultrasound (IVUS). The maximum frame rate and pull-back speed of the M2 OCT system are 15.6 f/sec and 2 mm/sec, respectively. The M3 OCT system featured improvements in the frame rate and pull-back speed. The maximum frame rate and pull-back speed of the M3 OCT system were raised to 20 f/sec and 3 mm/sec, respectively. Since light-scattering occurs from red blood cells which may cause significant signal attenuation, complete removal of blood from the target vessel is required to acquire the images. By limitation of pullback speed, TD-OCT generally needs over-the-wire type catheter with an occlusion balloon (Helios™ Goodman, Co. ltd., Nagoya, Japan) to obtain continuous long sectional images. The blockage of blood flow by employing temporary balloon occlusion increases the complexity of procedures, leads to transient myocardial ischemia, and limits the evaluation of coronary ostial lesions. The recently proposed continuous-flushing mode of acquisition does not require proximal balloon occlusion. During imaging, low molecular weight dextran plus lactated Ringer’s solution is infused through guiding catheter at rate between 2.5 and 4.5 ml/sec by a power injector. The continuous-flushing technique simplifies the procedure and makes it possible to observe proximal lesions of coronary arteries [1–4]. However, this method is not appropriate for exploring long lesions due to the limitation of infusion time.
2nd generation optical coherence tomography
Recently, C7-XR imaging system (LightLab Imaging, Inc., Westford, MA, USA) has been made commercially available. The C7 system uses a novel swept frequency laser as a light source, and is also called the frequency domain OCT (FD-OCT). The image is formed with a 15 μm axial resolution. The max frame rate is 100 f/sec, and it reaches to five times of previous generation OCT systems. The nominal pull-back speed of the C7 system is 20 mm/sec, which is ten times higher than the M2 OCT systems. Table 1 shows the comparison between FD-OCT and TD-OCT system [5]. The C7 system has the potential to scan the entirety of target coronary vessel within a few seconds. This feature permits the use of a single bolus flush to produce a blood-free environment, eliminates the need for balloon occlusion and makes it possible to observe proximal lesions. Furthermore, the C7 system uses a monorail type catheter which allows for faster and easier deployment into coronary arteries without wire replacement. In addition, Takarada et al. [6] reported that the FD-OCT system has potential to yield a higher rate of clear image segments compared to the conventional TD-OCT system. The disadvantages of the M2 OCT system, such as balloon occlusion and more complicated procedures, have limited the use of the current OCT system in clinical applications. Terumo (Terumo Corporation, Tokyo, Japan) and Volcano (Volcano Corporation, Tokyo, Japan) are also developing a new FD-OCT system. We envisage that the introduction of new technology will further expand the potential of OCT in clinical applications.
Image Interpretation
Artifacts
Artifact from residual blood
Incomplete clearance of blood cells reduces the brightness of the vessel wall image. Moreover, the remaining blood cells might be observed as a membrane (Fig. 1a). It is often difficult to distinguish between the blood adherent to vessel wall and the intima. Incomplete clearance occurs especially in vessels with rich collateral blood supply. For TD-OCT, vessel sizes of more than 4 mm in diameter may lead to incomplete occlusion of blood flow.
Artifacts of optical coherence tomography images. a Artifact from residual blood: residual blood is observed as a membrane. b Bubble shadows: there is a darkened area of which the apex is the image probe. c Sew-up artifact: slight elliptical distortion with visible stitch. d Ghost reflections: ghost reflections appear at a fixed distance away from the primary image of an object
Bubble shadows
There is a silicon lubricant between the sheath and imaging probe. A gap can form due to the uneven distribution of the silicon liquid, and this can scatter the light beam emitted from image wire. This artifact causes a darkened sector of which the apex is image probe (Fig. 1b). We can also see such darkened sectors when something adheres to image wire.
Sew-up artifact
Figure 1c shows a typical sew-up artifact, which is a misalignment of the vessel wall border. This is produced by the rapid movement of image wire or vessels during imaging acquisition and increases at low frame acquisition rates.
Ghost reflections
Ghost reflections are caused by the light reflecting multiple times between the image wire and highly reflective objects, like stent struts. Ghost reflections appear at a fixed distance away from the primary image of an object (Fig. 1d).
Z-offset drift
Optical fiber is stretched during auto pullback and the Z-offset calibration will drift. Therefore, it is necessary to adjust the Z-offset calibration again before taking detailed measurements. Z-offset can be readjusted by using the outside edge of sheath, which is not stretched during auto pullback as a reference.
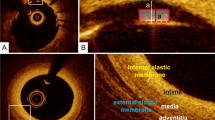
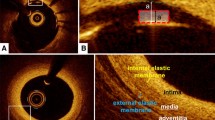
Normal coronary artery
OCT can visualize the trilaminar structure of a normal vessel wall (Fig. 2). The innermost bright band is intima-internal elastic lamina, and then from inside to outside are the dark layer of media, bright layer of external elastic lamina and the outermost dark band of adventitia. Despite the thickness measuring below 20 μm histologically, the internal and external elastic lamina are depicted as layers with a thickness around 20 μm in OCT images.
Atherosclerotic coronary artery
Atherosclerotic coronary lesions, which appear as thickened areas of tissue in OCT images, are composed of fibrous tissue, lipid, calcium, thrombus, etc. OCT encounters difficulty in imaging the trilaminar structure of vessel walls at coronary atherosclerotic lesions because the near-infrared light signals are attenuated. However, the different components of the visualized atherosclerotic lesion can be identified by their distinctive borderlines and the intensity, uniformity and attenuation of the associated signal. Fibrous plaques consist of homogeneous high back-scattering areas with indistinct borderlines. Lipid plaques are also diffusely bordered, but exhibit lower signal intensity with high attenuation. Calcifications within plaques are identified by the presence of well-delineated, low back-scattering heterogeneous regions (Fig. 3). Previous postmortem studies have demonstrated excellent sensitivity and specificity in the discrimination of plaque characteristics by OCT [7, 8]. The plaque disruption and thrombi, which are frequently found within culprit lesions of patients with acute coronary syndrome, can also be identified by OCT. Thrombi are identified by OCT as well delineated masses with high signal intensity that are attached to the vessel wall and protrude into the lumen. OCT can even discriminate between red thrombus (high-backscattering protrusions with signal free shadowing) and white thrombus (low-backscattering protrusions) (Table 2) [9]. Moreover, Tearney et al. [10] have reported methods that allow for the quantification of macrophages within fibrous caps of atherosclerotic plaques.
Coronary artery immediately after intervention
Previous studies have demonstrated higher sensitivity of OCT compared with IVUS for detection and assessment of dissection, plaque protrusion and incomplete sent apposition after intracoronary stenting [11]. Figure 4 presents an OCT image immediately after stent placement. The stent strut reflections appear as high signal band. There are black shadows behind stent struts, because the near-infrared signal of OCT cannot penetrate the metal. The form of stent reflection varies according to the state of stent strut and the position of image wire. The stent reflection is formed by the aggregation of short tangential lines to a circle around the image probe. Typical reflections of the strut surface are high intensity lines with thickness of about 30 μm which are arranged in a circular fashion when image probe is positioned at the center of the stent (Fig. 5).
Vascular healing after coronary stenting
Early after the stent implantation, there are thrombus formations and fibrin deposition around the stent struts. Then the macrophage and dedifferentiated vascular smooth muscle cells (VSMC) with expression of α-actin accumulate adjacent to the stent struts, resulting in the early neointimal formation. Thereafter, organization of mural thrombus, deposition of fibrin and extracellular matrix together with proliferation of VSMC and inflammatory cells finally lead to endothelial regeneration. An excessive proliferation results from the formation of a restenotic lesion. Drug-eluting stents (DES) decrease the risk of restenosis by inhibiting the proliferative reaction. On the other hand, the risk of late stent thrombosis represents a concern for the patients treated with DES. Delayed arterial healing after stenting is commonly observed in pathologic specimens of vessels treated with DES. Incomplete neointimal coverage has been suggested as a predictor of late stent thrombosis.
OCT enables in vivo visualization of coronary vessel response to endovascular stents with a unique ultra-high resolution. Notably, OCT has a high ability to detect incomplete neointimal coverage, and has provided information on the risk of stent thrombosis. OCT is useful not only for quantitative analysis of neointima but also for evaluating the morphology of neointima. Recently, several comparative studies between OCT and pathology findings have been reported for the elucidation of the various image patterns of neointima by OCT. Templin et al. [12] demonstrated that optical density measurements reveal a significant difference between fibrin covered and neointima covered struts. The median of normalized optical density of stent strut coverage was significantly lower for fibrin-covered than neointima-covered stent struts. Low optical density areas are often detected around stent struts within the developed neointima. Teramoto et al. [13] reported that peri-stent low intensity areas might represent the existence of fibrinoid and proteoglycans. Furthermore, OCT analysis has demonstrated that neointima within the bare-metal stent (BMS) often transform into lipid-laden tissue over an extended period of time, and that the expansion of neovascularization from peri-stent to intra-intima contributes to atherosclerotic progression of neointima (Fig. 6) [14].
Up to now, investigators have clarified that the vascular healing courses vary among stents by using OCT. Takano et al. [15] elucidated a delayed arterial healing after sirolimus-eluting stent (SES) implantation compared with BMS. At 3-month follow-up, the frequency of uncovered struts was higher in the SES group than the BMS group (15% vs. 0.1%; p < 0.0001). Furthermore, the incidence of complete stent struts coverage is still low at 3 years after SES implantation [16, 17]. The degree of neointimal growth in paclitaxel-eluting stent (PES) is generally greater compared with SES. Some investigators indicated that PES had a non-uniform neointimal thickness, more peri-strut low-density areas and more in-stent thrombus compared with SES at 6 months [18, 19]. Zotarolimus-eluting stent (ZES) has different characteristics, with biocompatible polymer and rapid drug-elution, compared with the first-generation DES. Kim et al. demonstrated that most of the stent struts were covered with neointima, and malapposition was not found at 3 months after ZES implantation [20]. It was reported that overlapping SES and PES were associated with poor endothelial coverage in some animal models [21]. However, ODESSA Trial Investigators demonstrated a similar impact of DES on stent coverage at overlapping and nonoverlapping sites. This result suggests the safety of overlapping DES in humans. In addition, they showed a heterogeneous vascular response according to DES type, with higher rates of uncovered or malapposed struts in PES and SES compared with low rates observed in BMS and ZES [22]. On the other hand, OCT examinations have also showed different vascular response after DES implantation between acute coronary syndrome (ACS) and stable angina pectoris (SAP) patients. DES implanted for ACS had a higher frequency of incompletely apposed struts and uncovered struts at follow-up compared with DES implanted for SAP [23–25]. The vascular response of ACS patients after DES implantation were variable among the DES type. The patterns of neointimal coverage and malapposition at 9 months after ZES implantation were not different between ACS and SAP [26]. Gonzalo et al. [27] evaluated the morphologic characteristics of DES restenosis by OCT. OCT examinations could help in understanding the mechanism of not only late thrombosis but stent restenosis.
Quantitative analysis
OCT provides measurements of detailed vascular structure superior to those of any other currently available modality. The thin-cap fibroatheroma (TCFA), one type of vulnerable plaque, is characterized histologically by the following features: thin fibrous cap (<65 μm), large lipid pool, and activated inflammatory cells near or within the fibrous cap. The OCT findings can be used to identify certain types of vulnerable plaque. The fibrous cap thickness of the lipid plaque as measured by OCT and histological assessment shows close correlation (Fig. 7) [28]. Furthermore, it was demonstrated that lipid lowering therapy with statins increased the fibrous cap thickness covering the lipid core in OCT analysis [29]. OCT can be anticipated to serve as an investigational tool to understand and identify the vulnerable plaque [30–34].
The high resolution of OCT provides advantages for the evaluation of stent apposition. In patients implanted with SES, incomplete apposition is associated with the presence of OCT-detected thrombus at follow up, and such incomplete apposition has the possibility to cause late stent thrombosis [35]. The stent apposition is usually evaluated by comparing the distance between the middle of strut reflection and vessel wall with the actual stent strut thickness. Since the measured points of vessel wall are often invisible in the stent struts shadow, we need to trace the extended line of nearby vessel wall (Fig. 8). However, the universal definition of incomplete stent apposition as determined by OCT has not been established. Sawada et al. [36] evaluated the factors that influence the measurement of stent apposition, and proposed the optimum methods for the measurement of stent apposition by using a phantom model. Increased errors of OCT measurements were associated with low frame acquisition rate and eccentrically positioned image wire. They proved that the distance between the middle of strut reflection and vessel wall should be measured to evaluate the stent apposition accurately. Furthermore, they proposed that the stent strut should be identified as incomplete apposition or malapposition when the distance between middle stent surface reflection and the vessel wall is greater than the axial resolution plus the actual stent thickness including the outer polymer layer. However, there is also an in vitro study which demonstrated that the inner polymer layer of DES may influence OCT measurement for the stent strut [37]. We need to establish the universal definition of incomplete stent apposition.
Measurement of stent apposition. The distance between the middle of strut reflection and vessel wall is measured to evaluate stent apposition. Since the measured points of the vessel wall are often in the stent strut shadow and not viewable, we need to extended the line of the nearby vessel wall (dashed line)
Neointimal thicknesses can be measured more accurately by OCT than IVUS [38–41]. Although the reflection surface does not stand for the actual strut surface, the neointimal thickness is usually measured as the distance between the endo-luminal surface of stent refection and the vessel wall. This measuring line is always drawn towards the center of the vessel lumina. Figure 9 shows OCT images of the neointimal coverage of stent struts. The distance between the reflection surface and the vessel wall varies according to the measuring site. Moreover, the stent reflection orientation varies according to the position of image wire as mentioned above. Therefore, it is presumably appropriate to define the border of each strut as the axial and lateral center of the stent strut reflection to evaluate neointimal thickness.
OCT does not have adequate penetration depth to evaluate the whole vessel cross-section in all cases. Therefore, OCT is not appropriate for the measurement of plaque area and arterial remodeling. The vessel diameter of distal coronary artery measured by TD-OCT tends to be smaller than that measured by IVUS [42]. This phenomenon is presumably caused by the reduction in intravascular pressure during blood flow occlusion.
Limitations of OCT
OCT does not have adequate penetration depth to evaluate the whole vessel cross-section in all cases. Large vessel sizes may lead to “scale out” loss of images. The combined use of OCT and other devices might be helpful in intervention therapy by clarifying the structure and tissue characteristics of whole vessels cross-sections.
Stent analysis needs a very delicate and intensive labor. Practical realization of an automated stent analysis system is anticipated.
Conclusions
OCT allows detailed structural analysis of the coronary vessel with extremely high resolution far superior to any other currently available intravascular imaging modality. Furthermore, OCT findings are well correlated with pathological findings. OCT is useful for comprehending the morphology of coronary arteries and vascular healing after coronary stenting.
References
Prati F, Cera F, Ramazzoti V, Imola F, Guidice R, Albertucci M. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. Eurointervention. 2007;3:365–70.
Kataiwa H, Tanaka A, Kitabata H, Imanishi T, Akasaka T. Safety and usefulness of non-occlusive image acquisition technique for optical coherence tomography. Circ J. 2008;72:1536–7.
Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, Giudice M, et al. From bench to bedside: a novel technique of acquiring OCT images. Circ J. 2008;72:839–43.
Kataiwa H, Tanaka A, Kitabata H, Matsumoto H, Kashiwagi M, Kuroi A, et al. Head to head comparison between the conventional balloon occlusion method and the non-occlusion method for optical coherence tomography. Int J Cardiol. 2009;5 (epub ahead of print).
Kubo T, Asakura T. Optical coherence tomography imaging: current status and future perspectives. Cardiovasc Interv Ther. 2010;25:2–10.
Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A, et al. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75:202–6.
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–5.
Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, et al. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol. 2006;97:1172–5.
Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97:1713–7.
Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–9.
Diaz-Sandoval LJ, Bouma BE, Tearney GJ, Jang IK. Optical coherence tomography as a tool for percutaneous coronary interventions. Catheter Cardiovasc Interv. 2005;65:492–6.
Templin C, Meyer M, Müller MF, Djonov V, Hlushchuk R, Dimova I, et al. Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur Heart J. 2010;31:1792–801.
Teramoto T, Ikeno F, Otake H, Lyons JK, van Beusekom HM, Fearon WF, et al. Intriguing peri-strut low-intensity area detected by optical coherence tomography after coronary stent deployment. Circ J. 2010;74:1257–9.
Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol. 2009;55:26–32.
Xie Y, Takano M, Murakami D, Yamamoto M, Okamatsu K, Inami S, et al. Comparison of neointimal coverage by optical coherence tomography of a sirolimus-eluting stent versus a bare metal stent 3 months after implantation. Am J Cardiol. 2008;102:27–31.
Takano M, Yamamoto M, Inami S, Murakami D, Seimiya K, Ohba T, et al. Long-term follow up evaluation after sirolimus-eluting stent implantation by optical coherence tomography: do uncovered struts persist? J Am Coll Cardiol. 2008;51:968–9.
Ishigami K, Uemura S, Morikawa Y, Soeda T, Okayama S, Nishida T, et al. Long-term follow-up of neointimal coverage of sirolimus-eluting stents—evaluation with optical coherence tomography. Circ J. 2009;73:2300–7.
Miyoshi N, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, et al. Comparison by optical coherence tomography of paclitaxel-eluting stents with sirolimus-eluting stents implanted in one coronary artery in one procedure. 6 month follow-up. Circ J. 2010;74:903–8.
Murakami D, Takano M, Yamamoto M, Inami S, Ohba T, Seino Y, et al. Advanced neointimal growth is not associated with a low risk of in-stent thrombus. Optical coherence tomographic findings after first-generation drug-eluting stent implantation. Circ J. 2009;73:1627–34.
Kim JS, Jang IK, Fan C, Kim TH, Kim JS, Park SM, et al. Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography: the ENDEAVOR OCT trial. JACC Cardiovasc Interv. 2009;2:1240–7.
Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–10.
Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, et al. ODESSA Trial Investigators. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2010;3:531–9.
Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, et al. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent 3 months after implantation. Am J Cardiol. 2007;99:1033–8.
Kubo T, Imanishi T, Kitabata H, Kuroi A, Ueno S, Yamano T, et al. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2008;1:475–84.
Gonzalo N, Barlis P, Serruys PW, Garcia-Garcia HM, Onuma Y, Ligthart J, et al. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2:445–52.
Kim JS, Fan C, Choi D, Jang IK, Lee JM, Kim TH, et al. Different patterns of neointimal coverage between acute coronary syndrome and stable angina after various types of drug-eluting stents implantation; 9-month follow-up optical coherence tomography study. Int J Cardiol. 2009;24 (epub ahead of print).
Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia-Garcia HM, van Soest G, et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–93.
Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, et al. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152:e1–4.
Takarada S, Imanishi T, Kubo T, Tanimoto T, Kitabata H, Nakamura N, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. 2009;202:491–7.
Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–5.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–9.
Takano M, Jang IK, Inami S, Yamamoto M, Murakami D, Okamatsu K, et al. In vivo comparison of optical coherence tomography and angioscopy for the evaluation of coronary plaque characteristics. Am J Cardiol. 2008;101:471–6.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Implication of plaque color classification for assessing plaque vulnerability: a coronary angioscopy and optical coherence tomography investigation. JACC Cardiovasc Interv. 2008;1:74–80.
Fujii K, Kawasaki D, Masutani M, Okumura T, Akagami T, Sakoda T, et al. OCT assessment of thin-cap fibroatheroma distribution in native coronary arteries. JACC Cardiovasc Imaging. 2010;3:168–75.
Ozaki Y, Okumura M, Ismail TF, Naruse H, Hattori K, Kan S, et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010;31:1470–6.
Sawada T, Shite J, Negi N, Shinke T, Tanino Y, Ogasawara D, et al. Factors that influence measurements and accurate evaluation of stent apposition by optical coherence tomography. Assessment using a phantom model. Circ J. 2009;73:1841–7.
Mizuno M, Takano M, Mizuno K. The effects of drug-eluting stent polymer on measurement of strut thickness by optical coherence tomography: in vitro comparison with bare-metal stent. Int J Cardiol. 2010;145:48–9.
Suzuki Y, Ikeno F, Koizumi T, Tio F, Yeung AC, Yock PG, et al. In vivo comparison between optical coherence tomography and intravascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. JACC Cardiovasc Interv. 2008;1:168–73.
Prati F, Zimarino M, Stabile E, Pizzicannella G, Fouad T, Rabozzi R, et al. Does optical coherence tomography identify arterial healing after stenting? An in vivo comparison with histology, in a rabbit carotid model. Heart. 2008;94:217–21.
Terashima M, Rathore S, Suzuki Y, Nakamura Y, Kaneda H, Nasu K, et al. Accuracy and reproducibility of stent-strut thickness determined by optical coherence tomography. J Invasive Cardiol. 2009;21:602–5.
Murata A, Wallace-Bradley D, Tellez A, Alviar C, Aboodi M, Sheehy A, et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. JACC Cardiovasc Imaging. 2010;3:76–84.
Yamaguchi T, Terashima M, Akasaka T, Hayashi T, Mizuno K, Muramatsu T, et al. Safety and feasibility of an intravascular optical coherence tomography imagewire system in the clinical setting. Am J Cardiol. 2008;101:562–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inami, S., Wang, Z., Ming-juan, Z. et al. Current status of optical coherence tomography. Cardiovasc Interv and Ther 26, 177–185 (2011). https://doi.org/10.1007/s12928-011-0057-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-011-0057-0