Abstract
How the NPs effect the growth and physiological response like the release of organic acids along the root exudates is largely unknown yet. In this study, the effects of titanium dioxide (TiO2) and silicon dioxide (SiO2) nanoparticles (NPs) treatments (1000 mg/L) on maize seedlings for 6 days were examined. Plant biomass, pigment, malondialdehyde (MDA), reactive oxygen species (ROS) production, and contents of organic acids in root exudates were analyzed. SiO2 NPs significantly reduced (p < 0.05) shoot length, roots, and shoot fresh weight. TiO2 NPs showed significant differences (p < 0.05) in pigment contents compared to the CK. Chlorophyll a, b, total chlorophyll content, and chlorophyll/carotenoid ratio decreased by 27.8%, 29%, 28.1%, and 46.1%, respectively, while the content of carotenoid increased by 33.6% (p < 0.05). As concerns SiO2 NPs treatment, there was no significant increase (p > 0.05) in chlorophyll a content compared to the CK, while chlorophyll b increased by 28.9% (p < 0.05), and chlorophyll a/b ratio and content of carotenoid decreased by 16.8% and 54.7% (p < 0.05), respectively. MDA content significantly diminished in roots and leaves under SiO2 NPs. However, O2·ˉ production increased in roots by 17.2% and 23.8% (p < 0.05), respectively, under TiO2 and SiO2 NPs treatment. The pH of root exudates was declined by 17.4% and 14.2% (p < 0.05) respectively under both NPs treatment. Organic acid contents under TiO2 NPs significantly heightened (p < 0.05) by 60.7%, 31.2%, and 50.5% for citric, lactic, and fumaric acid, respectively, while formic and oxalic acid decreased by 27.8% and 26.4% respectively compared to the CK. In SiO2 NPs case, oxalic acid increased by 41.1% (p < 0.05), while malic and citric acid decreased by 62.6% and 45.7% respectively compared to the CK. In conclusion, both NPs treatments showed alternative impacts on maize seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is an increasing interest in the impact of the inevitable environmental nanoparticles on plant growth, even the entire food chain [1]. From an ecotoxicological point of view, titanium dioxide (TiO2) and silicon dioxide (SiO2) NPs are by far the most investigated metal oxide nanoparticles [2, 3].
Root, shoot length, and weight are morphological displays of plant health that has been shown in previous investigation. Along with it, the different size and concentration of TiO2 heightened and reduced the fresh biomass of wheat [4]. In addition, the chlorophyll content in leaves of cucumber and Phaseolus vulgaris were reduced under TiO2 NPs treatment [5, 6]. Plants developed antioxidant defense systems to prevent the negative effects of reactive oxygen species (ROS) based on toxicity, throughout their production [7]. While, the 10 and 30 ppm treatment of TiO2 NPs boosted the antioxidant enzyme activities in P. vulgaris [5]. As reported, TiO2 NPs controlled the nitrogen metabolism by improving enzyme activities and the conversion of inorganic nitrogen into organic nitrogen in the form of protein and chlorophyll [8, 9].
An elevated level of SiO2 NPs in the environment can lead to the physiological effect on living organisms. A research study based on SiO2 NPs improved maize seed germination by providing better available nutrients and pH in the culture medium [10]. Further, with salinity tension, SiO2 NPs ameliorates the dry and fresh weight of the leaves, chlorophyll content, and proline accumulation. An increase in the accumulation of proline, free amino acids, nutrient content, and activity of antioxidant enzymes by SiO2 NPs is thereby improving the tolerance of plants to abiotic stress [11]. Some studies revealed that SiO2 NPs improved the growth and development of plants by increasing the parameters of gaseous exchange and chlorophyll fluorescence, such as the net photosynthetic rate, transpiration rate, stomatal conductance, potential activity of PSII, effective photochemical efficiency, electron transport rate, and photochemical quench [11]. On number of well-characterized SiO2 NPs, it was concluded that they were not phytotoxic to Arabidopsis thaliana [12]. At the same time, however, they proposed to allow an indirect negative effect of the SiO2 NPs by the adsorption of nutrients by particles, which are therefore not available for uptake and transport, leading to physiological disturbances in the plant. Both positive and negative effects have been reported when applied to higher plants, but in the soil, the microbial ecosystem can also be influenced by nanoparticles [13].
The rhizosphere is an active microenvironment, in which water and nutrients are absorbed and many substances such as organic acids, amino acids, sugar, endogenous hormones, enzymes, and certain metabolites are constantly secreted from the roots [14]. Organic acids can either stimulate the solubility or immobility of trace and toxic metals, depending on the nature and concentration of organic acids, soil properties, and other environmental factors [15]. In general, organic acids are released in the form of anions and their release is balanced by releasing the cations. In addition, the metal dependence of proton efflux can be coupled after the damage of H+-ATPase pumping activities as indicated in some plants, e.g., Cucumis sativus [16]. Most plants are able to emit some low molecular weight organic acids under stress, such as oxalic, malic, citric, and succinic acids [17]. For example, tartaric is the most important organic acid in root exudates of Ricinus communis under Cu stress [18]. Succinic acid filled 43.7–73.6% in the secretion from Capsicum annuum roots, and tartaric and acetic citric can be observed under Cd stress [19]. Oxalic acid can be well separated as the most low molecular weight organic acids secreted from mangrove plant Kandelia obovata roots under Cd stress [20]. Based on the number of carboxylic groups, the accession of organic acids may cause soil acidosis, as well as the decline of soil pH [21]. However, little is known about the comparative effects of TiO2 and SiO2 NPs on root exudates.
In this study, we hypothesized that relevant concentration of TiO2 and SiO2 NPs would induce physiological responses in maize seedling. To evaluate this, we shall study plant–nanoparticle interactions in hydroponic growth medium. Therefore, this study will focus on the growth and physiological (ROS, MDA, chlorophyll) response of maize seedlings and the release of organic acids along the root exudates of maize seedlings under TiO2 and SiO2 NPs stress.
2 Materials and Methods
2.1 Characterization of TiO2 and SiO2 NPs
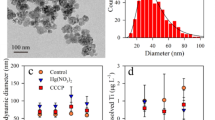
TiO2 and SiO2 NPs were bought from DK Nano Technology Co., Ltd., Beijing, China, which had nominal particle size of 30 nm and 99.9% purity provided by producer. Scanning electron microscopy (SEM, S-4800, Hitachi, Ltd. Japan) was applied to examined morphology of TiO2 and SiO2 NPs. The SEM image of TiO2 and SiO2 NPs is shown in Figs. 1 and 2 respectively without further purification, while the mean size of particles was 30 nm, claimed by the producer [22].
2.2 Preparation of TiO2 and SiO2 NPs Suspension
The suspension of concentration on 0 (CK), 1000 mg/L (TiO2), and 1000 mg/L (SiO2) NPs respectively was prepared for distilled water. To avoid assemblage, the TiO2 and SiO2 NPs suspensions were sonicated for 1 h (VWR 75T Aquasonic sonicator, 30 °C, 100 W, 40 kHz) before the use [23]. Small magnetic bars were located in the suspension for invoking to avoid aggregation of the particles [24].
2.3 Plant Material, Growth, and Treatment Condition
Maize (Zea mays L.) seeds were purchased from Agriculture Sciences Academy of Hubei, China. Selected healthy seeds thoroughly washed with distilled water to remove debris. Seeds were firstly sterilized in 70% ethanol for 5 min, then in 10% (v/v) H2O2 solution for 15 min followed by washing with distilled water to ensure surface sterility, finally imbibed in distilled water for 24 h. Then, the soaked seeds were placed on moist filter papers in a Petri dish in dark at 28 °C for germination. After 3 days, uniform seedlings with same radicle protrusion of 2 cm were selected and transferred onto the black Styrofoam sheet which was placed on the top of plastic box (top 19.5 × 13 cm, bottom 17.5 × 11 cm) filled with 1/2 strength Hoagland solution [25]. Each treatment box had fifteen seedlings and grown at an environmentally controlled growth room, where maintained with temperature of 25–27 °C, humidity of 50–70%, 12/12-h day/night photoperiod, and light intensity at 800–1000 μmol m−2 s−1. After 3 days in order to facilitate adaptation and appearance of second leaf, they were exposed with full strength Hoagland solution with NPs treatments of 0 (CK), 1000 mg/L (TiO2), and 1000 mg/L (SiO2) respectively for 6 days. Each treatment had three replicates. Stock solutions of TiO2 and SiO2 NPs were sonicated as above mentioned method. The culture solutions were replaced at every third day and completion of the treatment seedlings was harvested for the further study [26].
2.4 Plant Growth Measurement
After exposure to TiO2 and SiO2 NPs treatment for 6 days, maize seedlings were randomly selected and smoothly uprooted; the root system was washed under running tap water. Data of 5 seedlings from each treatment with three replications were measured for shoot and root length, shoot, and root fresh weight. Fresh materials were oven-dried at 70 °C for 72 h and root and shoot dry weights were recorded [27]. An electronic balance (Model BS 223S, Sartorius, Germany) was used for weight measurement.
2.5 Pigment Content in Leaves
Photosynthetic pigments were extracted from 0.3 g of leaves cut into pieces as sample in 10 ml acetone of 80% and left for 2 days in the dark with periodic shaking. After 2 days, the samples were whirled and centrifuged at 12,000 rpm for 10 min. The absorbance of the supernatant at 663, 645 and 470 nm was measured using spectrophotometer (Varian Cary 50 UV-VIS, Varian, Palo Alto, CA, USA) [28]. The content of chlorophyll a and b and carotenoids was calculated using the following formula [29].
The contents of pigments were carried as mg/g per fresh weight.
2.6 Determination of Malondialdehyde
The membrane lipid peroxidation was determined in relation to thiobarbituric acid reactive substances (TBARS) [30]. The root and leaf samples (0.2 g) were homogenized in phosphate buffer of 1 ml of 50 mM (pH 7.8) in an ice bath, centrifuged at 10,000 g and 4 °C for 15 min. The reaction mixture, which contained 0.4 ml of the supernatant, 0.65 ml of 0.5% in the TBA in 20% TCA was incubated for 20 min at 95 °C in a water bath, and then cooled to room temperature. Lastly, the mixture was centrifuged at 10,000 g for 15 min and the absorbance of the supernatant was measured at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm and 450 nm. The malondialdehyde (MDA) concentration was calculated as
2.7 Determination of Reactive Oxygen Species (ROS)
Hydrogen peroxide (H2O2) content measurement was performed in spectrophotometer [30]. The roots and leaves samples (0.2 g) were homogenized in 1 ml of 5% trichloroacetic acid (TCA) and centrifuged at 12,000 g, 4 °C for 15 min. The reaction mixture consisted of 0.6 ml supernatant, 0.5 ml potassium phosphate buffer of 10 mM (pH 7.0), 0.4 ml of 5% TCA, and 0.5 ml potassium iodide (KI) of 1 M. The reaction was kept in the darkness for 1 h and the absorbance was measured at 390 nm. The content of H2O2 was calculated from the standard curve prepared with the known concentrations of H2O2.
The superoxide radical (O2·−) content was measured with a minor modification [30]. The roots and leaves samples (0.2 g) were homogenized in mortar and pestle placed in an ice bath with 1 ml of phosphate buffer (pH 7.8), centrifuged at 12,000 g, 4 °C for 15 min. The supernatant of 0.25 ml reacted with 0.75 ml of 1 mM hydroxylamine hydrochloride for 1 h, 1 ml of α-naphthylamine from 7 mM stock and then 1 ml of p-aminobenzene sulfonic acid from 17 mM stock was added. The reaction mixture was kept at 25 °C for 20 min, and the optical density of the solution was measured with a spectrophotometer at 530 nm. For the standard curve, NaNO2 was used instead of the supernatant.
2.8 Root Exudate Collection
After 6 days of exposure to TiO2 and SiO2 NPs treatments with full strength Hoagland nutrient solution, the root exudates were collected [31] with little modifications. At 09:00 am, equal size of seedlings was gently taken out from the black Styrofoam sheet which covered on treatment medium, washed with tap water to remove the ions and NPs for 2 min followed by 1 min sterilized distilled water. The 4 seedlings from each 3 replicates were transferred to 50 ml sterilized plastic vial wrapped by aluminum foil to maintain the roots in the dark [32]. It was contained 40 ml of sterilized distilled water to submerge the whole root system of seedlings. The seedlings were placed for 24 h in a plant growth room, where maintained temperature at 25–27 °C, humidity 50–70%, 12/12-h day/night photoperiod, and light intensity 800–1000 μmol m−2 s−1 [33]. After time interval, the roots were gently removed, then washed with extra 10 ml deionized water to collect all exudates in final volume of 50 ml. After collection, the exudates were quickly evaporated from 50 ml up to 2 ml with help of rotatory evaporator machine at 40 °C [34]. Root exudates (organic acids) concentrated volume of 2 ml were filtered through 0.22 μm sterile syringe filters (Sartorius, Minisart, Gottingen, Germany), transferred into the dark red automatic sample bottles of 1.5 ml and stored at − 20 °C refrigerator for further analysis in HPLC.
2.9 Measurement of pH and Organic Acid Content of Root Exudates
The pH of collected root exudates was analyzed with a pH meter (ORION 3 Star, USA). After collection of root exudates, organic acid was analyzed by HPLC machine (Shimadzu made in Japan) fixed with an ion-exclusion column (ThermoScientific ODS Hypersil Dim (mm) 250 × 4.6). The mobile phase A was 25 mM KH2PO4 solution at pH 2.4 and phase B was methanol with flow rate of 1.0 ml/min, detection wavelength 210 nm, inject 20 μl, and time 10 min per sample. The positive identification of organic acids was performed by comparison of retention time with addition of 8 standard curves for each organic acid. The different retention times were 2.6, 3.0, 3.3, 3.7, 4.3, 4.6, 5.7, and 6.6 min for oxalic, tartaric, formic, malic, lactic, acetic, citric, and fumaric acids, respectively.
2.10 Statistical Analysis
The data were analyzed using Excel 2013 and GraphPad Prism 5.00. The data were expressed as mean ± standard error (SE) (n = 3). Statistical significances of differences among treatments were determined using t test (and nonparametric tests) followed by unpaired t test at a significance level of 0.05 (significantly different from the CK: *p < 0.05, **p < 0.01, and ***p < 0.001).
3 Results and Discussion
3.1 Effects of TiO2 and SiO2 NPs on Seedling Growth
The aim of this study is to raise awareness about the effect of TiO2 and SiO2 NPs on the growth of maize seedlings. In this regard, high dose of TiO2 and SiO2 NPs was selected to clarify the maximum potential impressions. It is to be noted that the NPs concentration contained in this study is much higher than that of nature [35], so the results obtained are considered a mechanistic study rather than a simulation of environmental processes. To date, previous studies on the effects of NPs on plant growth have shown that the stimulation or inhibition on plant growth depends on the types of NPs, exposure concentrations, and plant species [36]. For example, 20 mg/L of the iron oxide (ã-Fe2O3) NPs stimulates the root length of maize (Zea mays), but at 50 and 100 mg/L, NPs inhibits root growth [28].
In the present study, the plant root/shoot elongation and fresh/dry biomass were quantified as indicators of plant growth. Root/shoot length and biomass of maize seedlings showed significant (p < 0.05) or non-significant changes (p > 0.05) under TiO2 and SiO2 NPs treatment as compare to the CK (Fig. 3a–f). There was not such a major negative effect of TiO2 and SiO2 NPs treatment on maize seedling except under SiO2 treatment in Fig. 3b–d. Over all change TiO2 NPs treatment showed no significant effect on maize seedling growth and biomass. Phytotoxicity of NPs is somewhat rarified to determination, due to the quick dissolution of metallic ions from the NPs along with the potential toxicity of the NPs themselves [37].
In case of TiO2 NPs treatment, that the roots, shoot length, and fresh biomass of Fenugreek (Trigonella foenum-graecum) and rice (Oryza sativa L.) exposed under different TiO2 NPs treatments, had no impact with respect to control [38, 39]. Same results were seen in our data belong to TiO2 treatment (Fig. 3a–f). Oppositely, aggregation of TiO2 NPs at the cell wall surfaces led to the root reducing the water transport capacity and cell wall pore size, thereby TiO2 NPs had little repressive depressions on the root and shoot fresh weight (Fig. 3c, d) of maize seedlings [40].
Shoot height showed significant decrease by 10.6% (p < 0.05) at SiO2 NPs in maize seedlings compare to CK (Fig. 3b). As equate to CK (Fig. 3c, d), root and shoot fresh weight reduced by 34.7% and 26.9% (p < 0.05) at SiO2 NPs respectively compare to CK. An agreement of the researchers related to our SiO2 NPs treatment results on seedling growth structure such as the effects of SiO2 NPs on the development of both non-transgenic and Bt-transgenic cotton, disclosed that exposure like 0, 10, 100, 500, and 2000 mg/L for 3 weeks, significantly decreased the plant height, root and shoot fresh and dry biomasses [41]. While root length showed no significant difference (Fig. 3a), that is similar to as shown where there is no significant impact of the SiO2 NPs treatment on wheat and lupin root lengths compared to the controlled plants [42].
Besides, root fresh weight at SiO2 was significantly (p < 0.05) reduced by 22.4% as compare to TiO2 NPs treatment (Fig. 3c). Alternatively in (Fig. 3e, f), the root and shoot dry weight have no significant (p > 0.05) decrease in maize seedlings exposed to TiO2 and SiO2 NPs as associated to CK. These results showed the little phytotoxicity of SiO2 NPs in terms of reduction of maize seedlings growth, while TiO2 had little increased the growth rate in seedlings as compared SiO2 treatment.
3.2 Changes in the Pigment Contents
Chlorophyll content is the most common indicator of the photosynthetic pigment of plant, which is one of the most important determinants of its growth, and chlorophyll level can be a significant indicator of NPs toxicity to plants [36]. Within plant cells, oxidative damage could occur in chloroplast through interactions with metal-based NPs that may finally interrupt the biosynthesis of chlorophyll or cause the abasement of chlorophyll in leaves [43]. In our study, pigment content in maize seedlings changed between TiO2 and SiO2 NPs treatment compare to the CK. Under TiO2’s exposure, chlorophyll a 27.8%, chlorophyll b 29%, total chlorophyll content 28.1%, and chlorophyll/carotenoid ratio 46.1% significantly (p < 0.05) decreased (Fig. 4a, b, c, f). While carotenoid content (p < 0.05) increased by 33.6% (Fig. 4e). Other research shows that there is no significant impact of TiO2 NPs on the growth in terms of biomass of fenugreek seedlings, but leaf chlorosis is observed under 100 mg/L of TiO2 NPs by decrease in chlorophyll a, b, and carotenoid content [38]. Similar study on annual soil-grown herb plants (Clarkia unguiculata) exposed for 8 weeks with TiO2, CeO2, or Cu (OH)2 concentrations at unlike levels of light and nutrient, further it showed that TiO2 and CeO2 reduced photosynthetic rate and CO2 assimilation efficiency, possibly through the interruption of energy transfer from PSII to the Calvin cycle [44].
On the contrary, the SiO2 NPs have non-significantly changed chlorophyll a, total chlorophyll content, and chlorophyll/carotenoid ratio (Fig. 4a, c, f). While, chlorophyll b increased by 28.9%. And ratio of chlorophyll a/b 16.8% and carotenoid content 54.7% respectively (p < 0.05) decreased in maize seedling under exposure to SiO2 NPs compare to the CK (Fig. 4b, d–e). In case of SiO2 NPs, other authors pointed out that samples of maize leaves harvested from experimental plots (20 days) showed the gradual increase in chlorophyll a and b values depending on the concentration gradient of SiO2 NPs in contrast to bulk counterpart (0.012 and 0.03 μg/mL) of 20-day-old samples of maize leaves. In addition, a higher chlorophyll content is obtained at N15 (15 kg/ha porous silica NPs) and N20 (20 kg/ha porous silica NPs) (0.045 and 0.047 μg/mL, respectively) than in other regimes of silica treatments [10]. Research declared that the SiO2 NPs increased the accumulation of silicon in leaves and chloroplasts which led to increased photosynthetic activity [42]. In addition of SiO2 NPs has been found to reduce the accumulation of arsenic in maize variety and hybrid seedlings, resulting in better photosynthetic performance [45]. Former research display that diminishing in carotenoid content is a common reaction to the metal toxicity [46], but the gain is due to the vital role of this pigment in the detoxification of ROS [47]. Dissimilarity in total chlorophyll/carotenoids is proposed as a good sign of stress in plants [48]. In brief, our study showed various effects of TiO2 and SiO2 NPs on photosynthetic pigment compare to the CK.
3.3 Lipid Peroxidation and ROS Changed Under TiO2 and SiO2 NPs Stress
Plants suffer from environmental stress by producing malondialdehyde (MDA). The MDA is creditworthy for damage of cell membrane and is produced by the peroxidation of polyunsaturated fatty acids with free radicals, and as previously mentioned, it is utilized as a marker of oxidative stress, or MDA content is a parameter to assess cell membrane integrity and its content represents the membrane injury in the presence of stresses or pollutants [49]. The lipid peroxidation enhanced as observed by the other researchers is in demarcation to our result, where treatment denseness or time duration dependence increased/decreased in MDA was detected. This discrepancy might be due to the different plant species or varieties used in the experiments. Two scientists [50, 51] work on bean, tomato, and green pea; they demonstrated ZnO NPs treatments increased the lipid peroxidation in comparison to the control. However, in our study, lipid peroxidation in terms of MDA content exhibited non-significantly reduction under TiO2 treatment compared with the CK (Fig. 5a, b), suggesting there were no serious oxidative stress taking place in roots and leaves of maize seedlings under TiO2 NPs. An agreement with this para is that a non-significant gain of MDA in Allium cepa roots was disclosed under different concentrations of TiO2 NPs [1]. Furthermore TiO2 NPs did not induce physiological significant changes in wheat (Triticum aestivum) measured by lipid peroxide [52]. Similar notice was described in tolerant varieties of different plant species. For example, in tolerant Zea mays (Golden Variety), no MDA accumulation was found under 400 or 800 mg/kg CeO2 NPs treatments in soil [53]. Alternative to TiO2 NPs treatment, the accumulation of MDA in the maize seedlings roots and leaves significantly (p < 0.05) decreased by 28.9% and 49.2% at SiO2 NPs treatment, respectively, compared to the CK (Fig. 5a, b). Similarly, the addition of silica NPs reduced the accumulation of arsenic in maize variety and hybrid seedlings, resulting in improved photosynthetic performance, reduced levels of oxidative stress markers (MDA), and an improved antioxidant defense system [45].
Effects of TiO2 and SiO2 NPs on ROS content and lipid peroxidation in terms of malondialdehyde (MDA) content in maize seedlings. a MDA content in roots. b MDA content in leaves. c H2O2 content in roots. d H2O2 content in leaves. e O2·− content in roots. f O2·− content in leaves. Data are means ± SE from three replicates (n = 3)
Reactive oxygen species (ROS) are a group of responsive free radicals that appear due to oxidative stress. Further ROS are associated the growth reduction, there is a significant amount of evidence that ROS is important for cell division and cell extension [54]. ROS are generated by mitochondria through its dysfunction [55]. The production of ROS is a fundamental reaction of the plants to biotic and abiotic stress [56]. ROS, especially for hydrogen peroxide (H2O2) and superoxide (O2·−), induced by NPs could cause oxidative stress in plants which means ROS level is a great indicator of oxidative stress in plants [57]. In our study, the TiO2 and SiO2 NPs treatments did not make any significant effect on the production of H2O2 in the roots and leaves of maize seedlings (Fig. 5c, d). Similar to our results, one scientist [52] pointed out that the harmless impressions of TiO2 NPs were as well confirmed for wheat (Triticum aestivum), assessed by hydrogen peroxide production. Further relative to our results, mesoporous silica nanoparticles have no influence on the level of H2O2 in treated wheat and lupin plants [42]. However, in the case of superoxide radical O2·−, one of the important ROS increased significantly (p < 0.05) by 17.2% (p < 0.05) and 23.8% (p < 0.05) respectively in the roots under TiO2 and SiO2 NPs treatment compare to the CK (Fig. 5e), while it was of no significance in the leaves (Fig. 5f). Related to our results, there is no oxidative potential of SiO2 NPs agglomerations measured with conventional oxidative stress biomarkers [1].
Though, factors such as the size of NPs, form surface coating and denseness change depending greatly on the studies that sometimes lead to contradictory reports. In addition, plant species or varieties tend to differ in their response to NPs exposure, some show positive effects of the increase in NPs, while many others show negative effects [58]. The variances in the reaction to the exposure under NPs between plants and animals can be assigned to the cell structure. Plants, fungi, and bacteria have cell walls that form a primary site for interactions and an obstacle to the entry of NPs into the cells [40]. A comparable statement was proposed in the case of the L. minor water plant where TiO2 NPs were observed for leaves, but no cellular internalization was seen [28]. So in brief, it is widely acknowledged that acquaintance to NPs outcomes in cellular generation of ROS results in positive or negative effects on plant growth and development.
3.4 Effect of TiO2 and SiO2 NPs on pH and Organic Acid Content of Root Exudates
The pH or organic acids changed under TiO2 and SiO2 NPs treatments are of the great interest in this study. Low molecular weight organic acids are the main component of root exudates which function in belowground plant defense responding to biotic stress, and abiotic stress like heavy metal stress.
The pH value of the rhizosphere tends to be more acidic than the surrounding soil due to the release of protons by the roots to promote the absorption of soil ions and to counterbalance [59]. In our research (Fig. 6g), the pH value of the root excretion solution was CK 5.64 ± 0.03, which is generally lower than that of distilled water. In addition, the pH of root exudates was decreased by 17.4% and 14.2% (p < 0.05) respectively compare to the CK under TiO2 and SiO2 NPs treated maize seedling (Fig. 6g); the pH decrease in the soil is related to the increased production of organic acids by root secretion or other sources [60]. Conferring to previous study, applying the Cu stress to castor (Ricinus communis), the pH value of root secretion in treatments of 100, 250, and 500 μmol/L Cu decreased by 0.11, 0.26, and 0.32 pH units compared to the CK, respectively [18]. Among them, organic acids are the main component of root excretion. Briefly it has been confirmed that root secretion can close the soil to make it more acidic.
Numerous elements may affect root secretions of the plant, such as the status of the plant nutrient [61], metal or environmental stress, soil type (pH value, organic substance content, soil structure), presence of soil microorganism [18, 60], and plant species [62]. The plant roots constantly reacted and changed their immediate environment in the rhizosphere by conveying the chemicals separately from the roots [63]. Almost 5 to 21% of all photosynthetic solid carbon is transferred by root excretion to the rhizosphere, which helps the plant in soil nutrient gain in the development of microbiome in the rhizosphere [64]. The excretion of phytochemicals from the roots is a crucial means for plants to react to their stressed environment. It is an effective exclusion mechanism that reduces metal absorption and enables plant development at a high level of contamination [61, 65]. However, whether organic acid changed under TiO2 and SiO2 NPs exposure is not clear yet.
Although the root exudation rates are likely to rise when plants are grown in solid substrates, but in the absence of soil, the composition and quantity of organic acids mainly depend on plant species and metal doses [65]. From this perspective, a hydroponic method was selected in the current study to facilitate the exchange of nutrient solutions and a complete retake of root exudates. Six types of organic acid such as formic, oxalic, malic, citric, lactic, and fumaric acids were identified by high performance liquid chromatography (HPLC) machine by the peak area and the retention time method in the root exudates of maize seedlings treated by TiO2 and SiO2 NPs in hydroponic medium for 6 days (Fig. 6a–f). The results of our study indicate that LMWOAs in the root exudates from maize seedlings can be changed in quality and quantity by TiO2 and SiO2 NPs treatment. Among those organic acids, the content of formic acid was reduced by 27.8% (p < 0.05) at TiO2 NPs, while SiO2 NPs showed no significant change as compare to the CK (Fig. 6a). The oxalic as well as citric acids are known as reducing and chelating agents for metal cations [66]. Oxalic acid content was decreased by 26.4% at TiO2 NPs exposure and increased by 41.1% at SiO2 NPs exposure, respectively, compare to the CK (Fig. 6b), while oxalic acid content at SiO2 NPs treatment was higher by 91.8% (p < 0.05) than that of TiO2 NPs treatment. Our results from oxalic acid were similar with the study of a scientist [67] who worked on rice and found that the content of oxalic acid was reduced under the Cd or Zn stress, while oxalic increased in the addition of silicon. Malic acid showed no significant change under TiO2 NPs treatment compare to the CK; however, it decreased by 62.6% (p < 0.05) and 55.9% (p < 0.05) under SiO2 NPs treatment compare to the CK and TiO2 NPs treatment, respectively (Fig. 6c). The content of citric acid exhibited 60.7% increase (p < 0.05) and 45.7% decrease (p < 0.05), under TiO2 and SiO2 NPs respectively compare to the CK, while its content under SiO2 NPs exposure was lowered by 66.2% (p < 0.05) than that of TiO2 NPs treatment (Fig. 6d). Lactic acid content significantly (p < 0.05) increased by 31.2% at TiO2 NPs exposure compare to the CK, while it showed no significant change under SiO2 NPs treatment (Fig. 6e). Besides, the content of lactic acid under SiO2 NPs was lower by 24.9% (p < 0.05) than that of TiO2 NPs exposure (Fig. 6e). Related consequences were described that increase in certain organic acids, such as citric, lactic, and acetic acid, was found to improve the confrontation of the Cd [20, 68]. Furthermore studies found the secretion of citrate, malate, lactic acids, and other related organic acids by K. candel and crop plants are the main detoxifying mechanism in response to heavy metal stress [65, 67, 69]. Fumarate is at medium level in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) [70]. Here, fumaric acid increased by 50.5% (p < 0.05) under TiO2 NPs exposure compare to the CK, and there was no significant change under SiO2 NPs treatment compare with the CK and TiO2 NPs as well (Fig. 6f). Therefore, high level of citric, lactic, and fumaric acids (Fig. 6d–f) implies that the antioxidant function of these organic acids plays an important role in response to TiO2 NPs, while oxalic acid (Fig. 6b) response to SiO2 NPs stress in maize seedlings. In comparison between TiO2 and SiO2, malic, citric, and lactic acids significantly (p < 0.05) increased, while oxalic acid decreased (p < 0.05) and formic and fumaric acid have not any significance at TiO2 NPs treatment compare to SiO2 NPs treatment (Fig. 6a–f). These results showed that organic acids play diverse functions when reacting to unlike stress. Furthermore, root exudates unlike root length, for example, in metallophyte plants, the organic acid secretion was enhanced yet without root elongation, though the agricultural plants exuded citric acid at constant levels [65].
Taken together, the exudation of citric, lactic, fumaric, and oxalic acids from maize seedlings was likely understood as an adaptation to adverse environment, especially to toxic TiO2 and SiO2 NPs concentrations.
These results demonstrated that high dose of TiO2 and SiO2 NPs can change the secretions of root exudates in order to tolerate or accumulate further. These findings suggest that organic acids act as a representative of maize seedling responding to TiO2 and SiO2 NPs stress.
4 Conclusion
In the present study, we examined the effects of TiO2 and SiO2 NPs on plant biomass, photosynthetic pigments, MDA, ROS production, and organic acid content and pH of root exudates from hydroponically grown maize plants for 6 days. TiO2 NPs treatment did not affect plant growth and biomass, while SiO2 NPs reduced shoot length, shoot fresh weight, and dry root weight. Pigment content was decreased at TiO2 NPs exposure, while it has the positive role under SiO2 NPs treatment compare to the CK. Membrane lipid peroxidation in terms of MDA content in roots and leaves had no significant difference between CK and TiO2 NPs but it decreased under SiO2 NPs. Contents of H2O2 have not significant change in roots and leaves. O2˙ˉ production significantly increased in roots and no significant alteration in leaves is seen, respectively, under TiO2 and SiO2 NPs treatment compare to the CK. The pH of root exudates was declined (p < 0.05) by mentioned treatment, which means the rhizosphere was acidified under TiO2 and SiO2 NPs treatment. The contents of formic and oxalic acids in root exudates significantly diminished under TiO2 NPs compared to the CK; however, citric, lactic, and fumaric acid increased (p < 0.05), while malic acids have no changes under TiO2 NPs treatment compared to the CK. In case of SiO2 NPs, the content of oxalic acids increased and citric and malic acids decreased (p < 0.05) and formic, lactic, and fumaric acids have no significant variation compare to the CK. Taken together, TiO2 and SiO2 NPs’s treatments bring variation to maize seedling growth, chlorophyll contents, content of carotenoid, MDA production, reducing the pH and contents of different organic acids, in the form of the root exudation.
References
Koce, J. D., Drobne, D., Klancnik, K., Makovec, D., Novak, S., & Hocevar, M. (2014). Oxidative potential of ultraviolet—a irradiated or nonirradiated suspensions of titanium dioxide or silicon dioxide nanoparticles on Allium cepa roots. Environmental Toxicology & Chemistry, 33(4), 858–867. https://doi.org/10.1002/etc.2496.
Menard, A., Drobne, D., & Jemec, A. (2011). Ecotoxicity of nanosized TiO2: Review of in vivo data. Environmental Pollution, 159, 677–684. https://doi.org/10.1016/j.envpol.2010.11.027.
Passagne, I., Morille, M., Rousset, M., Pujalte, I., & L’azou, B. (2012). Implication of oxidative stress in size-dependent toxicity of silica nanoparticles in kidney cells. Toxicology, 299, 112–124. https://doi.org/10.1016/j.tox.2012.05.010.
Vittori, A. L., Carbone, S., Gatti, A., Vianello, G., & Nannipieri, P. (2014). Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environmental Science & Pollution., 22(3), 1841–1853. https://doi.org/10.1016/j.scitotenv.2019.05.265.
Jacob, D. L., Borchardt, J. D., Navaratnam, L., Otte, M. L., & Bezbaruah, A. N. (2015). Uptake and translocation of Ti from nanoparticles in crops and wetland plants. International Journal of Phytoremediation, 15(2), 142–153. https://doi.org/10.1080/15226514.2012.683209.
Servin, A. D., Morales, M. I., Castillo-Michel, H., Hernandez-Viezcas, J. A., Muniz, B., Zhao, L., Nunez, J. E., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2013). Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environmental Science & Technology, 47, 11592–11598. https://doi.org/10.1021/es403368j.
Schmitt, F. J., Renger, G., Friedrich, T., Kreslavski, V. D., Zharmukhamedov, S. K., Los, D. A., Kuznetsov, V. V., & Allakhverdiev, S. I. (2014). Reactive oxygen species: Reevaluation of generation, monitoring and role in stress-signaling in phototropic organisms. Biochimica et Biophysica Acta, 1837, 835–848. https://doi.org/10.1016/j.bbabio.2014.02.005.
Yang, F., Hong, F., You, W., Liu, C., Gao, F., Wu, C., & Yang, P. (2006). Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biological Trace Elements Research, 110(2), 179–190. https://doi.org/10.1385/bter:110:2:179.
Mishra, V., Mishra, R. K., Dikshit, A., & Pandey, A.C. (2014). Interactions of nanoparticles with plants. Emerging technologies and management of crop stress tolerance. Oxford: Elsevier Inc, pp. 159–180. https://doi.org/10.1016/B978-0-12-800876-8.00008-4.
Suriyaprabha, R., Karunakaran, G., Yuvakkumar, R., Prabu, P., Rajendran, V., & Kannan, N. (2012). Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. Journal of Nanoparticles Research, 14(12), 1–14. https://doi.org/10.1007/s11051-012-1294-6.
Siddiqui, M. H., & Al-Whaibi, M. H. (2014). Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum Mill.). Saudi Journal of Bioliological Science, 21(1), 13–17. https://doi.org/10.1016/j.sjbs.2013.04.005.
Slomberg, D. L., & Schoenfisch, M. H. (2012). Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environmental Science & Technology, 46, 10247–10254. https://doi.org/10.1021/es300949f.
Monica, R. C., & Cremonini, R. (2009). Nanoparticles and higher plants. Caryologia, 62(2), 161–165. https://doi.org/10.1080/00087114.2004.10589681.
Zeng, F., Chen, S., Miao, Y., Wu, F., & Zhang, G. (2008). Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress. Environmental Pollution, 155(2), 284–289. https://doi.org/10.1016/j.envpol.2007.11.019.
Ding, Y. Z., Song, Z. G., Feng, R. W., & Guo, J. K. (2014). Interaction of organic acids and pH on multi-heavy metal extraction from alkaline and acid mine soils. International Journal of Environmental Science & Technology, 11, 33–42. https://doi.org/10.1007/s13762-013-0433-7.
Janicka-Russak, M., Kabala, K., Burzynski, M., & Klobus, G. (2008). Response of plasma membrane H+-ATPase to heavy metal stress in Cucumis sativus root. Journal of Experimental Botany, 59, 3721–3728. https://doi.org/10.1093/jxb/ern219.
Javed, M. T., Stoltz, E., Lindberg, S., & Greger, M. (2013). Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environmental Science Pollution Research, 20(3), 1876–1880. https://doi.org/10.1007/s11356-012-1413-z.
Huang, G., Guo, G., Yao, S., Zhang, N., & Hu, H. (2016). Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress. International Journal of Phytoremediation, 18, 33–40. https://doi.org/10.1080/15226514.2015.1058333.
Xin, J. L., Huang, B. F., Dai, H. W., Zhou, W. J., Yi, Y. M., & Peng, L. J. (2014). Roles of rhizosphere and root-derived organic acids in Cd accumulation by two hot pepper cultivars. Environmental Science & Pollution Research, 22(8), 6254–6261. https://doi.org/10.1007/s11356-014-3854-z.
Xie, X., Weiss, D. J., Weng, B., Liu, J., Lu, H., & Yan, C. (2013). The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata (S., L.) Yong) roots. Environmental Science & Pollution Research, 20, 997–1008. https://doi.org/10.1007/s11356-012-1031-9.
Zhi, A. L., Bi, Z., Han-Ping, X., Yong-Zhen, D., Wan-Neng, T., & Sheng-Lei, F. (2008). Role of low-molecule-weight organic acids and their salts in regulating soil pH. Pedosphere, 18(2), 137–148. https://doi.org/10.1016/S1002-0160(08)60001-6.
Zhang, R., Zhang, H., Tu, C., Hu, X., Li, L., Luo, Y., & Christie, P. (2015). Phytotoxicity of ZnO nanoparticles and the released Zn(II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environmental Science and Pollution Research, 22, 11109–11117. https://doi.org/10.1007/s11356-015-4325-x.
Lin, D., & Xing, B. (2007). Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environmental Pollution, 150, 243–250. https://doi.org/10.1016/j.envpol.2007.01.016.
Zhang, D., Hua, T., Xiao, F., Chen, C., Gersberg, R. M., Liu, Y., Stuckey, D., Wun Jern Ng, W. J., & Tan, S. K. (2015). Phytotoxicity and bioaccumulation of ZnO nanoparticles in Schoenoplectus tabernaemontani. Chemosphere, 120, 211–219. https://doi.org/10.1016/j.chemosphere.2014.06.041.
Hoagland, D. R., & Arnon, D. I. (1950). The water-culture method for growing plants without soil. California Agricultural Experimental Station Circular, 347, 357–359.
Wang, Z. Y., Xie, X. Y., Zhao, J., Liu, X. Y., Feng, W. Q., White, J. C., & Xing, B. S. (2012). Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environmental Science & Technology, 46, 4434–4441. https://doi.org/10.1021/es204212z.
Lin, D., & Xing, B. (2008). Root uptake and phytotoxicity of ZnO nanoparticles. Environmental Science & Technology, 42, 5580–5585. https://doi.org/10.1021/es800422x.
Li, J., Hu, J., Ma, C., Wang, Y., Wu, C., Huang, J., & Xing, B. (2016). Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere, 159, 326–334. https://doi.org/10.1016/j.chemosphere.2016.05.083.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts—Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15. https://doi.org/10.1104/pp.24.1.1.
Chen, J., Wang, W. H., Wu, F. H., You, C. Y., Liu, T. W., Dong, X. J., He, J. X., & Zheng, H. L. (2013). Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant and Soil, 362, 301–318. https://doi.org/10.1007/s11104-012-1275-7.
Pan, X., Yang, J., Mu, S., & Zhang, D. (2012). Fluorescent properties and bifenthrin binding behavior of maize (Zea mays L.) seedling root exudates. European Journal of Soil Biology, 50, 106–108. https://doi.org/10.1016/j.ejsobi.2012.01.001.
Valentinuzzi, F., Cesco, S., Tomasi, N., & Mimmo, T. (2015). Influence of different trap solutions on the determination of root exudates in Lupinus albus L. Biology and Fertility of Soils, 51, 757–765. https://doi.org/10.1007/s00374-015-1015-2.
Zhang, N., Wang, D., Liu, Y., Li, S., Shen, Q., & Zhang, R. (2014). Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant and Soil, 374, 689–700. https://doi.org/10.1007/s11104-013-1915-6.
Chen, J., Liu, X., Wang, C., Yin, S. S., Li, X. L., Hu, W. J., Simon, M., Shen, Z. J., Chu, Q., Xiao, C. C., Peng, X. X., & Zheng, H.-L. (2015). Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. Journal of Hazardous Materials, 297, 173–182. https://doi.org/10.1016/j.jhazmat.2015.04.077.
Gottschalk, F., Sonderer, T., Scholz, R. W., & Nowack, B. (2009). Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environmental Science & Technology, 43, 9216–9222. https://doi.org/10.1021/es9015553.
Ma, C., Chhikara, S., Minocha, R., Long, S., Musante, C., White, J. C., Xing, B., & Dhankher, O. P. (2015). Reduced silver nanoparticle phytotoxicity in Crambe abyssinica with enhanced glutathione production by overexpressing bacterial γ-glutamylcysteine synthase. Environmental Science & Technology, 49, 10117–10126. https://doi.org/10.1021/acs.est.5b02007.
Ma, X. M., Geiser-Lee, J., Deng, Y., & Kolmakov, A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation role of root hairs and lateral roots in silicon uptake by rice. Science of the Total Environment, 408(16), 3053–3061. https://doi.org/10.1016/j.scitotenv.2010.03.031.
Missaoui, T., Smiri, M., Chmingui, H., & Hafiane, A. (2017). Effects of nanosized titanium dioxide on the photosynthetic metabolism of fenugreek (Trigonella foenum-graecum L.). Comptes Rendus Biologies, 340, 499–511. https://doi.org/10.1016/j.crvi.2017.09.004.
Cai, F., Wu, X., Zhang, H., Shen, X., Zhang, M., Chen, W., Gao, Q., White, J. C., Tao, S., & Wang, X. (2017). Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). NanoImpact, 5, 101–108. https://doi.org/10.1016/j.impact.2017.01.006.
Asli, S., & Neumann, P. M. (2009). Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell & Environment, 32(5), 577–584. https://doi.org/10.1111/j.1365-3040.2009.01952.x.
Le, V. N., Rui, Y., Gui, X., Li, X., Liu, S., & Han, Y. (2014). Uptake, transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. Journal of Nanobiotechnology, 12, 50–64. https://doi.org/10.1186/s12951-014-0050-8.
Sun, D., Hussain, H. I., Yi, Z., Rookes, J. E., Kong, L., & Cahill, D. M. (2016). Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere, 152, 81–91. https://doi.org/10.1016/j.chemosphere.2016.02.096.
Ma, C., White, J. C., Dhankher, O. P., & Xing, B. (2015b). Metal-based nanotoxicity and detoxification pathways in higher plants. Environmental Science & Technology, 49, 7109–7122. https://doi.org/10.1021/acs.est.5b00685.
Conway, J. R., Beaulieu, A. L., Beaulieu, N. L., Mazer, S. J., & Keller, A. A. (2015). Environmental stresses increase photosynthetic disruption by metal oxide nanomaterials in a soil-grown plant. ACS Nano, 9(12), 11737–11749. https://doi.org/10.1021/acsnano.5b03091.
Tripathi, D. K., Singh, S., Singh, V. P., Prasad, S. M., Chauhan, D. K., & Dubey, N. K. (2016). Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Frontiers of Environmental Science & Engineering, 4, 46–60. https://doi.org/10.3389/fenvs.2016.00046.
Rout, G. R., Samantaray, S., & Das, P. (2001). Aluminium toxicity in plants: A review. Agronomie, 21, 3–21. https://doi.org/10.1051/agro:2001105.
Chandra, R., Bharagava, R. N., Yadav, S., & Mohan, D. (2009). Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. Journal of Hazardous Materials, 162, 1514–1521. https://doi.org/10.1016/j.jhazmat.2008.06.040.
Hendry, G. A. F., & Price, A. H. (1993). Stress indicators chlorophyll and carotenoids. In Methods in comparative plant ecology (pp. 148–152). London: Chamman and Hall.
Nair, P. M., & Chung, I. M. (2014). Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere, 112, 105–113. https://doi.org/10.1016/j.chemosphere.2014.03.056.
Mukherjee, A., Peralta-Videa, J. R., Bandyopadhyay, S., Rico, C. M., Zhao, L., & Gardea-Torresdey, J. L. (2014). Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics, 6, 132–138. https://doi.org/10.1039/c3mt00064h.
Garcia-Gomez, C., Obrador, A., Gonzalez, D., Babin, M., Dolores, & Fernandez, M. (2017). Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Science of the Total Environment, 589, 11–24. https://doi.org/10.1016/j.scitotenv.2017.02.153.
Larue, C., Laurette, J., Herlin, N., Khodja, H., Fayard, B., Flank, A. M., Brisset, F., & Carriere, M. (2012). Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Science of the Total Environment, 431, 197–208. https://doi.org/10.1016/j.scitotenv.2012.04.073.
Zhao, L., Peng, B., Hernandez-Viezcas, J. A., Rico, C., Sun, Y., Peralta-Videa, J. R., Tang, X., Niu, G., Jin, L., Varela-Ramirez, A., Zhang, J. Y., & Gardea-Torresdey, J. L. (2012). Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano, 6(11), 9615–9622. https://doi.org/10.1021/nn302975u.
Singh, R., Singh, S., Parihar, P., Mishra, R. K., Tripathi, D. K., Singh, V. P., Devendra, K., Chauhan, D. K., & Prasad, S. M. (2016). Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Frontiers in Plant Science, 7, 1299. https://doi.org/10.3389/fpls.2016.01299.
Ahmed, B., Dwivedi, S., Abdin, M. Z., Azam, A., Al-Shaeri, M., Khan, M. S., Saquib, Q., Al-Khedhairy, A. A., & Musarat, J. (2017). Mitochondrial and chromosomal damage induced by oxidative stress in Zn (2+) ions, ZnO-bulk and ZnO-NPs treated Allium cepa roots. Scientific Reports, 7, 40685. https://doi.org/10.1038/srep40685.
Ee, S. F., Oh, J. M., Noor, N. M., Kwon, T. R., Mohamed-Hussein, Z. A., Ismail, I., & Zainal, Z. (2013). Transcriptome profiling of genes induced by salicylic acid and methyl jasmonate in Polygonum minus. Molecular Biology Report, 40(3), 2231–2241. https://doi.org/10.1007/s11033-012-2286-4.
Mostofa, M. G., Hossain, M. A., Fujita, M., & Tran, L. S. (2015). Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Scientific Reports, 5, 11433. https://doi.org/10.1038/srep11433.
Cox, A., Venkatachalam, P., Sahi, S., & Sharma, N. (2017). Reprint of: Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiology and Biochemistry, 110, 33–49. https://doi.org/10.1016/j.plaphy.2016.08.007.
Brady, N. C., & Weil, R. R. (2003). Elements of the nature and properties of soils (2nd ed.pp. 736–738). Upper Saddle River, NJ: Pearson Education.
Israr, D., Mustafa, G., Khan, K. S., Shahzad, M., Ahmad, N., & Masood, S. (2016). Interactive effects of phosphorus and Pseudomonas putida on chickpea (Cicer arietinum L.) growth, nutrient uptake, antioxidant enzymes and organic acids exudation. Plant Physiology & Biochemistry, 108, 304–312. https://doi.org/10.1016/j.plaphy.2016.07.023.
Montiel-Rozas, M. M., Madejón, E., & Madejón, P. (2016). Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environmental Pollution, 216, 273–281. https://doi.org/10.1016/j.envpol.2016.05.080.
Chiang, P. N., Wang, M. K., Chiu, C. Y., & Chou, S. Y. (2006). Effects of cadmium amendments on low-molecular-weight organic acid exudates in rhizosphere soils of tobacco and sunflower. Environmental Toxicology, 21, 479–488. https://doi.org/10.1002/tox.20210.
Badri, D. V., & Vivanco, J. M. (2009). Regulation and function of root exudates. Plant Cell & Environment, 32(6), 666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x.
Clemmensen, K. E., Bahr, A., Ovaskainen, O., Dahlberg, A., Ekblad, A., Wallander, H., Stenlid, J., Finlay, R. D., Wardle, D. A., & Lindahl, B. D. (2013). Roots and associated Fungi drive long-term carbon sequestration in boreal forest. Science, 339(6127), 1615–1618. https://doi.org/10.1126/science.1231923.
Meier, S., Alvear, M., Borie, F., Aguilera, P., Ginocchio, R., & Cornejo, P. (2012). Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicology & Environmental Safety, 75, 8–15. https://doi.org/10.1016/j.ecoenv.2011.08.029.
Ryu, S. R., Jeon, E. K., & Baek, K. (2016). A combination of reducing and chelating agents for electrolyte conditioning in electrokinetic remediation of as-contaminated soil. Journal of the Taiwan Institute of Chemical Engineers, 70, 252–259. https://doi.org/10.1016/j.jtice.2016.10.058.
Fan, X., Wen, X., Huang, F., Cai, Y., & Cai, K. (2016). Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiologiae Plantarum, 38(8), 1–9. https://doi.org/10.1007/s11738-016-2221-8.
Ehsan, S., Ali, S., Noureen, S., Mahmood, K., Farid, M., Ishaque, W., Shakoor, M. B., & Rizwan, M. (2014). Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicology & Environmental Safety, 106, 164–172. https://doi.org/10.1016/j.ecoenv.2014.03.007.
Lu, H., Yan, C., & Liu, J. (2007). Low-molecular-weight organic acids exuded by mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environmental & Experimental Botany, 61, 159–166. https://doi.org/10.1016/j.envexpbot.2007.05.007.
Moharregh-khiabani, D., Linker, R. A., Gold, R., & Stangel, M. (2009). Fumaric acid and its esters: An emerging treatment for multiple sclerosis. Current Neuropharmacology, 7(1), 60–64. https://doi.org/10.2174/157015909787602788.
Acknowledgments
This study was financially supported by the National Key Research and Development Program of China (2017YFC0506102), the Natural Science Foundation of China (NSFC) (31870581, 31570586), and Marine Scholarship by State Ocean Administration, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Humans and Animals Statement
None.
Informed Consent
All the authors agree and approve the submission of the manuscript.
Funding
This study was funded by the National Key Research and Development Program of China (2017YFC0506102), the Natural Science Foundation of China (NSFC) (31870581, 31570586) and Marine Scholarship by State Ocean Administration, China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghoto, K., Simon, M., Shen, ZJ. et al. Physiological and Root Exudation Response of Maize Seedlings to TiO2 and SiO2 Nanoparticles Exposure. BioNanoSci. 10, 473–485 (2020). https://doi.org/10.1007/s12668-020-00724-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-020-00724-2