Abstract
Mindfulness meditation (MM) and EEG-alpha neurofeedback (NFB) have both been shown to improve attentional performance and increase full 8–12-Hz EEG alpha amplitude, but no studies have compared MM and NFB on their effects for modulating EEG alpha or attentional control. Sixty-one university students were randomized to a 15-min single-session MM (n = 24), NFB (n = 17), or sham-NFB (SHAM; n = 20) intervention and were compared on EEG alpha full and sub-band amplitudes during completion of a single 15-min session of either intervention across 5 successive 3-min epochs, as well as during performance of the Stroop test. MM and NFB participants demonstrated higher global full-band alpha amplitude when compared with SHAM participants during the final intervention epoch, whereas no group differences were observed for sub-band amplitudes. In the absence of group differences in behavioral performance, MM participants exhibited a lower ERD of the upper alpha-band within frontal cortex 200–400 ms post-stimulus on the Stroop task, an effect that correlated with upper alpha amplitudes demonstrated during the intervention. Future research directions are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In its simplicity, focused attention forms of mindfulness meditation (MM) essentially involve teaching an individual to better sustain their attention toward an intended object (e.g., the sensation of respiration) and away from external (e.g., sounds) or internal sources of distraction (e.g., thoughts, “mind wandering”). Upon becoming aware of lapses away from the intended object, the MM practitioner non-judgmentally redirects attention back to the intended source. Studies in cognitive neuroscience find that repeated practice of this seemingly relatively simple series of events can develop one’s capacity for attentional control (e.g., Lutz et al. 2008) and other cognitive functions (Eberth and Sedlmeier 2012; Sedlmeier et al. 2012). For example, in experienced MM practitioners, performance of the Stroop task correlated with average daily minutes spent meditating (Chan and Woollacott 2007) and individual differences in self-reported measures of mindfulness (Moore and Mailnowski 2009).
The most replicated electro-neurophysiological correlates of MM include tonic increases in the amplitude of EEG alpha (8–12 Hz) oscillations during MM practice and increased resting EEG alpha amplitude (reviewed by Cahn and Polich 2006; Chiesa and Serretti 2010; Lomas, Ivtzan, and Fu 2015; e.g., Aftanas and Goloshekin 2003). Thus, MM practitioners not only exhibit dynamic shifts associated with being in a mindful state during meditation practice but also stable elevations in baseline alpha rhythm, often seen over posterior, central, and midline frontal cortex (Cahn and Polich 2006; Chiesa and Serretti 2010; Lomas, Ivtzan, and Fu 2015; e.g., Lagapolous et al. 2009). The alpha rhythm is known to play a role in attentional processes including internalized attention and top-down attentional control (Cooper et al. 2003; Klimesch et al. 2007); therefore, these findings are also consistent with a hypothesized role for MM in regulating attentional function. However, research attributes unique neurocognitive functions to lower (8–10 Hz) and upper (10–12 Hz)-alpha sub-bands (Klimesch 1999). As reviewed by Klimesch et al. (2007), whereas 8–10-Hz oscillations are known to desynchronize during task periods that require selective attention, alertness, and vigilance, 10–12-Hz oscillations desynchronize during semantic and working memory processes.
A further question of interest is whether the direct self-regulation of EEG alpha oscillations, via brain-computer interface technology, would have similar benefits to MM for attentional processes. EEG neurofeedback (NFB) involves recording and displaying to participants their EEG in real-time in the form of visual and/or auditory feedback stimuli, thereby providing the opportunity for participants to attempt to modulate such signals through various attentional strategies. Indeed, NFB rewarding an increase of the amplitude of the full alpha band (8–12 Hz) successfully produced increases in alpha amplitude in prior research (van Boxtel et al. 2012; Dekker et al. 2014; Egner and Gruzelier 2004; Zoefel et al. 2011), while NFB rewarding a decrease of alpha amplitude-suppressed alpha amplitude relative to sham (placebo) control (Ros et al. 2013). Referring to attentional outcomes, improvements following NFB have also been documented in Stroop performance and sustained attention (go/no-go task) (Angelakis et al. 2007) and in a mental rotation working-memory task (Hanslmayr et al. 2005; Zoefel et al. 2011).
Whereas most intervention studies of the effects of MM and NFB involve multiple sessions taking place over the course of several weeks, an emerging literature has investigated the subjective and neural effects of brief, single-session interventions. With regard to MM, a few studies have described successful modulation of emotional regulation capacities (Arch and Craske 2006), deautomization (Wenk-Sormaz 2005), and resistance to the sunk-cost fallacy (Hafenbrack, et al. 2014) after only single, 15–20-min sessions of MM practice. However, attentional capacities were not primarily investigated in these studies. Indeed, research on the benefits of MM on attention used a minimum of 5 days of training, in addition to combining MM with other elements (Tang et al. 2007). Referring to NFB, single 20-min NFB training sessions on increasing the upper alpha band amplitude have been reported to improve cognitive performance in the mental rotation task (Hanslmayr et al. 2005) as well as psychomotor reactivity (Bazanova et al. 2007). Moreover, Ros et al. found that a single session of NFB rewarding a decrease of alpha amplitude was associated with increased states of wellbeing, decreased alpha amplitude, and modulation of resting-state functional connectivity between neural networks associated with mind wandering in a neuroimaging study of first-time NFB practitioners (Ros et al. 2013). Of particular relevance to the present study, van Lutterveld et al. (2016) also found that participants were able to modulate the real-time NFB signal within the gamma-frequency range within posterior cortex (posterior cingulate) using meditation practices, where a reduction in amplitude was associated with the experience of effortless awareness; correlated effects were also observed within the alpha frequency range, among others.
Taken together, we are aware of no previous studies that examined the effects of MM practice separately for the lower and upper alpha sub-bands. In addition, to our knowledge, the form of change in alpha full- and sub-band amplitudes across the duration of a MM sitting, whether linear or non-linear, has not yet been studied. Finally, no prior studies have directly compared MM and EEG-alpha NFB therapies with regard to their effects on neurocognition, especially regarding the potential for a single 15-min session to unfold potential advantages in attentional capacity. We therefore conducted a randomized controlled pilot trial evaluating the immediate outcomes of a single 15-min session of either MM or EEG-alpha NFB with sham (“placebo”) NFB control on EEG alpha oscillations and attentional Stroop performance. Outcomes on the EEG were investigated separately for the full- (8–12 Hz), lower (8–10 Hz), and upper (10–12 Hz) alpha sub-bands across five 3-min epochs across the course of each intervention, allowing investigation of the trajectory of change, whether linear or non-linear (in the present case, restricted to investigation of quadratic curves). Referring to response to the Stroop task, we measured indicators of behavioral performance (accuracy and reaction time) as well as measures of neurocognitive processing, specifically, alpha event-related desynchronization (ERD) in the lower and upper alpha sub-bands. Finally, where corresponding effects were found to be significant in the case of both amplitude change over the course of intervention epochs and behavioral performance or ERD, we correlated these measures to determine whether changes occurring during the intervention were directly associated with neurocognitive outcomes for attentional control. In general, in comparison with the sham (“placebo” NFB) group, we predicted that participants randomized to either MM and true NFB would experience a more positive emotional state and exhibit greater EEG alpha during their intervention, as well as demonstrate improved behavioral performance and a diminished ERD in response to the Stroop task. By contrast, comparisons between the MM and NFB groups were exploratory.
Method
Participants
Seventy-four healthy adults aged 18–25 were recruited from the University of Western Ontario (UWO) undergraduate psychology research participation pool and received partial course credit for completing the study. Inclusion criteria were a lack of prior experience with MM practice or NFB. Participants were randomly assigned to either the mindfulness meditation group (MM; n = 25), EEG-alpha NFB group (NFB; n = 25), or were randomized to a control (“placebo”) sham-NFB group (SHAM; n = 24)1. Participants were excluded from final analyses based on obtained z-scores ≥3.0 relative to the sampling distribution for any variable. As such, a single participant was removed from the MM group (due to EEG contamination), 8 individuals were removed from the NFB group (4 due to EEG contamination, 3 due to DASS, and 2 due to POMS), and 4 were removed from the SHAM group (all due to EEG contamination). Therefore, after addressing outliers, a total of 61 participants were included in the final analysis (MM, n = 24; NFB, n = 17; SHAM, n = 20). The study procedure was administered within a dedicated electrophysiology laboratory. Participants voluntarily consented to participate after being provided with detailed information regarding the potential benefits and risks of the study interventions. No participants were withdrawn from the study and no adverse events were recorded.
Procedure
Interventions
Mindfulness Meditation (MM)
Participants in the MM group were introduced to a simple mindful breathing meditation administered using previously published procedures (Frewen et al. 2008, 2011, 2014). Participants were instructed to focus their attention toward the sensation of their breathing at their nostrils. They were asked to refrain from manipulating their breathing in any form and instead to allow their natural breathing rhythm to occur. They were instructed that whenever they became aware that their attention had wandered from a focus on breathing sensation, they should simply redirect their attention back to the sensation of their breathing without judging themselves critically. In addition to focusing their attention toward their breath, participants were instructed to observe any distracting thoughts, feelings, or sensations without judging, evaluating, or elaborating on them. These meditation instructions are consistent with recent psychological conceptualizations of MM that emphasize the development of attentional abilities combined with a specific, non-judgmental attitude toward the different mental experiences that may arise during MM (Lutz et al. 2008; Slagter et al. 2011). The MM was performed with eyes closed in seated position for 15-min. At approximate 3-min intervals, while keeping their eyes closed, participants also pressed a computer keyboard button resting upon their laps upon hearing a meditation bell, indicating whether or not, at such moments, they were attending toward their breathing or if instead they had become distracted, in accordance with published procedures for collecting Meditation Breath Attention Scores (Frewen et al. 2008, 2011, 2014). Three consecutive meditation bells marked the beginning and ending of the MM. Of the 24 participants in the MM group, 3 did not correctly respond to the MBAS bell. The mean MBAS score for the remaining participants was 3.48 (SD = 0.93).
EEG-Alpha Neurofeedback (NFB)
Participants in the NFB group were trained to enhance their EEG-alpha amplitude at their scalp Pz site (midline parietal cortex, in accordance with the International 10–20 system), where the EEG-alpha rhythm is typically maximal (e.g., Ergenoglu 2004). Prior to electrode placement, skin was prepared with a mildly abrasive skin cleaner to help improve impedance and conductance of electrodes. Electrodes were affixed with adhesive conductive paste. The electrode was connected to a Spectrum4 amplifier (J&J Engineering, USA) interfacing with EEGer 4.3 neurofeedback software (EEG Spectrum Systems, CA). Ground and reference electrodes were placed on the right and left earlobes, respectively. Impedances were checked to be ≤5 kΩ. Each session involved 15 min of continuous neurofeedback, where participants were asked to close their eyes for the duration of the training. For the purpose of NFB training, the raw EEG signal at Pz was bandpass filtered using the infinite impulse response function to extract the alpha (8–12 Hz) amplitude with an epoch size of 0.5 s.
The protocol was such that participants were guided toward continually increasing their absolute EEG-alpha amplitude beyond a moving threshold calculated every 0.5 s. Thresholds in NFB are typically set in such a way that the participant achieves a certain level of success that is neither too high nor too low (Demos 2005). As such, the initial threshold was set such that their EEG-alpha amplitude would temporarily exceed the moving threshold at random 65 % of the time above the initial 1-min average; by contrast, participants would fail to receive feedback 35 % of the time. Positive feedback was provided as a low-frequency auditory tone; being that the sounding of the tone itself is not intrinsically rewarding, it must be assumed that participants are motivated by their own self-efficacy and/or the intrinsically rewarding properties of the targeted neurophysiological state (i.e., an increased 8–12-Hz amplitude within their EEG). Participants, with their eyes closed, were not given explicit strategies for producing the tones but were instead asked to focus their attention continuously toward the tones for guidance.
Sham Neurofeedback (SHAM)
All set-up and training procedures applied to the SHAM group were identical to those for the real EEG-alpha NFB group. Instructions were also identical and all participants completed 15 min of sham NFB in which participants similarly attempted to produce the audio tones. However, whereas the real NFB group heard auditory feedback that validly reflected their own brain activity, the SHAM group heard a pre-recorded session that involved the identical tones that the real NFB group was exposed to (Raymond et al. 2005). Pre-recorded sessions were created by placing a digital voice recorder beside the computer speaker during alpha-NFB training sessions, recording their auditory feedback tones. The pre-recorded session was then played back to SHAM participants using Windows Media Player (Microsoft, USA) after digital processing omitted background noise and ensured an identical audio volume as actual feedback sessions. In this way, the feedback given to the sham group bore no relation to the participants’ actual own brain activity but still mimicked the feedback that would typically occur during a true NFB session.
Self-Report Measures
For descriptive purposes, participants completed standard, well-validated measures of symptoms of depression, anxiety, and stress (Depression Anxiety Stress Scales [DASS]; Lovibond and Lovibond 1995) and mindfulness-related traits (Five Facet Mindfulness Questionnaire [FFMQ]; Baer et al. 2006). In order to assess the immediate outcomes of the interventions on mood and state mindfulness, participants also completed the Profile of Mood States-Short Form (POMS; McNair et al. 1971; Curran et al. 1995) and the Toronto Mindfulness Scale (TMS; Lau et al. 2006).
Study Protocol
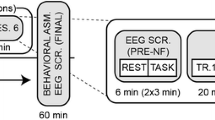
Participants were randomly assigned to MM, NFB, or SHAM. Whereas participants were blinded with regard to their randomization to NFB vs. SHAM, experimenters were not blinded. Pre-intervention baseline DASS, POMS, and FFMQ were administered following EEG electrode cap setup. The EEG cap was worn throughout the session, allowing for continuous EEG recording for all conditions. Additionally, participants in the NFB and SHAM groups were affixed with three additional electrodes at the Pz site, left, and right earlobes, used for NFB/SHAM. A pre-intervention baseline EEG measurement was recorded for 3 min, where participants were asked to close their eyes and allow their minds to naturally wander. Each participant then underwent their respective interventions for 15 min. All interventions were conducted with eyes closed. Participants then completed the POMS and TMS self-reports, followed by the Stroop task.
Measures
Behavioral Measures: Stroop Task Design and Stimuli
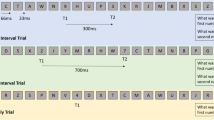
Stimuli in the Stroop task were the four color words “RED”, “BLUE”, “GREEN,” and “YELLOW.” These words were presented in the same color-ink as the written word in congruent trials (e.g., RED presented in red ink) and in different colors for incongruent trials (e.g., RED presented in blue ink). The task was presented on a 21-inch CRT-monitor (100Hz vertical refresh rate, 1024 × 768 resolution) and running in the E-Prime 2.0 environment (Psychology Software Tools Inc., USA). Words were presented in Arial font (font size 48 pt) and viewed at a distance of approximately 70 cm. Incongruent stimuli appeared in each of the three other colors with equal frequency, whereas the ratio of congruent to incongruent trials was 1:1. Participants were instructed to indicate the color each word was presented in, while ignoring the semantic meaning of the word, as fast and as accurately as possible. Four keys on a standard QWERTY keyboard were used to enter their responses. The keys were color coded using circular colored stickers, with the key “s” for red, “c” for yellow, “m” for blue, and “l” for green. The keys were chosen to provide optimum comfort for the participant while responding with the index and middle finger of both hands. Stimuli were presented on the screen for 1500 ms, followed by a variable ITI ranging between 1500 and 1800 ms, during which a centrally located fixation cross was presented. The stimulus word always appeared centrally on the screen, replacing the fixation cross.
The experiment began with a color-to-key acquisition phase which consisted of 48 trials presenting the four words but in black ink only (e.g. RED in black ink); completion of such trials resulted in all participants learning the key-color associations with high speed and accuracy. Indeed, all the participants were able to improve their overall accuracy and reaction time from the first 12 trials (accuracy M = 0.91, SD = 0.17; reaction time M = 771.5 ms, SD = 228.27 ms) to the last 12 trials (accuracy M = 0.94, SD = 0.12; reaction time M = 580.66 ms, SD = 112.56 ms). This was followed by a practice phase where 32 trials were presented to the participant, which were identical to those used in the experimental blocks. During the acquisition and practice phases, response accuracy feedback was given following each trial. The experimental phase consisted of three blocks of 48 trials, for a total of 144 trials, with 72 congruent and 72 incongruent trials. The entire task lasted for approximately 8 min.
Electrophysiological Measures: EEG Recording
EEG was recorded using a custom elastic cap and the ActiveTwo BioSemi amplifier system (BioSemi, Amsterdam, Netherlands). Cap sizes varied and were chosen based on participant head circumference. Recordings were taken from 32 Ag/AgCl electrodes following the international 10–20 system. Two electrodes were placed on the left and right mastoids. Electrooculogram generated from blinks and eye movements was recorded from five facial electrodes: two approximately 1 cm above and below the participant’s left eye, one on the nose bridge, one approximately 1 cm to the left of the left eye, and one approximately 1 cm to the right of the right eye. As per BioSemi’s design, the ground electrode during acquisition was formed by the common mode sense-active electrode and the driven right leg-passive electrode (see www.biosemi.com/faq/cms&drl.htm for details). For further offline analysis, the average reference was used. All bioelectric signals were digitally filtered at 0.1–100 Hz (24 dB/octave roll-off) and amplified on a laboratory computer using ActiView software (BioSemi), sampled at 512 Hz and stored for offline analysis. With respect to impedances, BioSemi uses an active electrode system in which the electrode itself provides for impedance transformation. EEG voltages are typically not influenced due to the high input impedance, whereas the output impedance is very low (<1 Ohm). As such, interference currents are prevented from generating significant voltages due to current flow via low impedances of the active electrode output. EEG recording occurred during a pre-intervention 3-min baseline and continued through the study duration.
Data Analyses
Note that all results are reported as exploratory and without correction for multiple comparisons.
Data Reduction and Offline Analyses
Following EEG recording, all EEG data were preprocessed using routines available via EEGLab v12, an open source toolbox running in the MATLAB environment for electrophysiological signal processing (Delorme and Makeig 2004; http://sccn.ucsd.edu/eeglab/). After being imported into MATLAB, the continuous EEG data were re-referenced using a common-average head reference algorithm, where an average of EEG activity at every electrode site is used as a reference, thereby removing noise common to all sites. Data were then digitally filtered depending on our experimental condition as will be described.
EEG Baseline Analyses
Baseline continuous EEG measurements taken before, during, and after the interventions were filtered with a low cutoff value of 1 Hz and a high cutoff value of 30 Hz using a finite impulse response (FIR) filter. Continuous EEG data were then segmented into 1-s epochs used for artifact rejection. We excluded epochs with abnormally large amplitudes (over ±75 μV). Epochs contaminated by spurious gross-movement and other non-stereotyped artifacts were also identified by visual inspection and additionally rejected. Participants were included in analyses of EEG baselines if they retained >40 % of the 1-s epochs after artifact rejection and pre-processing, calculated within each 3-min segment independently (e.g., pre-intervention 3-min baseline, first 3 min of intervention, etc.). Full data were available for 24 MM, 17 NFB, and 20 SHAM participants.
Spectral Analysis for Continuous EEG at Baseline and During Intervention
EEG power was calculated by using Welch’s power spectral density estimate in the Neurophysiological Biomarker Toolbox, an open-source toolbox running in MATLAB (NBT; Hardstone et al. 2012; www.nbtwiki.net). Continuous EEG was fast Fourier transformed (FFT) and averaged in the frequency domain using a hamming window (1024 sampling points). The FFTs were then grouped into lower (8–10 Hz), upper (10–12 Hz), and overall alpha (8–12 Hz) frequency bands and log-transformed. Amplitude measures during the 15-min intervention were calculated in five 3-min segments.
For the purposes of presentation, the 32-channel EEG data were collapsed into nine clusters, resulting in regional means: left frontal (Fp1, AF3, F7, F3), mid frontal (Fz, FC1, FC2), right frontal (Fp2, AF4, F8, F4), left central (T7, FC5, C3, CP5), mid-central (Cz), right central (T8, FC6, C4, CP6), left posterior (P7, P3, PO3, O1), mid-posterior (CP1, CP2, Pz), and right posterior (P8, P4, PO4, O2). The average amplitude values across the respective electrode sites were calculated for these regional means for lower (8–10 Hz), upper (10–12 Hz), and full-band alpha (8–12 Hz) frequencies as observed during each experimental condition. In statistical analyses, effects for location (left hemisphere [LH], midline, and right hemisphere [RH]) and lobe (frontal, central, posterior) were determined independently.
Event-Related Desynchronization During Stroop Task
Event-related changes in the EEG-alpha band power were calculated using the ERD method originally proposed by Pfurtscheller and Lopes da Silva (1999). Before calculating ERD, data were digitally bandpass filtered, squared (in order to obtain simple power estimates), and averaged separately between congruent vs. incongruent trials. ERD is defined as the percentage of a decrease (ERD; desynchronization) or increase (ERS; synchronization) in the band (alpha) power during a post-stimulus interval (A) as compared to a baseline reference interval (R) as follows: ERD/S% = (A − R) / R × 100 %. As such, positive values reflected an increase in alpha power following stimulus presentation relative to pre-stimulus baseline, termed ERS, whereas negative values reflected a decrease in alpha power, termed ERD, in percentage units of the alpha power observed during the pre-stimulus baseline. The time window of −750 to −250 ms prior to stimulus onset was used as the baseline reference interval. Post-stimulus test intervals were two equivalent consecutive (short and late) time intervals between 200 and 600 ms post-stimulus onset (i.e., 200–400, and 400–600 ms). The 400–600-ms time period was used as this usually pertains to the late negative ERP component that typically reflects the behavioral interference effect in the Stroop task and tends to correlate with behavioral performance (Hanslmayr et al. 2008; Liotti et al. 2000). Conversely, the 200–400-ms time period was aimed at observing the earlier aspects of stimulus processing that, in themselves, may not be a source of behavioral Stroop interference effect (Ilan and Polich 1999). For statistical comparisons, data were collapsed into the lower alpha (8–10 Hz) and upper alpha (10–12 Hz) sub-bands. ERD values were measured separately for the nine cortical regions as described previously.
Statistical Analysis
Missing data was noted as follows: two individuals failed to complete either the POMS or DASS (one each from the NFB and SHAM groups), three individuals failed to complete the TMS (two from the MM and one from the SHAM group), and two individuals failed to complete the Stroop (one each from the MM and SHAM groups). Referring to those remaining participants for which complete data was available, prior to statistical analysis confirmed that no significant differences between groups were observed at baseline in reference to depression, anxiety, or stress symptoms (DASS), trait mindfulness (FFMQ), mood state (POMS), or global EEG alpha.
Results
Group Differences at Baseline
Table 1 reports participant demographics and group differences in self-reported depression, anxiety, stress scales (DASS), profile of mood states (POMS), and EEG alpha-power at baseline, pre-intervention.
Effects of Intervention on Self-Reported Mood and State Mindfulness
Figure 1 shows that, across groups, participants reported less depression, vigor, anger, tension, confusion, and fatigue following the interventions on the POMS (all univariate main effects had ps ≤ .05). Main effects for group randomization were non-significant and, contrary to our hypotheses, all interactions with group were also null except in the case of POMS confusion (F(2, 53) = 3.40, p = .041, η2-partial = 0.11). Follow-up tests of the latter effect indicated that, whereas pre-post differences were statistically significant for all three groups, confusion decreased more so in the MM and SHAM groups than in the NFB group. Group differences were neither observed post-intervention on the TMS referring to self-reported state mindful curiosity (p = .71) or decentering (p = .83). We therefore conclude that, overall, MM and NFB failed to influence self-reported mood and state mindfulness more so than did SHAM.
Profile of mood state (POMS) self-report scores before and after each respective intervention: a MM, b NFB, and c SHAM. Across all groups, participants reported less depression (D), vigor (V), anger (A), tension (T), confusion (C), and fatigue (F) following respective interventions (univariate main effects all p < 0.05). However, main effects and interactions for group randomization were not significant. In regard to the Toronto Mindfulness Scale (TMS), post-intervention self-reports on states of mindful curiosity (Cr) and decentering (Dc) showed no group differences
Effects of Intervention on EEG-Alpha Amplitudes
Figure 2 illustrates effects observed for the global (whole-brain) and lobe-specific alpha amplitudes across the five 3-min intervention epochs. Table 2 summarizes the observed effects on alpha-amplitudes between- and within-group during the intervention; Supplementary Fig. S1 further differentiates these effects by location (left, right, midline) into the nine corresponding ROIs. Multivariate main effects and various interactions were statistically significant for intervention epoch, lobe, and location across groups (ps < .01). Group interacted significantly at the multivariate level with epoch × lobe × hemisphere (F(96, 20) = 1.49, p = .002, η 2 = 0.05). At the univariate level, an effect approached significance only in the case of lower (8–10 Hz) alpha amplitudes (F(32, 896) = 1.75, p = .073, η 2 = 0.06) and upper (10–12 Hz) alpha amplitudes (F(32, 896) = 1.45, p = .10, η 2 = 0.05). Nevertheless, follow-up tests were performed at the final 3-min intervention period, showing that both MM participants (t(41) = 1.65, p = .05, Cohen’s d = 0.52) and NFB participants (t(33) = 1.75, p = .04, Cohen’s d = 0.61) demonstrated higher global full-band alpha amplitude when compared with SHAM participants, whereas MM and NFB participants did not differ (t(38) = 0.17, p = .87). In comparison, there were no significant differences between groups at the first epoch (all ps ≥ .489). We therefore conclude that, consistent with hypotheses, by the end of the intervention, MM and NFB had modulated EEG full-band alpha amplitude more so than did SHAM. However, differences in alpha magnitude between first and last 3-min periods between groups revealed no significant differences (all p’s > 0.05).
Observed effects of global (whole brain) and lobe-specific a full alpha 8–12 Hz, b lower alpha sub-band 8–10 Hz, and c upper alpha sub-band 10–12 Hz power during the 15-min intervention, divided into five 3-min epochs (Global: G1–G5; Frontal: F1–F5; Central: C1–C5; Parietal: P1–P5). Note axes are scaled to maximize visualization of EEG-alpha changes during intervention. *p < 0.05: between group effects indicate that by the end of the intervention, MM and NFB full alpha band (8–12 Hz) amplitudes were greater than SHAM
In comparison, and contrary to hypotheses, analyses of between-group differences in reference to lower and upper alpha amplitudes yielded non-significance at all electrode sites. Significant interactions were therefore investigated within-groups. In brief, within-group follow-up tests in MM participants revealed that lower alpha amplitudes demonstrated significant linear decreases across all electrode sites except for within left central cortex for which p < .10; quadratic effects were observed only in the case of mid-central cortex (F(1, 23) = 4.79, p = .04, η 2 = 0.17). In comparison, upper alpha amplitudes differed across intervention epochs only within posterior cortex, where both linear decreases (F(1, 23) = 8.06, p = .009, η 2 = 0.26) and quadratic effects were observed (F(1, 23) = 5.45, p = .029, η 2 = 0.19). We therefore conclude MM practice was associated with linear or quadratic decreases in both lower and upper alpha sub-bands depending on electrode location.
Interestingly, largely similar effects were obtained in the case of the SHAM intervention. Specifically, the three-way interaction was significant in SHAM participants with reference to lower alpha amplitudes (F(16, 288) = 3.06, p = .02, η 2 = 0.15). However, in the case of upper alpha amplitude intervention, epoch interacted only with lobe across location (F(8,144) = 3.59, p < .01, η 2 = 0.17). Referring to lower alpha amplitudes, linear decreases, or quadratic effects were observed across frontal electrode sites, and linear decreases were observed within posterior electrode sites, whereas no significant effects were observed at central electrode sites. In comparison, referring to upper alpha amplitudes, amplitudes differed across intervention epochs only within posterior cortex, where both a linear decrease (F(1, 18) = 13.85, p = .002, η 2 = 0.44) and a marginally significant quadratic effect were observed (F(1, 18) = 4.25, p = .054, η 2 = 0.19). Thus, once again, we conclude that SHAM-NFB was associated with linear or quadratic decreases in both lower and upper alpha sub-bands depending on electrode location. However, in striking contrast with results obtained from MM and SHAM participants, no intervention effects were observed for NFB on either the lower or upper alpha sub-bands.
Effects of Intervention on Stroop Task
Stroop Behavioral Performance
As can be expected, main effects for Congruency were found for both reaction time (F(1, 56) = 128.24, p <0.001, η 2 = 0.70) and accuracy (F(1, 56) = 25.29, p < 0.001, η 2 = 0.31), with incongruent trials associated with increased errors and slower reaction time. However, in contrast with hypotheses, the main effect of Group and the interaction between Group and Condition were non-significant. We must therefore conclude that MM and NFB failed to influence the behavioral indicators of attentional control more so than did SHAM.
Stroop Event-Related Alpha Desynchronization and Synchronization (ERD/S)
Referring to ERD, in reference to both the lower and upper alpha-bands, various multivariate main effects and interactions were statistically significant across groups as referring to time of measurement (200–400, or 400–600 ms post-stimulus), trial type (congruent/incongruent), lobe, and hemisphere (ps < .01). In summary, results indicated an overall stronger ERD for the lower than upper alpha sub-band, a stronger ERD within posterior than frontal or central cortex, and a stronger ERD for midline electrodes than for those placed at left or right lateral cortex. Concerning the effect of time of measurement post-stimulus, effects in central and posterior cortex tended to be stronger for the later 400–600-ms measurement, whereas this temporal effect was less prominent within frontal cortex.
Referring to the effects of group, only a non-significant multivariate interaction was obtained with measurement time, trial type, and hemisphere, across lobe (F(8, 200) = 1.86, p = .07, η 2 = 0.07). Further, at the univariate level, the interaction approached significance only in the case of upper alpha ERD (F(4, 100) = 2.30, p = .07, η 2 = 0.08) while not for lower alpha ERD (F(4, 100) = 1.88, p = .13). However, further follow-up tests suggested that the effect of measurement time by hemisphere on upper alpha ERD was not statistically significant in the case of congruent trials (F(4, 100) = 0.62, p = .65) or in the case of incongruent trials (F(4,100) = 0.62, p = .99).
It should be noted, however, that the strongest effect involving group at the univariate level was rather an interaction with time of measurement and lobe, irrespective of trial type (F(4, 100) = 2.73, p = .03, η 2 = 0.10). Examining the effect of group on time of measurement separately within each lobe, it was found that, whereas frontal cortex amplitude approached significance (F(2, 51) = 2.85, p = .07, η 2 = 0.10), effects were clearly null within central (F(2, 51) = 0.20, p = .82) and posterior cortex (F(2, 51) = 0.45, p = .64). Follow-up tests of the effect within frontal cortex within each intervention group indicated that, whereas MM participants demonstrated a lesser upper alpha ERD at the earlier than at the later measurement period (t(21) = 2.84, p = .01, Cohen’s d = 1.24), no such effect was observed in the NFB participants (t(13) = 1.26, p = .23) or SHAM participants (t(17) = 0.37, p = .72). The upper alpha frontal ERD observed 200–400 ms post-stimulus in MM participants was also less significant than that observed in central (t(21) = 4.36, p < .001, Cohen’s d = 1.90) or posterior cortex (t(21) = 4.36, p < .001, Cohen’s d = 1.90). Finally, between-group tests indicated that the MM group demonstrated less ERD in frontal cortex 200–400 ms post-stimulus in comparison with both SHAM (t(38) = 1.71, p = .048, Cohen’s d = 0.55) and NFB participants (t(34) = 1.65, p = .055, Cohen’s d = 0.57), whereas the latter two groups did not differ (t(30) = 0.32, p = .98). At the later stage of post-stimulus processing (400–600 ms), however, no group differences approached statistical significance. We therefore conclude that, in partial support for our hypothesis, MM (but not NFB) modulated the upper alpha ERD during Stroop performance relative to SHAM, although effects depended on electrode location and time of measurement post-stimulus. These results are illustrated in Fig. 3.
Event-related desynchronization (ERD) for a lower 8–10 Hz and b upper 10–12 Hz alpha sub-bands, either 200–400 or 400–600 ms post-stimulus in the Stroop task across frontal (F), central (C), and parietal (P) electrode sites. Only congruent trials are displayed. MM participants displayed lower ERD in their frontal cortex during 200–400 ms post-stimulus, relative to the SHAM group (p = 0.048)
Correlations Between Alpha Amplitudes During Intervention and ERD
Given that group effects on ERD during performance of the Stroop were observed only in reference to the upper alpha band within frontal cortex, we examined correlations between upper alpha sub-band frontal-ERD during performance of the Stroop and upper alpha sub-band frontal amplitudes observed across intervention epochs; significant correlations were observed at both the 200–400-ms (r = −.31, p = .01) and 400–600-ms, (r = −.25, p = .04) measurement time points, post-stimulus onset. Taking into account the negative sign of an ERD effect, these negative correlation coefficients indicate that greater upper alpha amplitudes measured during the intervention were associated with greater ERD of the upper alpha amplitude during performance of the Stroop task. This association was evident when measured separately at each of the five intervention epochs (rs ranged from −.20 to −.34) and within all intervention groups.
Discussion
The results of this pilot study contribute to a growing literature associating the practice of MM and NFB with effects on the EEG-alpha rhythm and attentional processing. Whereas the practice of MM is thought to directly train attention while indirectly modulating the EEG alpha rhythm, NFB directly modulates the alpha rhythm through a brain-computer interface with anticipated downstream effects on attentional processing. A novel aspect of this pilot study was to examine the intervention effects of both MM and NFB (vs. SHAM-NFB) not only for the full (8–12 Hz) but further for the lower (8–10 Hz) and upper (10–12 Hz) alpha sub-bands, which have been attributed to different cognitive functions (Klimesch 1999; Klimesch et al. 2007). We also assessed the immediate effects of each intervention for attentional control by administering the Stroop task. Preliminary conclusions of this pilot study include that, although insufficient to influence subjective mood state, state mindfulness, or behavioral performance indicative of attentional control more so than SHAM, single 15-min sessions MM and NFB were sufficient to modulate EEG alpha activity during intervention performance. Moreover, MM modulated the upper alpha ERD during performance of the Stroop task more so than did NFB or SHAM, while the degree of the obtained upper alpha ERD significantly correlated with alpha amplitudes observed at the conclusion of the intervention.
In reference to the full alpha band, consistent with hypotheses, by treatment end, MM and NFB participants demonstrated higher amplitudes than did SHAM participants when directly comparing the final 3 min of intervention. However, as alternative analyses of alpha-change differences between the first and last periods of the intervention revealed no significant group differences, this conclusion is limited and warrants further investigation. However, among the most significant findings of the current study is the contrast of effects observed across the three interventions for the full versus upper and lower alpha sub-bands. Specifically, whereas full-band alpha amplitudes demonstrated clear quadratic effects across the intervention epochs particularly within MM and NFB participants, first decreasing from 3 to 6 min and then increasing particularly in the final epoch between 12 and 15 min, lower sub-band amplitudes tended to decrease linearly with time but particularly so across early intervention epochs, while upper band amplitudes either demonstrated null effects or similarly decreased with time, particularly at posterior sites. These distinct effects of the interventions on the full vs. lower and upper alpha sub-bands suggest the importance of further differentiating intervention effects on the sub-bands in future studies of MM and NFB. Interestingly, within-group analyses tended to reveal greater similarities between MM and NFB participants with regard to full-band alpha amplitudes, while greater similarities (i.e., amplitude decreases) were observed between MM and SHAM participants in reference to the lower and upper alpha sub-bands, where null effects were observed for NFB participants. Given that our NFB intervention targeted increasing the full-band alpha amplitude, it is interesting that NFB effects appeared to be relatively selective to that brain rhythm. Whether NFB can differentially modulate the full- vs. sub-bands of the alpha frequency range when directly targeted is therefore a question for future research.
Although our study was underpowered to detect between-group differences simply as a result of low sample size, that significant between-group differences on subjective and behavioral measures failed to emerge may also allude to the need for multiple sessions of intervention training in order to induce lasting and detectable changes on self-report and behavioral indices that are correlated with effects on the EEG. Moreover, stronger effects on the EEG itself may also require repeated training. Indeed, MM practice is usually linked with increases in EEG-alpha amplitude in studies sampling participants that have received multiple sessions of meditation practice or are experienced meditators from a wide array of contemplative practices and techniques (Cahn and Polich 2006). This is also true in reference to NFB; most studies have suggested that multiple sessions are needed for the participant to establish associative relations between modifications in their EEG-alpha amplitude and changes to internal states (Vernon et al. 2009; Konareva 2005). This is consistently reported in studies describing changes in EEG-alpha after multiple NFB sessions taking place over a period of weeks (Angelakis et al. 2007; van Boxtel et al. 2012; Dekker et al. 2014; Zoefel et al. 2011). Nevertheless, a recent study reported significant changes in the full EEG-alpha band following a single NFB session involving alpha-suppression training (Ros et al. 2013), and others have reported successful single-session NFB training of the alpha rhythm as well (Bazanova et al. 2007; Hanslmayr et al. 2005).
Probably the most salient feature distinguishing the single-session NFB paradigm used in our study from these others, however, is that of an eyes-closed vs. eyes-open NFB training protocol, where previous single-session studies have tended to use the latter condition. Given that the alpha amplitude is normally seen as a function of reduced sensory input from the thalamic nuclei to the cortex (e.g., Vernon et al. 2009), it has long been known that keeping the eyes open will naturally suppress alpha amplitude relative to an eyes-closed condition, providing a lower baseline from which to attempt to increase the alpha amplitude, thereby presumably more amenable to intervention effects via NFB. Aware of such considerations, we nevertheless elected to conduct NFB with eyes-closed to ensure comparability with MM which is most often practiced with eyes closed. Future studies would do well to compare MM with NFB in both eyes-open and eyes-closed conditions.
Moreover, the present study did not take note of participants’ individual alpha frequency (IAF). The use of the IAF, defined as the frequency exhibiting the greatest EEG amplitude within the extended alpha range (Klimesch 1999), has become increasingly popular as an estimate of alpha activity (Angelakis et al. 2007; Hanslmayr et al. 2005; Zoefel et al. 2011). This is primarily due to the high inter-individual variability in alpha frequency. Indeed, studies have noted that the alpha rhythm varies across a wider frequency range than the traditional 8–12-Hz band, and by using this fixed frequency band, bias may be introduced against certain subjects, especially those with an IAF beyond this band (Haegens et al. 2014). The present study nevertheless opted to define the alpha band using the traditional 8−12-Hz frequency range, primarily in keeping with the greater meditation literature which has generally investigated effects on the 8–12-Hz band (Cahn and Polich 2006). We acknowledge the exclusion of implementing the IAF as a potential study limitation, and it will be necessary to incorporate IAF-informed upper and lower alpha sub-band designation in future trials of the effects of MM vs. NFB.
Referring to performance of the Stroop, overall we replicated the well-known behavioral pattern of facilitation and interference that has been described for congruent vs. incongruent trials, as well as a greater ERD for incongruent trials. Additionally, participants who had practiced MM demonstrated less frontal upper alpha ERD during early stages of cognitive processing (200–400 msec) than did NFB or SHAM participants, while degree of frontal upper alpha ERD was correlated with the same amplitude when observed across intervention epochs. Although we observed such effects in the absence of intervention effects on behavioral performance, these results suggest that the modulation of EEG alpha rhythms is a possible partial mediator of the effects of MM on attentional control. However, the extent of ERD is typically seen to positively correlate with cognitive performance (Klimesch 1999; Klimesch et al. 2007), interpreted as a correlate of increased cellular excitability in thalamocortical systems during cortical information processing (e.g., Pfurtscheller and Lopes da Silva 1999). In this context, previous studies have interpreted reductions in ERD as decreased cognitive effort necessary to perform a task (Pfurtscheller and Lopes da Silva 1999; Romero et al. 2008), in the present study, possibly also reflecting a reduction in effort needed by MM participants to perform the Stroop task adequately. To corroborate such an interpretation, future studies should administer neurocognitive tasks with a greater sensitivity to performance-linked changes in EEG parameters. It is worthwhile to note that in the framework of MM studies, our findings are in line with a study by Lutz et al. (2009) who also showed a reduction in ERD for MM practitioners during a selective attention dichotic listening task relative to controls which was also interpreted as possibly indicative of correspondingly reduced cognitive effort, effected via more efficient brain resource allocation as produced by MM training (Slagter et al. 2007). It should be noted, however, that in the present study, MM effects on ERD were observed for earlier (200–400 ms) rather than later (400–600 ms) stages of processing, and that it is rather EEG measures obtained during the later post-stimulus time period that best predict the Stroop interference effect (Ilan and Polich 1999; Liotti et al. 2000). Given the absence of an MM effect on behavioral performance of the Stroop, the similar absence of an effect of MM practice on the ERD observed at later temporal stages of processing, however, thus provides congruent findings.
In addition to those already mentioned, further consideration of the limitations of the current pilot study can assist in providing possible directions for future research. First, our sample size was small and, as we have noted previously, was underpowered to detect between-group differences that were less than large in effect size; indeed a number of results reported herein fall below conventionally accepted thresholds for statistical significance. The susceptibility of our analyses to type-2 errors thus seems large. This point notwithstanding, we also conducted several analyses without correction for multiple comparisons, rendering susceptibility to type-1 error also large. As our study was underpowered to examine effects following further statistical control for multiple comparisons, replication in larger samples is strongly advisable. Moreover, our sample was composed only of primarily educated, non-meditating young adults; future studies should evaluate the generalizability of the present results to groups of a differing demographic, including practiced meditators. Additionally, a certain number of our participants had to be removed due to outlying scores, the influence of which on our present results, and ability to fully generalize to populations of interest, is presently unknown. Moreover, as already noted, we recommend future studies evaluate participants across multiple intervention sessions using a longitudinal design rather than only as a single-session trial and, in particular, conduct of a randomized within-subject cross-over design would provide the opportunity to evaluate the potential benefit of NFB following MM practice and vice versa. In addition, whereas the present study employed only single blinding of participants randomized to NFB vs. SHAM, a stronger design would involve blinding of clinicians administering the interventions as well. Inclusion of long-term MM and NFB practitioners would also help elucidate the significance of effects observed in novices here. Finally, use of a computerized version of the Stroop task, with collection of manual button presses rather than vocal responses as the dependent measure, can also be considered a limitation given that several authors have highlighted that response formats implemented when administering the Stroop task influence behavioral performance and the sensitivity of interference effects in particular (Kindt et al. 1996; Salo et al. 2001). Although verbal responses are more likely to generate EEG artifacts, Liotti et al. (2000) showed that different response formats in the Stroop task (verbal, covert, or button press responses) yield differential scalp distributions of ERPs.
We conclude that, to our knowledge, this pilot study is the first to demonstrate together the independent outcomes of a single session of MM and NFB distinctly on the full vs. lower and upper sub-bands of the EEG-alpha rhythm, as well as in regard to neurocognitive performance of the Stroop task. We recommend further investigation of the potential of treatments such as MM and NFB that target the EEG-alpha rhythm to improve attentional abilities and other neurocognitive functions.
References
Aftanas, L. I., & Goloshekin, S. A. (2003). Changes in cortical activity in altered states of consciousness: the study of meditation by high-resolution EEG. Human Physiology, 28(2), 143–151.
Angelakis, E., Stathopoulou, S., Frymiare, J. L., Green, D. L., Lubar, J. F., & Kounios, J. (2007). EEG neurofeedback: a brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clinical Neuropsychology, 21, 110–129.
Arch, J. J., & Craske, M. G. (2006). Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Beahviour Research and Therapy, 44, 1849–1858.
Baer, R. A., Smith, G. T., Hopkins, J., Krietemeyer, J., & Toney, L. (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13, 27–45.
Bazanova, O. M., Verevkin, E. G., & Shtark, M. B. (2007). Biofeedback in optimizing psychomotor reactivity II: the dynamics of segmental alpha-activity characteristics. Human Physiology, 33(6), 695–700.
Cahn, B. R., & Polich, J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132, 180–211.
Chan, D., & Woollacott, M. (2007). Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? Journal of Alternative and Complementary Medicine, 13, 651–657.
Chiesa, A., & Serretti, A. (2010). A systematic review of neurobiological and clinical features of mindfulness meditations. Psychological Medicine, 40, 1239–1525.
Cooper, N. R., Croft, R. J., Dominey, S. J. J., Burgess, A. P., & Gruzelier, J. H. (2003). Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implication for idling and inhibition hypotheses. International Journal of Psychophysiology, 47, 65–74.
Curran, S. L., Andrykowski, M. A., & Studts, J. L. (1995). Short form of the profile of mood states (POMS-SF): psychometric information. Psychological Assessment, 7(1), 80–83.
Dekker, M. K. J., Sitskoorn, M. M., Denissen, A. J. M., & van Boxtel, G. J. M. (2014). The time-course of alpha neurofeedback training effects in healthy participants. Biological Psychology, 95, 70–73.
Delorme, A., & Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21.
Demos, J. N. (2005). Getting started with neurofeedback (p. 79). New York: W.W. Norton & Company, Inc.
Eberth, J., & Sedlmeier, P. (2012). The effect of mindfulness meditation: a meta-analysis. Mindfulness, 3, 174–189.
Egner, T., & Gruzelier, J. H. (2004). EEG Biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related brain potentials. Clinical Neurophysiology, 115, 131–139.
Ergenoglu, T. (2004). Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Research. Cognitive Brain Research, 20(3), 376–383.
Frewen, P. A., Evans, E. M., Maraj, N., Dozois, D. J. A., & Partridge, K. (2008). Letting go: mindfulness and negative automatic thinking. Cognitive Therapy and Research, 32, 758–774.
Frewen, P. A., Lundberg, E., MacKinley, J., & Wrath, A. (2011). Mindfulness, 2, 254–269.
Frewen, P. A., Unholzer, F., Logie-Hagan, K. R. J., & MacKinley, J. D. (2014). Meditation breath attention scores (MBAS): test-retest reliability and sensitivity to repeated practice. Mindfulness, 5(2), 161–169.
Haegens, S., Cousijn, H., Wallis, G., Harrison, P. J., & Nobre, A. C. (2014). Inter- and intra-individual variability in alpha peak frequency. NeuroImage, 92, 46–55.
Hafenbrack, A. C., et al. (2014). Debiasing the mind through meditation: mindfulness and the sunk-cost bias. Psychological Science, 25(2), 369–376.
Hanslmayr, S., Sauseng, P., Doppelmayr, M., Schabus, M., & Klimesch, W. (2005). Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Applied Psychophysiology and Biofeedback, 30(1), 1–10.
Hanslmayr, S., Pastotter, B., Bauml, K. H., Gruber, S., Wimber, M., & Klimesch, W. (2008). The electrophysiological dynamics of interference during Stroop task. Journal of Cognitive Neuroscience, 20, 215–225.
Hardstone, R., Poil, S.-S., Schiavone, G., Nikulin, V. V., Mansvelder, H. D., & Linkenkaer-Hansen, K. (2012). Detrended fluctuation analysis: a scale-free view on neuronal oscillations. Frontiers in Physiology, 3, 450.
Ilan, A. B., & Polich, J. (1999). P300 and response time from a manual Stroop task. Clinical Neurophysiology, 110, 367–373.
Kindt, M., Bierman, D., & Brosschot, J. F. (1996). Stroop versus Stroop: comparison of a card format and a single-trial format of the standard color-word Stroop task and the emotional Stroop task. Personality and Individual Differences, 21, 653–661.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews, 29, 169–195.
Klimesch, W., Sauseng, P., & Hanslmayr, S. (2007). EEG alpha oscillations: the inhibition-timing hypothesis. Brain Research Reviews, 52, 63–88.
Konareva, I. N. (2005). Modifications of the EEG frequency pattern in humans related to a single neurofeedback session. Neurophysiology, 37, 388–395.
Lagapolous, J., et al. (2009). Increased theta and alpha EEG activity during nondirective meditation. The Journal of Alternative and Complementary Medicine, 15(11), 1187–1192.
Lau, M., Bishop, S. R., Segal, Z. V., Buis, T., Anderson, N. D., Carlson, L., Shapiro, S., Carmody, J., Abbey, S., & Devins, G. (2006). The Toronto mindfulness scale: development and validation. Journal of Clinical Psychology, 62, 1445–1467.
Liotti, M., Woldorff, M. G., Perez, R., & Mayberg, H. S. (2000). An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia, 38, 701–711.
Lomas, T., Ivtzan, I., & Fu, C. H. Y. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience & Biobehavioral Reviews, 57, 401–410.
Lovibond, P. F., & Lovibond, S. H. (1995). The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33, 335–343.
Lutz, A., Slagter, H. A., Dunne, J. D., & Davidson, R. J. (2008). Attention regulation and monitoring in meditation. Trends in Cognitive Sciences, 12(4), 163–169.
Lutz, A., Slagter, H. A., Rawlings, N. B., Francis, A. D., Greischar, L. L., & Davidson, R. J. (2009). Mental training enhances attentional stability: neural and behavioural evidence. The Journal of Neuroscience, 29, 13418–13427.
McNair, D. M., Lorr, M., & Droppleman, L. F. (1971). EITS manual for the profile of mood states. San Diego: Educational and Industrial Testing Service.
Moore, A., & Mailnowski, P. (2009). Meditation, mindfulness, and cognitive flexibility. Consciousness and Cognition, 18, 176–186.
Pfurtscheller, G., & Lopes da Silva, F. H. (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology, 110, 1842–1857.
Raymond, K., Varney, C., Parkinson, L. A., & Gruzelier, J. H. (2005). The effects of alpha/theta neurofeedback on personality and mood. Cognitive Brain Research, 23, 287–292.
Romero, S. G., McFarland, D. J., Faust, R., Farrell, L., & Cacace, A. T. (2008). Electrophysiological markers of skill-related neuroplasticity. Biological Psychology, 78, 221–230.
Ros, T., Théberge, J., Frewen, P. A., Kluetsch, R., Densmore, M., Calhoun, V. D., & Lanius, R. A. (2013). Mind over chatter: plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. NeuroImage, 15, 324–335.
Salo, R., Henik, A., & Robertson, L. C. (2001). Interpreting Stroop interference: an analysis of differences between task versions. Neuropsychology, 15, 462–471.
Sedlmeier, P., Eberth, J., Schwarz, M., Zimmerman, D., Haarig, F., & Kunze, S. (2012). The psychological effects of meditation: a meta-analysis. Psychological Bulletin, 138, 1139–1171.
Slagter, H. A., Lutz, A., Greischar, L. L., Francis, A. D., Nieuwenhuis, S., Davis, J. M., & Davidson, R. J. (2007). Mental training affects distribution of limited brain resources. PLoS Biology, 5(6), 1228–1236.
Slagter, H. A., Davidson, R. J., & Lutz, A. (2011). Mental training as a tool in the neuroscientific study of brain and cognitive plasticity. Frontiers in Human Neuroscience, 5, 17.
Tang, Y., Ma, Y., Wang, J., Fan, Y., Feng, S., Lu, Q., et al. (2007). Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of the Sciences, 104, 17152–17156.
van Boxtel, G. J. M., Denissen, A. J. M., Jager, M., Vernon, D., Dekker, M. J. K., & Sitskoorn, M. M. (2012). A novel self-guided approach to alpha activity. International Journal of Psychophysiology, 83, 282–294.
van Lutterveld, R., Houlihan, S. D., Pal, P., Sacchet, M. D., McFarlane-Blake, C., & Brewer, J. D. (2016). Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation. NeuroImage. doi:10.1016/j.nueorimage.2016.02.047.
Vernon, D., Dempster, T., Bazanova, O., Rutterford, N., Pasqualini, M., & Andersen, S. (2009). Alpha neurofeedback training for performance enhancement: reviewing the methodology. Journal of Neurotherapy, 13, 214–227.
Wenk-Sormaz, H. (2005). Meditation can reduce habitual responding. Advances, 21, 33–49.
Zoefel, B., Huster, R. J., & Herrmann, C. S. (2011). Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage, 54, 1427–1431.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Ontario Mental Health Foundation and the Lawson Health Research Institute.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(JPG 105 kb)
Rights and permissions
About this article
Cite this article
Chow, T., Javan, T., Ros, T. et al. EEG Dynamics of Mindfulness Meditation Versus Alpha Neurofeedback: a Sham-Controlled Study. Mindfulness 8, 572–584 (2017). https://doi.org/10.1007/s12671-016-0631-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-016-0631-8