Abstract
Electroencephalogram (EEG) studies of mindfulness have shown it can lead to increases in alpha power, which are similar to those obtained by alpha-based neurofeedback (NF) interventions. It has been hypothesized there may be relationships between mindfulness and NF in terms of the neural pathways through which they induce salutary outcomes. The aim of the study was to evaluate possible changes in mindfulness and cognitive functioning following an alpha-based NF intervention, and the role of alpha power as a mediator of improvements. A controlled, non-randomized, trial with 50 healthy participants was conducted with two experimental conditions: a six-session NF intervention and a waiting-list control group. Both groups were administered mindfulness questionnaires (Mindful Attention Awareness Scale (MAAS), Five Facet Mindfulness Questionnaire (FFMQ)) and cognitive measures (Paced Auditory Serial Addition Task (PASAT)), at pre- and post-test. The NF intervention focused on the up-regulation of upper alpha power. Differences among groups were estimated using ANCOVAs, and mediation assessment through path analyses. Compared to controls, the NF group showed enhanced task-related upper alpha power (effect size (ES) = 1.16, p < 0.001), mindfulness outcomes (MAAS: ES = 0.94, p = 0.004; FFMQ: ES = 1.38, p < 0.001), and a trend of cognitive functioning (PASAT time: ES = 0.59, p = 0.062). Upper alpha power had a mediating effect for cognitive functioning (PASAT errors: indirect effect = 0.81, 95% CI = 0.21–1.85), but not for mindfulness. These results demonstrate the effectiveness of NF for increasing mindfulness in healthy individuals with no previous experience in mindfulness or neurofeedback training, suggesting that NF may be an acceptable method of augmenting mindfulness-related capacities in the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mindfulness derives from Buddhist practice and is the process of remaining consciously aware of present-moment sensory and psychological experience (Van Gordon et al. 2015). Two of the most empirically investigated mindfulness-based interventions are mindfulness-based stress reduction (MBSR; Kabat-Zinn 2003) and mindfulness-based cognitive therapy (MBCT; Teasdale et al. 1995). Both of these interventions are associated with improvements in attention, body awareness, emotional regulation, and self-perspective (Holzel et al. 2011). MBSR and MBCT also have demonstrable efficacy for treating specific somatic and psychiatric disorders, including chronic pain, mood disorders, and anxiety disorders (Shonin et al. 2015).
A significant body of research has shown neurophysiological changes associated with mindfulness practices. Neuroimaging studies have reported neuroplastic changes in the anterior and posterior cingulate cortex, medial prefrontal cortex, insula, temporo-parietal junction, and the fronto-limbic and default mode network structures (Chiesa and Serretti 2010; Holzel et al. 2011). Electroencephalogram (EEG) effects have been extensively assessed mainly by power spectral analysis in different frequency bands (such as delta, theta, alpha, beta, and gamma) evaluating the power (or amplitude) differences during meditation in comparison to a control condition (mostly involving either closed- or open-eye resting state, although other tasks have been used). Contradictory results have been reported with mixed effects including power increases, decreases, or no differences. For example, several studies have reported increased alpha band amplitude during meditation (Ahani et al. 2014; Chow et al. 2017; Dunn et al. 1999; Hinterberger et al. 2014; Lagopoulos et al. 2009; Milz et al. 2014; Yu et al. 2011), while other studies reported no significant differences (Cahn et al. 2010; Lehmann et al. 2012) or decreased amplitude (Amihai and Kozhevnikov 2014). Recent meta-analyses—attempting to clarify this relationship—have concluded that the most consistent patterns associated with mindfulness practice are increased theta and alpha power although not all included studies were consistently conformed to these findings (Cahn and Polich 2006; Lomas et al. 2015).
This evidence has prompted proposals that meditation can be employed as a technique for cultivating psychosocially adaptive mental states, with similar effects to those elicited by neuromodulation techniques such as EEG-based neurofeedback (NF) (Brandmeyer and Delorme 2013). NF is a technique that promotes the self-regulation of brain patterns. It consists of monitoring brain activity, decoding brain patterns of interest, and providing users with real-time feedback (e.g., via audio or visual stimuli). It has been proposed as a suitable technique for treating disorders such as depression, anxiety, attention-deficit hyperactivity disorder, epilepsy, and insomnia (Niv 2013). NF and mindfulness practice present similar neurophysiological correlates because while mindfulness is principally associated with increased alpha power (Cahn and Polich 2006; Lomas et al. 2015), many NF protocols aimed at cognitive enhancement specifically target the up-regulation of alpha power in order to produce the desired effects (see review by Gruzelier 2014). Since the neuronal substrates of inhibitory mechanisms are hypothesized to be related to alpha oscillations, these NF protocols are supposed to enhance cognitive function by filtering non-relevant information or conflicting stimuli to the task being performed (Freunberger et al. 2011; Klimesch et al. 2007).
One of the most widely used NF training interventions to date for cognitive enhancement focuses on the up-regulation of the upper part of the alpha frequency band (Escolano et al. 2011, 2014; Gruzelier 2014; Hanslmayr et al. 2005; Zoefel et al. 2011), referred to as “upper alpha” (Klimesch 1999), in posterior locations of the scalp. This area shows the most prominent alpha activity (Niedermeyer and da Silva 2005), and it is commonly reported to be enhanced (but not topographically restricted) during meditation practice (Ahani et al. 2014; Dunn et al. 1999; Hinterberger et al. 2014; Lagopoulos et al. 2009; Yu et al. 2011). Moreover, there is a body of evidence showing a relationship between increased parieto-occipital alpha activity and cognitive function (Tuladhar et al. 2007; Van Dijk et al. 2008), pointing at inhibitory mechanisms of task-irrelevant brain regions (Freunberger et al. 2011; Jensen and Mazaheri 2010). Furthermore, it has been proposed that the division of the alpha band in two parts (lower and upper) reflects different cognitive processes, with the upper part specifically related to memory performance (Klimesch 1999).
The primary aim of the present study was to evaluate the effect of an upper alpha EEG-based NF intervention on mindfulness-related capacities and cognitive processing. It was hypothesized that NF training might produce improvements in mindfulness and also in cognitive processing and that possible enhancement in upper alpha power as a result of the NF training might mediate changes in mindfulness-related capacities and cognitive processing.
Method
Participants
Based on previous alpha-based neurofeedback studies (e.g., Escolano et al. 2014), a sample size of N = 50 healthy participants was considered adequate to find significant differences in the main outcome variables. Inclusion criteria were the following: (a) aged between 18 and 65 years and (b) able to speak and understand Spanish. Exclusion criteria were the following: (a) diagnosed with a neurological or psychological disorder and (b) having practiced mindfulness or a contemplative mind-body technique in the last 5 years.
No participants were ruled out of the study due to exclusion criteria. Fifty healthy participants were allocated to either the NF intervention (n = 27) or control condition (n = 23). However, three participants from the NF intervention and two from the control condition were excluded due to excessive artifacts in the EEG. Therefore, the final sample comprised 24 participants in the NF group and 21 in the control group. No significant differences between any socio-demographic variables at baseline were found between the two groups (Table 1). Intervention and control groups did not differ statistically in baseline individual alpha frequency (IAF): mean and SD IAF were 9.92(0.68) Hz for the intervention group and 10.17(0.67) Hz for the control group (t(43) = −1.26, p = 0.21).
Procedure
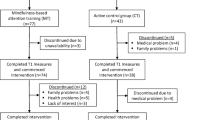
A pre-post, non-randomized, controlled trial was conducted with two experimental conditions: a six-session NF intervention and a waiting-list control group. A covariate-adaptive randomization (minimization) procedure (Hu et al. 2014) was employed to allocate participants to either the NF intervention or waiting-list control, matching them on sex, age, and education level, to balance the groups. The two allocation conditions performed a behavioral assessment (evaluating mindfulness-related capacities and cognitive function) and an EEG screening at both baseline and end-point phases (Fig. 1). EEG was not recorded during the execution of the behavioral assessments.
Experimental design of the study. The NF group and control group each completed a pre- and post-behavioral assessment and EEG screening within a 4-week time interval. The NF condition consisted of a total of six NF sessions (two sessions per week) that comprised five individual training trials (4 min each) and a pre- and post-EEG screening (6 min each). The EEG screenings included two recordings: eyes closed resting state and eyes open task-related activity
The NF intervention comprised a total of six sessions with a frequency of two sessions per week. Each session involved 20 min of NF training and an EEG screening at the beginning and end of the session. Each EEG screening consisted of two recordings: 3 min with closed eyes (resting state) and 3 min with open eyes in alert state (task-related activity). In the latter condition, participants were instructed to watch a computer screen and count the change of color tones in a square that progressively changed from gray to red or blue.
The researchers that conducted the behavioral assessments and EEG screenings were blinded as to allocation condition. The study was approved by the Aragon Ethical Committee (June 26, 2013; PI13/00077) and followed the recommendations of the Helsinki Declaration (and its subsequent modifications). Participants provided informed consent before engaging in any assessment.
EEG Recording
EEG data was recorded from 16 electrodes placed at FP1, FP2, F3, Fz, F4, C3, Cz, C4, P7, P3, Pz, P4, P8, O1, Oz, and O2 (subset of the 10/10system), with the ground and reference electrodes on FPz and left earlobe, respectively. EEG was amplified and digitized using a g.tec amplifier (Guger Technologies, Graz, Austria) at a sampling rate of 256 Hz, power-line notch-filtered at 50 Hz, and 0.5–60 Hz band-pass filtered. EEG recording and the NF procedure were administered using a locally developed software (Bit&Brain Technologies, Zaragoza, Spain).
Neurofeedback Procedure
The NF training focused on the increase of individual upper alpha power averaged over parieto-occipital locations (P3, Pz, P4, O1, and O2: referred to as the feedback electrodes). EEG power was calculated through a short-term fast Fourier transform (FFT) analysis with a 1s hamming window, 30 ms of overlapping, and zero-padded to 1024 points (0.25 Hz resolution). For each session, the pre-NF EEG screening was recorded and then used to calibrate the training for each participant and session. In this calibration step, we automatically filtered out the blinking component from the task-related activity by independent component analysis (ICA) using the FastICA algorithm (Hyvarinen 1999). Furthermore, we removed the epochs with amplitude larger than 200 uV at any electrode. The IAF was computed for each electrode on the power spectra of the reconstructed EEG data as the frequency bin with the maximum power value in the extended alpha range 7–13 Hz (Klimesch 1999). When no clear alpha peak was evident, the IAF was computed on resting state instead. The upper alpha band was thus defined as the [IAF, IAF+2] Hz interval (Klimesch 1999). The baseline was computed as the mean upper alpha power averaged across the feedback electrodes, and the 5th–95th percentiles established the lower and upper limits, respectively. After the calibration, participants performed the training trials. During online training, EEG data was online-filtered from blinking artifacts (through the aforementioned ICA filter) and a visual feedback was then displayed every 30 ms on a computer screen in the form of a square with changing saturation colors.
Measures
The following behavioral assessments (mindfulness and cognitive functioning questionnaires) were administered at both baseline and end-point phases:
The Five Facet Mindfulness Questionnaire (FFMQ; Baer et al. 2006) assesses five facets of mindfulness: observing, describing, acting with awareness, non-judging of inner experience, and non-reactivity to inner experience. It consists of 39 items rated on a Likert scale ranging from 1 (never or very rarely true) to 5 (very often or always true), with higher scores reflecting higher self-reported mindfulness skills. The Spanish version of the FFMQ—that has good levels of internal validity—was utilized in the present study by using a unique total score in order to simplify the total number of mindfulness assessments (Cebolla et al. 2012).
The Mindful Attention Awareness Scale (MAAS; Brown and Ryan 2003) assesses dispositional mindfulness and comprises 15 items that are rated on a scale from 1 (almost always) to 6 (almost never). Participants are asked to provide their responses based on everyday experience (i.e., rather than their experiences over a specified period of time). Individual item ratings are summed to provide a total score, with a higher score indicating higher levels of dispositional mindfulness. The present study employed the Spanish version of MAAS that has good psychometric properties (Soler et al. 2012).
The Paced Auditory Serial Addition Task (PASAT; Gronwall 1977) evaluates working memory and processing speed. The test is sensitive to minimal changes in neurocognitive performance and presents high levels of internal consistency and test-retest reliability (Tombaugh 2006). Test scores reflect the number of errors and elapsed time.
Data Analyses
EEG data from the initial and final EEG screenings was offline-inspected for the presence of artifacts such as eye blinks, eye movements, body movements, and electrocardiogram artifacts. Initially, the extended infomax ICA (Lee et al. 1999) was applied to the task-related activity to remove the eye-blinking component. Subsequently, both resting state and task-related activity were imported into EureKa! software (Congedo 2002) to eliminate the contaminated data by visual inspection. Participants with at least 30 s of artifact-free data were included in the analysis. EEG spectrum was computed following the same procedure as in the NF procedure. The main outcome for the EEG analysis was the pre-post study effects in the process variable (individual upper alpha power averaged across the feedback electrodes: P3, Pz, P4, O1, O2) measured as the power comparison in the initial versus final EEG screenings in both resting state and task-related activity.
For analyzing responses to socio-demographics, the Fisher test was used for categorical variables (sex, education level) and the Student t test for continuous variables (age). This was followed by an analysis of covariance (ANCOVA) for each mindfulness, cognitive, and EEG variable, with the factors of group (NF, control) and time (pre, post), and the baseline scores of each variable as a covariate. Effect sizes (ES) for the group × time interaction were calculated by means of Cohen’s d from partial eta-squared values (Cohen 1988). The rule of thumb for Cohen’s d is that 0.20 is small, 0.50 is medium, and 0.80 is large. Pearson or Spearman coefficients (depending on the nature of the variable distributions) were employed to test the correlations between the change scores of outcomes and their respective baseline values. We analyzed the direct and indirect relationships between treatment, EEG upper alpha values, and mindfulness and cognition outcomes using path analysis models. The treatment condition was the independent variable, the pre-post change scores in EEG upper alpha (process variable) was the mediator, and the dependent variables were the pre-post changes in outcome variables. The path analysis model for each outcome with significant correlation with the process variable was computed. The direct path between study condition and clinical outcome and the indirect effect through EEG upper alpha were tested. The model parameters were estimated using maximum likelihood (ML), and bootstrap values were used to compute the 95% bias-corrected confidence intervals (95% CI) for indirect effects. It was inferred that an indirect effect was statistically significant at the 5% significance level if the bootstrap 95% CI did not include zero. EEG variables (power values) were log-transformed prior to statistical testing.
The type I error was set at α = 0.05, taking into account Bonferroni’s criterion for multiple comparisons. All the analyses were performed with MATLAB-R2016A and SPSS-20.
Results
EEG
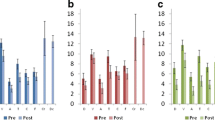
We evaluated the effects in the process variable (power in the individual upper alpha band averaged across the feedback electrodes) pre-post study in both resting state and task-related activity. As shown in Table 2, a significant [group × time] ANCOVA interaction (F(1,42) = 14.24, p < 0.001) was observed for task-related activity, favoring the NF group, with a large ES (d = 1.16). On the contrary, no significant [group × time] interaction (F(1,42) = 0.79, p = 0.378) was observed for the resting state activity.
Mindfulness and Cognitive Tests
As shown in Table 2, FFMQ showed a significant [group × time] ANCOVA interaction, (F(1,42) = 19.87, p < 0.001), favoring the NF group, with a large ES (d = 1.38). In the same way, the MAAS score demonstrated a significant [group × time] interaction (F(1,42) = 9.32, p = 0.004), favoring the NF group, with a large ES (d = 0.94). On the contrary, PASAT errors did not show a significant [group × time] interaction (F(1,42) = 2.56, p = 0.12), but presented a moderate ES (d = 0.49). Furthermore, PASAT time showed a trend in the [group × time] interaction (F(1,42) = 3.61, p = 0.06), with a moderately large ES (d = 0.59), favoring the NF group.
Correlations Between Baseline and Differential Scores
The change scores of EEG variables presented non-normal distributions, while the corresponding mindfulness and cognitive variables were normally distributed. The EEG variables at baseline did not show significant correlations with their respective change scores, but the mindfulness and cognitive outcomes showed significant inverse correlations (Table 3). The correlation analysis between differential scores identified significant associations between resting state activity and task-related activity (r = 0.35; p = 0.02); task-related activity and MAAS (r = 0.34; p = 0.02); task-related activity and PASAT errors (r = −0.45; p = 0.002); FFMQ and MAAS (r = 0.65; p < 0.001); FFMQ and PASAT time (r = −0.34; p = 0.02); FFMQ and PASAT errors (r = −0.32; p = 0.03); MAAS and PASAT errors (r = −0.34; p = 0.02); and PASAT time and PASAT errors (r = 0.47; p = 0.001).
Mediation Analyses
Two path models were tested to assess the possible mediation effect of task-related activity in MAAS and PASAT errors. The results did not support task-related activity change as a mediator of the changes in MAAS (bootstrapped indirect effect: mean = −0.37; SE = 0.91; 95% CI = −2.47–1.23), but there was a significant direct path, controlling for the task-related activity, between study condition and MAAS (beta = −7.21; SE = 2.25; t(43) = −3.20; p = 0.003). On the other hand, the study condition significantly predicted the change in task-related activity, which in turn predicted the change in PASAT errors (bootstrapped indirect effect: mean = 0.81; SE = 0.41; 95% CI = 0.21–1.85), being that there was not a significant direct path—after controlling for the task-related activity—between study condition and PASAT errors (beta = 0.36; SE = 0.87; t(43) = 0.41; p = 0.68).
Discussion
Although previous studies have described the effect of NF on cognitive functioning (Escolano et al. 2011, 2014; Gruzelier 2014), this study addresses the effect of NF on two of the most widely established mindfulness questionnaires (i.e., MAAS and FFMQ). The NF training focused on the up-regulation of the upper alpha power in posterior locations of the scalp. In the present study, cognitive functioning was assessed in addition to mindfulness outcomes in order to replicate previous findings and to ascertain whether any effect of NF in the aforementioned mindfulness and cognitive variables was mediated through upper alpha levels.
Due to the necessity to confirm that the NF intervention had produced the expected changes in EEG, we measured the effects in the trained parameter (upper alpha) in both resting state (eyes closed) and task-related activity (eyes open). Pre-post analysis revealed a significant upper alpha power enhancement during the task-related activity as a result of the NF training. These findings are consistent with EEG outcomes from similar NF interventions focusing on alpha up-regulation (Escolano et al. 2011, 2014; Zoefel et al. 2011). No significant changes were found in closed-eyes resting state activity—which are consistent with other studies (Escolano et al. 2014)—suggesting that the effects of the NF protocol on EEG do not translate to closed-eyes resting state. However, given that other studies (Nan et al. 2012) have not observed significant changes in pre-post baseline measures that combined closed-eyes and open-eyes resting state activity, the role of closed versus open eyes clearly needs to be evaluated.
The effects of NF on mindfulness were in the hypothesized direction. Given that NF has been shown to enhance various facets of cognitive functioning (Gruzelier 2014), it was expected that scores on the MAAS—a broad measure of dispositional mindfulness closely related to attentional processes (Bergomi et al. 2013)—would likewise improve. However, it was unexpected that the magnitude of improvements in mindfulness (effect sizes in the moderate-to-strong range) would be of a similar order to those observed following training in mindfulness using standard protocols such as MBSR (Martín Asuero et al. 2013). Improvements on the FFMQ (which arguably assesses a more comprehensive and targeted range of mindfulness attributes) remain to be decrypted across all five sub-scales. In the present study, an exploratory unique total score was measured for the FFMQ to avoid decreases in statistical power associated with multiple comparisons. Thus, the way in which different mindfulness facets are modified by NF training is a question that should be addressed in future research. In comparison to literature, Chow et al. (2017) investigated the effects of 15 min of either mindfulness meditation or alpha-based NF in mindfulness levels (as measured by the Toronto Mindfulness Scale) and reported no significant differences at post-intervention with regard to a sham-feedback control group, which suggests that a single intervention session might not produce observable effects in mindfulness-related capacities.
Although MAAS has been widely used in research (Osman et al. 2016), it has received several criticisms. One of the most important is that it has been shown to be unable to detect differences in mindfulness levels between meditators and non-meditators (MacKillop and Anderson 2007). In addition, MAAS is orientated more as a measure of “mindlessness” rather than mindfulness per se (Sauer et al. 2013). In respect of the FFMQ, one of its major shortcomings is that the methodology to construct the FFMQ mixes the dispositional and cultivated forms of mindfulness (Rau and Williams 2016). For instance, the MAAS is considered a tool to measure traits (MacKillop and Anderson 2007), whereas for example the Kentucky Inventory of Mindfulness Skills is used to assess mindfulness skills (Baer et al. 2004). Given that both questionnaires were included in the pool used to select the items of the FFMQ, using the same inventory to measure two different constructs may result in unreliable conclusions (Rau and Williams 2016).
While similar alpha-based NF interventions have reported significant improvements in cognitive functioning (Gruzelier 2014; Zoefel et al. 2011), and specifically in working memory (Escolano et al. 2011, 2014; Nan et al. 2012), these effects were less apparent in the present study. More specifically, we observed an improvement in processing speed as a marginal trend, and no significant enhancements in working memory as measured by the PASAT. Although the results were not significant, the ES obtained for processing speed and working memory was moderate, so we can assume that the use of a larger sample size might have allowed the detection of significant differences between groups in these cognitive variables. These results are in line with another study involving patients with depression and using the same evaluation test (Escolano et al. 2014). However, slightly lower ES were obtained in the present study, possibly because it involved healthy participants with better PASAT baseline measures, which might have led to a ceiling effect. This effect seems especially apparent in the PASAT error outcome (with a mean of around 3 in the present study and of 13 in depressive patients) (Escolano et al. 2014).
EEG upper alpha baseline values were not significantly related to their corresponding change scores, but mindfulness and cognitive baseline scores were inversely and significantly related to the observed differential values. This suggests that mindfulness and cognitive improvements were determined by their starting values, showing possible ceiling effects (Montero-Marin et al. 2016), while upper alpha enhancement might be free of that effect (or might have not been reached after six training sessions). An alternative explanation could be related to methodological artifacts of assessment, in the sense that the mindfulness and cognitive measures might not be sensitive to the pre-post changes of training in the case of high baseline scores. It is worth testing both possible explanations in future research, clarifying if the limits they point to arise due to the NF intervention, or the measurement methods.
Improvements in mindfulness arising due to NF training were not mediated by EEG upper alpha power, and thus, the mechanism of change remains unknown in respect of this outcome. Therefore, other EEG features should be explored as possible mediators of mindfulness improvement when using NF training. Nevertheless, improvements in cognitive processing measured by means of the number of errors of the PASAT were mediated by upper alpha power. This is consistent with the associations found between relative upper alpha power and working memory, by means of digit span measures (Nan et al. 2012).
In general, the observed improvements in mindfulness correlated with enhancements in cognitive functioning. Specifically, FFMQ change scores were related to both processing speed and number of errors, while MAAS change scores were only associated with the number of errors. This could be due to the fact that different cognitive functioning facets may depend on distinct mindfulness attributes, such as “focused attention” and “open monitoring” (Chiesa et al. 2011). Given that it is currently unclear which of these different attributes are targeted by NF, as well as the aforementioned limitations of the mindfulness questionnaires, further research would be required to explore this observation.
The use of EEG-based NF techniques to improve meditative awareness is not new as it has been successfully utilized to enhance “effortless awareness meditation” (Van Lutterveld et al. 2016). Other neurobiological techniques, such as repetitive transcranial magnetic stimulation, have also been found to increase mindfulness-related capacities (Leong et al. 2013). Given that some neurobiological interventions have even gone as far as administering hallucinogenic plants (i.e., ayahuasca) to augment mindfulness-related capacities (Soler et al. 2016), it seems reasonable to conclude that NF—a non-invasive EEG-based technique—could be an acceptable intervention for improving dispositional mindfulness.
Limitations
The present pilot study has several limitations. Firstly, this is a controlled but not a randomized controlled trial (participants were allocated to the intervention or control condition in order to find a homogeneous match between groups in socio-demographic variables). Secondly, other well-being variables associated with mindfulness (e.g., acceptance, compassion, non-attachment) were not assessed. Therefore, it remains unclear how improvements in mindfulness due to NF training are moderated/mediated by sophisticated variables that determine the therapeutic effects of mindfulness. Finally, there was no follow-up assessment. Consequently, it is difficult to know how mediating effects could work at follow-up and whether improvements in mindfulness and cognitive functioning would be maintained over time without the use of booster sessions.
The present study indicates in an exploratory way that mindfulness-related capacities can be improved via a short training in NF, without engaging in meditation practice. Furthermore, findings indicate that NF can improve mindfulness-related capacities in a shorter timescale compared to using standard mindfulness protocols such as MBSR. Such a conclusion is only as reliable as the scales employed to measure mindfulness, and it should be noted that an increasing number of researchers and Buddhist teachers have questioned the face validity of both the MASS and FFMQ (Grossman and van Dam 2011). Nevertheless, further research is warranted to explore the utility of NF for augmenting mindfulness and to ascertain whether NF-induced improvements in mindfulness (i) are correlated with increases in mindfulness-related variables such as non-attachment and acceptance, and (ii) reduce over time.
References
Ahani, A., Wahbeh, H., Nezamfar, H., Miller, M., Erdogmus, D., & Oken, B. (2014). Quantitative change of EEG and respiration signals during mindfulness meditation. Journal of Neuroengineering and Rehabilitation, 11, 87. doi:10.1186/1743-0003-11-87.
Amihai, I., & Kozhevnikov, M. (2014). Arousal vs. relaxation: a comparison of the neurophysiological and cognitive correlates of Vajrayana and Theravada meditative practices. PloS One, 9(7). doi:10.1371/journal.pone.0102990.
Baer, R. A., Smith, G. T., & Allen, K. B. (2004). Assessment of mindfulness by self-report: the Kentucky inventory of mindfulness skills. Assessment, 11, 191–206. doi:10.1177/1073191104268029.
Baer R. A., Smith G. T., Hopkins J., Krietemeyer J., Toney L. (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13, 27–45. doi: 10.1177/1073191105283504.
Brandmeyer, T., & Delorme, A. (2013). Meditation and neurofeedback. Frontiers in Psychology, 4, 3–5. doi:10.3389/fpsyg.2013.00688.
Bergomi, C., Tschacher, W., & Kupper, Z. (2013). Measuring mindfulness: first steps towards the development of a comprehensive mindfulness scale. Mindfulness, 4(1), 18–32. doi:10.1007/s12671-012-0102-9.
Brown, K. W., & Ryan, R. M. (2003). The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology, 84(4), 822–848. doi:10.1037/0022-3514.84.4.822.
Cahn, B. R., & Polich, J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132(2), 180–211. doi:10.1037/0033-2909.132.2.180.
Cahn, B. R., Delorme, A., & Polich, J. (2010). Occipital gamma activation during Vipassana meditation. Cognitive Process, 11(1), 39–56. doi:10.1007/s10339-009-0352-1.
Cebolla, A., García-Palacios, A., Soler, J., Guillen, V., Baños, R., & Botella, C. (2012). Psychometric properties of the Spanish validation of the Five Facets of Mindfulness Questionnaire (FFMQ). The European Journal of Psychiatry, 26(2), 118–126. doi:10.4321/S0213-61632012000200005.
Chiesa, A., & Serretti, A. (2010). A systematic review of neurobiological and clinical features of mindfulness meditations. Psychological Medicine, 40(08), 1239–1252. doi:10.1017/S0033291709991747.
Chiesa, A., Calati, R., & Serretti, A. (2011). Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review, 31(3), 449–464. doi:10.1016/j.cpr.2010.11.003.
Chow, T., Javan, T., Ros, T., & Frewen, P. (2017). EEG dynamics of mindfulness meditation versus alpha neurofeedback: a sham-controlled study. Mindfulness, 8(3), 572–584. doi:10.1007/s12671-016-0631-8.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2. Auflage). Hillsdale: Erlbaum.
Congedo, M. (2002). Eureka! (version3.0) [ComputerSoftware]. Knoxville,TN: Nova Tech EEG Inc. Available online at: www.NovaTechEEG.
Dunn, B. R., Hartigan, J. A., & Mikulas, W. L. (1999). Concentration and mindfulness meditations: unique forms of consciousness? Applied Psychophysiology and Biofeedback, 24(3), 147–165. doi:10.1023/A:1023498629385.
Escolano, C., Aguilar, M., & Minguez, J. (2011). EEG-based upper alpha neurofeedback training improves working memory performance. In 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (pp. 2327–2330). doi: 10.1109/IEMBS.2011.6090651.
Escolano, C., Navarro-Gil, M., Garcia-Campayo, J., Congedo, M., De Ridder, D., & Minguez, J. (2014). A controlled study on the cognitive effect of alpha neurofeedback training in patients with major depressive disorder. Frontiers in Behavioral Neuroscience, 8, 296. doi:10.3389/fnbeh.2014.00296.
Freunberger, R., Werkle-Bergner, M., Griesmayr, B., Lindenberger, U., & Klimesch, W. (2011). Brain oscillatory correlates of working memory constraints. Brain Research, 1375, 93–102. doi:10.1016/j.brainres.2010.12.048.
Grossman, P., & van Dam, N. T. (2011). Mindfulness, by any other name: trials and tribulations of sati in western psychology and science. Contemporary Buddhism, 12, 219–239. doi:10.1080/14639947.2011.564841.
Gronwall, D. M. A. (1977). Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills, 44(2), 367–373. doi:10.2466/pms.1977.44.2.367.
Gruzelier, J. H. (2014). EEG-neurofeedback for optimising performance. I: a review of cognitive and affective outcome in healthy participants. Neuroscience and Biobehavioral Reviews, 44, 124–141. doi:10.1016/j.neubiorev.2013.09.015.
Hanslmayr, S., Sauseng, P., Doppelmayr, M., Schabus, M., & Klimesch, W. (2005). Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Applied Psychophysiology and Biofeedback, 30(1), 1–10. doi:10.1007/s10484-005-2169-8.
Hinterberger, T., Schmidt, S., Kamei, T., & Walach, H. (2014). Decreased electrophysiological activity represents the conscious state of emptiness in meditation. Frontiers in Psychology, 5, 99. doi:10.3389/fpsyg.2014.00099.
Holzel, B. K., Lazar, S. W., Gard, T., Schuman-Olivier, Z., Vago, D. R., & Ott, U. (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science, 6(6), 537–559. doi:10.1177/1745691611419671.
Hu, F., Hu, Y., Ma, Z., & Rosenberger, W. F. (2014). Adaptive randomization for balancing over covariates. WIREs Computational Statistics, 6, 288–303. doi:10.1002/wics.1309.
Hyvarinen, A. (1999). Fast and robust fixed-point algorithms for independent component analysis. IEEE Transactions on Neural Networks, 10(3), 626–634. doi:10.1109/72.761722.
Kabat-Zinn, J. (2003). Mindfulness-based interventions in context: past, present, and future. Clinical Psychology: Science and Practice, 10(2), 144–156. doi:10.1093/clipsy.bpg016.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews, 29(2–3), 169–195. doi:10.1016/S0165-0173(98)00056-3.
Klimesch, W., Sauseng, P., & Hanslmayr, S. (2007). EEG alpha oscillations: the inhibition-timing hypothesis. Brain Research Reviews, 53(1), 63–88. doi:10.1016/j.brainresrev.2006.06.003.
Jensen, O., & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in Human Neuroscience, 4, 186. doi:10.3389/fnhum.2010.00186.
Lagopoulos, J., Xu, J., Rasmussen, I., Vik, A., Malhi, G. S., Eliassen, C. F., Arntsen, I. E., Saether, J. G., Hollup, S., Holen, A., Davanger, S., & Ellingsen, Ø. (2009). Increased theta and alpha EEG activity during nondirective meditation. The Journal of Alternative and Complementary Medicine, 15(11), 1187–1192. doi:10.1089/acm.2009.0113.
Lee, T. W., Girolami, M., & Sejnowski, T. J. (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Computation, 11(2), 417–441. doi:10.1162/089976699300016719.
Lehmann, D., Faber, P. L., Tei, S., Pascual-Marqui, R. D., Milz, P., & Kochi, K. (2012). Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. NeuroImage, 60(2), 1574–1586. doi:10.1016/j.neuroimage.2012.01.042.
Leong, K., Chan, P., Grabovac, A., Wilkins-Ho, M., & Perri, M. (2013). Changes in mindfulness following repetitive transcranial magnetic stimulation for mood disorders. Canadian Journal of Psychiatry, 58(12), 687–691. doi:10.1177/070674371305801206.
Lomas, T., Ivtzan, I., & Fu, C. H. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience & Biobehavioral Reviews, 57, 401–410. doi:10.1016/j.neubiorev.2015.09.018.
MacKillop, J., & Anderson, E. J. (2007). Further psychometric validation of the mindful attention awareness scale (MAAS). Journal of Psychopathology and Behavioral Assessment, 29(4), 289–293. doi:10.1007/s10862-007-9045-1.
Martín Asuero, A., Rodríguez Blanco, T., Pujol-Ribera, E., Berenguera, A., Moix, Q., & J. (2013). Effectiveness of a mindfulness program in primary care professionals. Gaceta Sanitaria., 27(6), 521–528. doi:10.1016/j.gaceta.2013.04.007.
Milz, P., Faber, P. L., Lehmann, D., Kochi, K., & Pascual-Marqui, R. D. (2014). sLORETA intracortical lagged coherence during breath counting in meditation-naïve participants. Frontiers in Human Neuroscience., 8(303). doi:10.3389/fnhum.2014.00303.
Montero-Marin, J., Puebla-Guedea, M., Herrera-Mercadal, P., Cebolla, A., Soler, J., Demarzo, M., Vazquez, C., Rodríguez-Bornaetxea, F., & García-Campayo, J. (2016). Psychological effects of a 1-month meditation retreat on experienced meditators: the role of non-attachment. Frontiers in Psychology, 12(7), 1935. doi:10.3389/fpsyg.2016.01935.
Nan, W., Rodrigues, J. P., Ma, J., Qu, X., Wan, F., Mak, P. I., Mak, P. U., Vai, M. I., & Rosa, A. (2012). Individual alpha neurofeedback training effect on short term memory. International Journal of Psychophysiology, 86(1), 83–87. doi:10.1016/j.ijpsycho.2012.07.182.
Niedermeyer, E., & da Silva, F. L. (Eds.). (2005). Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
Niv, S. (2013). Clinical efficacy and potential mechanisms of neurofeedback. Personality and Individual Differences, 54(6), 676–686. doi:10.1016/j.paid.2012.11.037.
Osman, A., Lamis, D. A., Bagge, C. L., Freedenthal, S., & Barnes, S. M. (2016). The mindful attention awareness scale: further examination of dimensionality, reliability, and concurrent validity estimates. Journal of Personality Assessment, 98(2), 189–199. doi:10.1080/00223891.2015.1095761.
Rau, H. K., & Williams, P. G. (2016). Dispositional mindfulness: a critical review of construct validation research. Personality and Individual Differences, 93, 32–43. doi:10.1016/j.paid.2015.09.035.
Sauer, S., Walach, H., Schmidt, S., Hinterberger, T., Lynch, S., Büsing, A., & Kohls, N. (2013). Assessment of mindfulness: a review on the state of the art. Mindfulness, 4, 3–17. doi:10.1007/s12671-012-0122-5.
Shonin, E., Van Gordon, W., & Griffiths, M. D. (2015). Does mindfulness work? British Medical Journal, 351, h6919. doi:10.1136/bmj.h6919.
Soler, J., Tejedor, R., Feliu-Soler, A., Pascual, J. C., Cebolla, A., Soriano, J., Alvarez, E., & Perez, V. (2012). Psychometric proprieties of Spanish version of Mindful Attention Awareness Scale (MAAS). Actas Españolas de Psiquiatría, 40(1).
Soler, J., Elices, M., Franquesa, A., Barker, S., Friedlander, P., Feilding, A., Pascual, J. C., & Riba, J. (2016). Exploring the therapeutic potential of ayahuasca: acute intake increases mindfulness-related capacities. Psychopharmacology, 233(5), 823–829. doi:10.1007/s00213-015-4162-0.
Teasdale, J. D., Segal, Z., & Williams, J. M. G. (1995). How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behaviour Research and Therapy, 33(1), 25–39. doi:10.1016/0005-7967(94)E0011-7.
Tombaugh, T. N. (2006). A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Archives of Clinical Neuropsychology, 21(1), 53–76. doi:10.1016/j.acn.2005.07.006.
Tuladhar, A. M., Huurne, N. T., Schoffelen, J. M., Maris, E., Oostenveld, R., & Jensen, O. (2007). Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping, 28(8), 785–792. doi:10.1002/hbm.20306.
Van Dijk, H., Schoffelen, J. M., Oostenveld, R., & Jensen, O. (2008). Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. Journal of Neuroscience, 28(8), 1816–1823. doi:10.1523/JNEUROSCI.1853-07.2008.
Van Gordon, W., Shonin, E., Griffiths, M. D., & Singh, N. N. (2015). There is only one mindfulness: why science and Buddhism need to work together. Mindfulness, 6(1), 49–56. doi:10.1007/s12671-014-0379-y.
Van Lutterveld, R., Houlihan, S. D., Pal, P., Sacchet, M. D., McFarlane-Blake, C., Patel, P. R., Sullivan, J. S., Ossadtchi, A., Druker, S., Bauer, C., & Brewer, J. A. (2016). Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation. NeuroImage, 151, 117–127. doi:10.1016/j.neuroimage.2016.02.047.
Yu, X., Fumoto, M., Nakatani, Y., Sekiyama, T., Kikuchi, H., Seki, Y., Sato-Suzuki, I., & Arita, H. (2011). Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. International Journal of Psychophysiology, 80(2), 103–111. doi:10.1016/j.ijpsycho.2011.02.004.
Zoefel, B., Huster, R. J., & Herrmann, C. S. (2011). Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage, 54(2), 1427–1431. doi:10.1016/j.neuroimage.2010.08.078.
Acknowledgments
We thank Red de Investigación en Actividades de Prevención y Promoción de la Salud (Research Network on Preventative Activities and Health Promotion) (REDIAPP-RD12/0005/0006) and Red de Excelencia PROMOSAM (PSI2014-56303-REDT) for their support in the development of this study.
Author information
Authors and Affiliations
Contributions
MN: designed and executed the study and assisted with the data analyses. CE: analyzed the data and collaborated with the design and writing of the study. JMM: analyzed the data and collaborated in the writing of the study. JMZ: collaborated with the design and writing of the study. ES: revised the initial draft and collaborated in the writing of the study. JG: designed the study and collaborated with the writing of the study.
Corresponding author
Ethics declarations
The study was approved by the Aragon Ethical Committee (June 26, 2013; PI13/00077).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic Supplementary Material
ESM 1
(DOCX 83 kb)
Rights and permissions
About this article
Cite this article
Navarro Gil, M., Escolano Marco, C., Montero-Marín, J. et al. Efficacy of Neurofeedback on the Increase of Mindfulness-Related Capacities in Healthy Individuals: a Controlled Trial. Mindfulness 9, 303–311 (2018). https://doi.org/10.1007/s12671-017-0775-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-017-0775-1