Abstract
Optical coherence tomography (OCT) is an optical analogue of intravascular ultrasound that provides high-resolution (10–20 μm) cross-sectional images of coronary arteries. The micron-scale resolution of OCT has an ability to capture in vivo what was previously seen only through a pathologist’s microscope. OCT can differentiate three types of atherosclerotic plaque components (fibrous, fibrocalcific and lipid-rich) with high sensitivity and specificity. Early in vitro and in vivo studies have demonstrated a possibility of OCT for identifying vulnerable plaque features, in particular the quantification of plaque rupture, intracoronary thrombus, thin-capped fibroatheroma and the distribution of macrophages within the fibrous cap. In addition, OCT has shown its effectiveness in imaging the short-term and long-term results of percutaneous coronary intervention. OCT can precisely assess stent strut malapposition, tissue protrusion, coronary artery dissection, and neointimal hyperplasia following stent implantation. Recently, next-generation OCT, called Fourier-domain OCT, has already been shown to be a powerful enabling technology for coronary imaging. The novel developments with high frame rate and fast pullback speed simplifies procedural requirements and will eventually eliminate limitations of current OCT systems such as need for proximal vessel balloon occlusion during image acquisition. This report details current and future developments in OCT imaging, which include exciting technological advancements that will consolidate the position of OCT as a key diagnostic tool to complement the armamentarium of the cardiologist well into the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optical coherence tomography (OCT) is a new intravascular imaging method using a fiber-optic technology. The greatest advantage of OCT is its extraordinary high-resolution about 10–20 μm, which is approximately ten times higher than that of intravascular ultrasound (IVUS). The high resolution afforded by this imaging modality is giving new insights into atherosclerotic plaque and the vascular responses after percutaneous coronary intervention (PCI) [1–3]. This report reviews the current and future developments of OCT and explores the clinical applications of OCT in coronary artery disease.
Current OCT technology
At present, three time-domain OCT systems have been developed for intravascular imaging: the M2 and M3 OCT system (LightLab Imaging, Westford, MA, USA); the Massachusetts General Hospital (MGH) OCT system; and CardioSpectra (Volcano Corporation, Rancho Cordova, CA, USA). The current OCT system employs a 0.016-inch fiber-optic imaging wire connected to a patient interface unit, which, in turn, hooks to the system console. The system console contains the optical engine and the computer (Fig. 1).
OCT is an optical analogue of IVUS using near-infrared light. The wavelength used is 1,310 nm, which minimizes absorption of the light waves by water, protein, lipids, and hemoglobin without tissue damage. Light is reflected by a tissue and by a moving mirror toward a detector. Information from the echo time delay measured by coherence interferometry and the intensity of backscatter of light from the tissue is used to create the image (Fig. 2a). The maximum imaging depth of the current OCT system is 1.0–2.0 mm, depending on tissue type, with axial and lateral resolutions of 10 and 20 μm, respectively. With a frame rate of approximately 20 f/ms and a pullback speed of 1.0–3.0 mm/s, high-resolution OCT images can be obtained without significant motion artifacts (Table 1).
Main components of time-domain OCT and Fourier-domain OCT. a In time-domain OCT, a broadband light source is divided by a beam splitter; part is sent to the tissue sample down the sample or measurement arm and the other down the reference arm to a moving mirror. The reflected signals are overlaid on a photo-detector. The intensity of interference is detected and used to create images. b In Fourier-domain OCT, the reference mirror does not move and the light source is a laser that sweeps its output rapidly over a broad band of wavelengths. Fourier transformation of the interference signals stored during a single sweep reconstructs the amplitude profile of the reflections, analogous to a single A-line in an ultrasound scan. Lasers with narrow line widths and wide sweep ranges enable the acquisition of Fourier-domain OCT images with high resolution over a wide range of depths
Procedural requirements for OCT imaging
As near-infrared light penetrates only a short distance through blood, temporary blood clearance is required for OCT imaging. To clear blood, a proximal occlusion balloon (Helios, Goodman Co. Ltd., Nagoya, Japan) is inflated to 0.5–0.7 atm, while simultaneously flushing physiological saline or Ringer’s lactate solution through the distal lumen of the balloon catheter at a rate of 0.5–1.0 ml/s. Yamaguchi et al. [4] evaluated the safety and feasibility of OCT in 76 patients. Procedural success rates were 97%, and no significant adverse events, including vessel dissection or fatal arrhythmia, were observed. Recently, a non-occlusive technique for OCT image acquisition has been developed alternative to the balloon-occlusion technique [5–7]. This non-occlusive technique based on manual infusion of contrast media or Dextran 40 and Ringer’s lactate solution (Low Molecular Dextran l Injection®; Otsuka Pharmaceutical Factory, Tokushima, Japan) through the guiding catheter simplifies the complex balloon-occlusion technique, leading to a marked reduction of procedural time. Barlis et al. [8] reported a large OCT registry data of 468 patients across 6 European medical centers. The OCT image acquisition was performed using occlusion balloon in 55% of cases, while the non-occlusive flush technique was used in the remaining 45%. Transient chest pain and QRS widening/ST-depression/elevation were observed in 48 and 46%, respectively. Major complications during OCT imaging included ventricular fibrillation (1.1%) due to balloon occlusion and/or deep guide catheter insertion, air embolism (0.6%) and vessel dissection (0.2%). There was no MACE within the 24 h after OCT examination.
Plaque characterization
The high resolution of OCT allows us to identify the boundary of the intima and media within the coronary arterial wall, which can not be distinguished by IVUS. In the OCT image, the intima is observed as a signal rich layer nearest the lumen, and media is visualized as a signal poor middle layer. The OCT measurement of intimal thickness is well correlated to histological examination [9]. OCT has an ability to evaluate subtle intimal thickening in vivo which is the early phase of coronary atherosclerosis.
Several histological examinations demonstrated that OCT is highly sensitive and specific for plaque characterization [10, 11] (Fig. 3). Yabushita el al [12] developed objective OCT image criteria for differentiating distinct components of atherosclerotic tissue. In their histology-controlled OCT study with 357 autopsy segments from 90 cadavers, fibrous plaques were characterized by homogeneous signal-rich regions, fibrocalcific plaques by signal-poor regions with sharp borders, and lipid-rich plaques by signal-poor regions with diffuse borders. Validation test revealed good intra- and inter-observer reliability (κ = 0.83–0.84) as well as excellent sensitivity and specificity: 71–79% and 97–98% for fibrous plaques; 95–96% and 97% for fibrocalcific plaques; and 90–94% and 90–92% for lipid-rich plaques, respectively. These definitions have formed the basis of plaque composition interpretation (Table 2). Using these definitions, Kawasaki et al. [13] reported that OCT has a best potential for tissue characterization of coronary plaques compared to integrated backscatter IVUS, and conventional IVUS (Fibrous tissue: sensitivity: 98% vs. 94% vs. 93%; specificity: 94% vs. 84% vs. 61%; Calcification: sensitivity: 100% vs. 100% vs. 100%; specificity: 100% vs. 99% vs. 99%; lipid pool: sensitivity :95% vs. 84% vs. 67%; specificity: 98% vs. 97% vs. 95%).
Vulnerable plaque detection
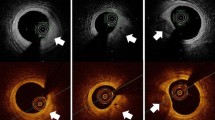
Rupture of vulnerable plaque is responsible for acute coronary events. Morphological features of vulnerable plaque include a large lipid core, a thin-capped fibroatheroma (TCFA: fibrous cap thickness <65 μm), and an accumulation of macrophages localized in the fibrous cap. Since OCT has a near-histological grade resolution, many in vitro and in vivo studies have been done to validate the capability of OCT to visualize these vulnerable plaque features [14–16] (Fig. 4).
Kubo et al. [17] used OCT, IVUS and angioscopy in patients with acute myocardial infarction (AMI) to assess the ability of each imaging method to detect the specific characteristics of vulnerable plaque. OCT was superior in detecting plaque rupture (73% vs. 40% vs. 43%, p = 0.021), erosion (23% vs. 0% vs. 3%, p = 0.003), and thrombus (100% vs. 33% vs. 100%, p < 0.001), as compared to IVUS and angioscopy. Intra- and inter-observer variability of OCT yielded acceptable concordance for these characteristics (κ = 0.61–0.83).
OCT might be the best tool available to detect TCFAs [18–20]. Kume et al. [21] examined the reliability of OCT for measuring the fibrous cap thickness. In the examination of 35 lipid-rich plaques from 38 human cadavers, there was a good correlation of the fibrous cap thickness between OCT and histological examination (r = 0.90; p < 0.001). In the clinical setting, Sawada et al. [22] compared the feasibility for detecting TCFA between OCT and virtual histology IVUS. Although the positive ratio of virtual histology IVUS for detecting TCFA was 45.9%, that of OCT was 77.8%. Jang et al. [23] analyzed OCT images among 57 patients who presented with stable angina pectoris (SAP), acute coronary syndrome (ACS), or AMI. The AMI group was more likely than the ACS group, who was more likely than the SAP group, to have a thinner cap, more lipid, and a higher percentage of TCFA (72% vs. 50% vs. 20% respectively, p = 0.012). Fujii et al. [24] performed a prospective OCT analysis of all 3 major coronary arteries to evaluate the incidence and predictors of TCFAs in patients with AMI and SAP. Multiple TCFAs were observed more frequently in AMI patients than in SAP patients (69% vs. 10%, p < 0.001). In the entire cohort, multivariate analysis revealed that the only independent predictor of TCFA was AMI (OR = 4.12; 95% CI = 2.35–9.87; p = 0.02,). On top of its reliability as a tool to measure the fibrous-cap thickness in vivo, a recent OCT study conducted by Takarada et al. [25] demonstrated that the lipid-lowering therapy with statin for 9 months significantly increased the fibrous-cap thickness in patients with hyperlipidemia (151 ± 110 to 280 ± 120 μm, p < 0.01). As therapies to prevent or make regression of atherosclerosis are developed, OCT can help to assess the treatment efficacy.
The OCT characteristics of coronary thrombi were studied by Kume et al. [26] in 108 coronary arterial segments at postmortem examination. White thrombi were identified as signal-rich, low-backscattering protrusions in the OCT image while red thrombi were identified as high-backscattering protrusions inside the lumen of the artery, with signal-free shadow. Using a measurement of the OCT signal attenuation within the thrombus, the authors demonstrated that a cut-off value of 250 μm in the 1/2 width of signal attenuation can differentiate white from red thrombi with a high sensitivity (90%) and specificity (88%).
A further unique aspect of OCT is its ability to visualize the macrophages. Tearney et al. [27, 28] proposed the potential of OCT to assess macrophage distribution within fibrous caps. There was a high degree of positive correlation between OCT and histological measurements of fibrous cap macrophage density (r < 0.84, p < 0.0001). A range of OCT signal standard deviation thresholds (6.15–6.35%) yielded 100% sensitivity and specificity for identifying caps containing >10% CD68 staining.
Guidance of coronary intervention
Considering the high resolution of OCT, it is not surprising that OCT provides more detailed morphological information for monitoring stent deployment than conventional imaging methods (Fig. 5). Kubo et al. [29, 30] demonstrated that OCT has an ability to detect stent edge dissection (40% vs. 16%, p = 0.005), tissue protrusion (58% vs. 20%, p < 0.001), and stent malapposition (47% vs. 18%, p < 0.001), at a level that is two to three times better than that of IVUS. Several studies also reported similar advantages of OCT in complex interventions such as bifurcation pecutaneous coronary intervention (PCI) and chronic total occlusions [31, 32]. Future prospective studies will need to address the consequences of these findings on the outcome after PCI. Recently, Tanaka et al. [33] investigated whether OCT could predict no-reflow after PCI in 83 patients with acute coronary syndrome. The size of lipid arc estimated by OCT was significantly greater in the no-reflow group than in the good-reflow group (166 ± 60 vs. 44 ± 63 degree, p < 0.001). Final TIMI blush grade deteriorated according to the increase in the lipid arc. A multivariable logistic regression model revealed that lipid arc was only an independent predictor of no-reflow (OR = 1.018; 95% CI = 1.004–1.033; p < 0.01). The authors concluded that OCT is useful tool for risk stratification of PCI.
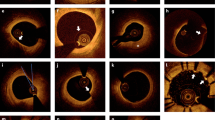
Moreover, OCT is capable of visualizing and quantifying thin neointimal hyperplasia after drug-eluting stent implantation (DES). Based on the data from animal studies of DES, the average late lumen loss for DES could be lower than 100 μm, which means this amount of corresponding intimal thickening is difficult to detect by IVUS. Several studies have recently been published highlighting the application of OCT in the detection of stent tissue coverage at follow-up [34]. Matsumoto et al. [35] studied 34 patients who underwent OCT examination at 6 months follow-up after sirolimus-eluting stent (SES; Cypher, Cordis Corp., Miami Lakes, Florida, USA) implantation. The median neointima thickness was 53 μm and the average rate of neointima-covered struts in an individual SES was 89%. Nine SES (16%) showed full coverage by neointima, whereas the remaining stents had partially uncovered strut lesions. Similarly, Yamamoto et al. [36] have recently reported 2 year follow-up OCT findings after SES implantation in 21 patients. Mean thickness of neointimal hyperplasia was 71 μm. Frequency of uncovered struts was 5%. In the bifurcations, 10% of stent struts located in the orifice of side branches were uncovered. In the overlapping segments, 7% of stent struts were uncovered. Prevalence of patients who had any uncovered struts was 81%. Furthermore, OCT identified in-stent thrombi in three patients, who had no abnormal findings on coronary angiograms. To determine when antiplatelet therapy could be discontinued, OCT provides important information about chronic drug-eluting stent status (Fig. 6).
OCT images of in-stent thrombosis at 2 months after paclitaxel-eluting stent implantation. a No abnormal findings were observed by coronary angiography in the stented lesion of the left ascending coronary artery. b Stent struts were fully covered by neointima in the distal part of the stent. c Thrombus was detected by OCT in the mid part of stent (arrowhead). d Some stent struts were mallapposed and not covered by neointima in the proximal part of the stent
Limitations
The current OCT system has some limitations for clinical use. First, an inherent limitation of OCT is the need for a blood-free imaging zone, because the near-infrared light signals are attenuated by red blood cells. The coronary occlusion for OCT image acquisition limits evaluation of left main or ostial coronary lesions. Second, the time constraint imposed by blood flow interruption as well as slow frame rate of current OCT system prevents scanning of a significant length of a coronary artery during a single flush. Third, the rate of flush may have a risk of underestimating the size of the vessel lumen. Finally, OCT has a relatively shallow axial penetration depth of 2 mm. The OCT signal does not reach the back wall of thick atherosclerotic lesions. The penetration depth of OCT depends on tissue characteristics. The OCT signal is attenuated more rapidly in the lipidic plaque compared to the fibrotic plaque. OCT is not appropriate for the visualization of whole vessel and the evaluation of arterial remodeling [37].
Current technology challenges
Recently, a second-generation OCT technology, termed Fourier-domain OCT, has been developed that solves the current time-domain OCT problems by imaging at much higher frame rates (100 frame/s), a faster pullback speed (20 mm/s), and a wider scan diameter (8.3 mm), without loss of image quality (Table 3). These advantages result from the elimination of mechanical scanning of the reference mirror, and signal-to-noise advantages of Fourier-domain signal processing (Fig. 2b). Imaging catheter of Fourier-domain OCT, which is designed for rapid-exchange delivery, has 2.5–2.8 Fr crossing-profile and can be delivered over a 0.014-inch guidewire through a 6 Fr or larger guide catheter. Injecting saline, angiographic contrast media, or a mixture or contrast and saline, through the guide catheter (4–6 ml/s, 2–3 s) can achieve effective clearing of blood for Fourier-domain OCT imaging. The high frame rate and fast pullback speeds of Fourier-domain OCT allows to image long coronary segments with minimal ischemia, eliminating the need for proximal vessel balloon occlusion during image acquisition.
To validate safety and feasibility of Fourier-domain OCT (C7, LightLab Imaging, Westford, MA, USA), a clinical study was commenced from September, 2008, at Wakayama Medical University, Wakayama Japan, in collaboration with LightLab Imaging. Figure 7 shows an example of a Fourier-domain OCT image extracted from pullback image sequence recorded from one of the patients. Recently, Tearney et al. reported that Fourier-domain OCT, called optical frequency-domain imaging (OFDI) by the author’s group, enables imaging of the 3-dimensional microstructure of long segments of coronary arteries [38]. In addition, Fourier-domain OCT facilitates the acquisition of spectroscopic and polarization, Doppler, and other imaging modes for plaque characterization [39, 40]. When Fourier-domain OCT is fully exploited, it has the potential to dramatically change the way that physicians and researchers understand coronary artery disease, which will in turn result in better diagnosis and treatment.
Conclusion
The high resolution of OCT provides histology-grade definition of the microstructure of coronary plaque in vivo. OCT allows a greater understanding of the pathophysiology of coronary artery disease and greater guidance of the appropriate patient-specific therapeutic approach. Whether OCT will have an established clinical role in vulnerable plaque detection and treatment must depend on the outcomes of future prospective natural history studies. Fourier-domain OCT with fast pullback speed and simple procedural requirements makes it more accessible to a greater number of centers and operators world-wide. With ongoing development of this technology, OCT has the potential to be an important clinical imaging modality, complementary to angiography and IVUS.
References
Kubo T, Akasaka T. Recent advances in intracoronary imaging techniques: focus on optical coherence tomography. Expert Rev Med Devices. 2008;5:691–7.
Kubo T, Akasaka T. OCT-ready for prime time? : clinical applications of optical coherence tomography. Cardiac Interve Today. 2009;4:35–7.
Raffel OC, Akasaka T, Jang IK. Cardiac optical coherence tomography. Heart. 2008;94:1200–10.
Yamaguchi T, Terashima M, Akasaka T, Hayashi T, Mizuno K, Muramatsu T, et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol. 2008;101:562–7.
Kataiwa H, Tanaka A, Kitabata H, Imanishi T, Akasaka T. Safety and usefulness of non-occlusion image acquisition technique for optical coherence tomography. Circ J. 2008;72:1536–7.
Prati F, Cera F, Ramazzotti V, Imola F, Giudice R, Albertucci M. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. Eurointervention. 2007;3:365–70.
Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, Giudice M, et al. From bench to bedside: a novel technique of acquiring OCT images. Circ J. 2008;72:839–43.
Barlis P, Gonzalo N, Di Mario C, Prati F, Buellesfeld L, Rieber J, et al. A multicentre evaluation of the safety of intracoronary optical coherence tomography. EuroIntervention. 2009;5:90–5.
Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, et al. Assessment of coronary intima-media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69:903–7.
Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, et al. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol. 2006;97:1172–5.
Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–9.
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–5.
Kawasaki M, Bouma BE, Bressner J, Houser SL, Nadkarni SK, MacNeill BD, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol. 2006;48:81–8.
Kitabata H, Kubo T, Akasaka T. Identification of multiple plaque ruptures by optical coherence tomography in a patient with acute myocardial infarction: a three-vessel study. Heart. 2008;94:544.
Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;118:2368–73.
Tanimoto T, Imanishi T, Tanaka A, Yamano T, Kitabata H, Takarada S, et al. Various types of plaque disruption in a culprit coronary artery visualized by optical coherence tomography in a patient with unstable angina. Circ J. 2009;73:187–9.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–9.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Implication of plaque color classification for assessing plaque vulnerability: a coronary angioscopy and optical coherence tomography investigation. JACC Cardiovasc Interv. 2008;1:74–80.
Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Kataiwa H, et al. Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2008;102:975–9.
Kume T, Okura H, Yamada R, Kawamoto T, Watanabe N, Neishi Y, et al. Frequency and spatial distribution of thin-cap fibroatheroma assessed by 3-vessel intravascular ultrasound and optical coherence tomography. Circ J. 2009;73:1086–91.
Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, et al. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152:e1–4.
Sawada T, Shite J, Garcia-Garcia HM, Shinke T, Watanabe S, Otake H, et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–46.
Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551a–5a.
Fujii K, Masutani M, Okumura T, Kawasaki D, Akagami T, Ezumi A, et al. Frequency and predictor of coronary thin-cap fibroatheroma in patients with acute myocardial infarction and stable angina pectoris a 3-vessel optical coherence tomography study. J Am Coll Cardiol. 2008;52:787–8.
Takarada S, Imanishi T, Kubo T, Tanimoto T, Kitabata H, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. 2009;202:491–7.
Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97:1713–7.
Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–9.
MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, et al. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44:972–9.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, et al. Comparison of vascular response after sirolimus-eluting stent implantation between unstable angina pectoris and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2008;1:475–84.
Kubo T, Akasaka T. Reply Letter to: optical coherence tomography to diagnose under-expansion of a drug eluting stent. JACC Cardiovasc Imaging. 2009;2:246.
Bouma BE, Tearney GJ, Yabushita H, Shishkov M, Kauffman CR, DeJoseph Gauthier D, et al. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89:317–20.
Takeda Y, Katoh D. OCT guided winning technique for chronic total occlusion. In: Regar E, van Leeuwen AMGJ, Serruys PW, editors. Optical coherence tomography in cardiovascular research. 1st ed. United Kingdom: Informa Healthcare, 2007:45.
Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, et al. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur Heart J. 2009;30:1348–55.
Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, et al. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am J Cardiol. 2007;99:1033–8.
Matsumoto D, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, et al. Neointimal coverage of sirolimus-eluting stents at six-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–7.
Takano M, Yamamoto M, Inami S, Murakami D, Seimiya K, Ohba T, et al. Long-term follow-up evaluation after sirolimus-eluting stent implantation by optical coherence tomography: do uncovered struts persist? J Am Coll Cardiol. 2008;51:968–9.
Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Matsumoto H, Arita Y, et al. Relationship between coronary arterial remodeling, fibrous cap thickness and high-sensitivity c-reactive protein levels in patients with acute coronary syndrome. Circ J. 2009;73:1291–5.
Tearney GJ, Waxman S, Shishkov M, Vakoc BJ, Suter MJ, Freilich MI, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc Imaging. 2008;1:752–61.
Giattina SD, Courtney BK, Herz PR, Harman M, Shortkroff S, Stamper DL, et al. Assessment of coronary plaque collagen with polarization sensitive optical coherence tomography (PS-OCT). Int J Cardiol. 2006;107:400–9.
Nadkarni SK, Pierce MC, Park BH, de Boer JF, Whittaker P, Bouma BE, et al. Measurement of collagen and smooth muscle cell content in atherosclerotic plaques using polarization-sensitive optical coherence tomography. J Am Coll Cardiol. 2007;49:1474–81.
Conflict of interest statement
The authors discloses that they hold no financial interest in any product or manufacturer mentioned herein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubo, T., Akasaka, T. Optical coherence tomography imaging: current status and future perspectives. Cardiovasc Interv and Ther 25, 2–10 (2010). https://doi.org/10.1007/s12928-009-0006-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-009-0006-3