Abstract
Soil contamination with toxic heavy metals (such as Cd or Zn) is becoming a serious problem worldwide because of the rapid development of social economy. Silicon plays a substantial role in alleviating heavy metal toxicity in crop plants. In this study, two rice varieties, Feng-Hua-Zhan and Hua-Hang-Si-Miao, were chosen to determine the effects of Si application on root morphological traits, cell structure and exudates of rice roots under Cd and/or Zn stress. Single or combined applications of Cd and Zn resulted in significant reduction of total root length, root surface area, root volume, average root diameter and root activity. However, 1.5 mM Si addition reversed these negative effects. Transmission electron microscopy observations showed that rice root cortex cells were heavily damaged under Cd and/or Zn stress for both two varieties, whereas Si addition resulted in improved cell structure integrity. In addition, lower levels of oxalic, acetic, tartaric, maleic and fumaric acids in root exudates were observed for Feng-Hua-Zhan under Cd and/or Zn stress, but addition of Si increased the acid levels. For Hua-Hang-Si-Miao, heavy metal treatments significantly reduced oxalic and fumaric acid levels and increased acetic, tartaric and maleic acid levels, whereas Si treatment showed opposite results. The above results indicated that Si could ameliorate the toxicity of heavy metals (Cd and Zn) for rice which resulted in improving root traits, cell structure and influencing root exudates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is one of the major crops in China, which provides an important contribution to economic and social stability. As a toxic metal, Cd can result in serious toxicity effects on organisms including humans when released to the environment, because of Cd bioaccumulation through the food chain (Song et al. 2009). Many studies have reported that excess Cd accumulation in plants prohibits plant growth, damages cell ultrastructure, and alters the primary and secondary metabolism of plants (Schutzendubel et al. 2001; Nwugo and Huerta 2008a, b; Da Cunha and do Nascimento 2009; Song et al. 2009). Zn is one of the essential trace elements for plants with important metabolic roles (Marschner 1995), but it can also cause severe phytotoxicity to plants and animals in excess. Cd and Zn have the same transporters in rice because of their chemical similarity. Evidences showed that OsHMA2 plays an important role in transporting Zn and Cd from root-to-shoot, which takes effects in loading Zn and Cd into the xylem in rice, and overexpression of OsHMA3 can reduce the accumulation of Cd in grains (Sasaki et al. 2014; Satoh-Nagasawa et al. 2012; Takahashi et al. 2012). Heavy metal ATPases (HMAs) are also important in translocating or detoxifying Zn and Cd in plants (Takahashi et al. 2014). Recent study found that Cd showed better mobility in Cd and Zn combined contamination (Ming et al. 2016).
In recent decades, silicon has been reported as a beneficial element in plants for its positive roles in alleviating environmental stresses (such as metal toxicity) (Ma 2004; Tripathi et al. 2012; Shen et al. 2014; Adrees et al. 2015). Nwugo and Huerta (2008a, b) showed that silicon application significantly reduced Cd concentration and improved photosynthetic traits and water use efficiency of Cd-stressed rice plants. Si can also reduce the metal toxicity to plants by inducing the secretion of flavonoid-type phenolics (Kidd et al. 2001) and the development of the apoplastic barrier in roots (Zhang et al. 2013). Similarly, Rizwan et al. (2016) reported that Si alleviates Cd toxicity may benefit from a root localized protection mechanism for reducing Cd translocation from roots to shoots. The absorption and translocation of Zn in plants reduced after Si addition because of the strong binding of Zn in cell wall (Gu et al. 2012; Lukacová et al. 2013). In addition, Lu et al. (2014) found that Si alleviates metal stress by increasing soil pH and reducing available Cd concentration in soil rather than reducing Cd accumulation. Moreover, Si improves the development of cortex and vascular tissues in roots, but it does not influence Cd distribution in apoplast and symplast (Vaculik et al. 2012). In Cd and Zn contaminated soil, Si can alter Cd and Zn distribution and allocation, therefore reduce the toxicity on soil (Patrícia Vieira Da Cunha et al. 2008). Based on the above findings, Si has important role in alleviating Cd or Zn toxicity, but the mechanisms of Si-mediated heavy metals resistance is not fully understood, especially in the roots. Our previous study found that silicon application can mitigate Cd and/or Zn toxicity and improve plant growth by increasing Si content and reducing Cd or Zn content in roots and shoots (Wen et al. 2011). Since organic acids secreted from roots have important roles in the heavy metal tolerance and detoxification (Ma et al. 2001), we hypothesize that if Si-alleviated metal toxicity is related to root secretion and improvement of cell structure stability. In this study, the effects of silicon addition on morphological traits, cell structure, and exudates of rice roots under Cd and/or Zn complex stress were studied, which will help further reveal the role of Si in mitigating heavy metal stress.
Materials and methods
Plant materials and grow conditions
Two rice varieties, Feng-Hua-Zhan and Hua-Hang-Si-Miao were used in this study. The rice seeds were sterilized using 10 % H2O2 for 10 min, and then soaked in distilled water for 48 h until they were germinated at 30 °C. After 2 days of seed germination and growth, fifteen rice seedlings were sown in a plastic pot with half concentrated Hoagland solution (pH 5.5–5.7), and each treatment had five pots. After 1 week, the solution was replaced by a full-strength solution and the solutions were renewed every day. The experiment was performed in an artificial climate chamber with the following conditions: 13 h light/11 h darkness, 25 °C/22 °C for day/night temperature, 80 %/60 % for day/night relative humidity, and 200 μmol m−2 s−1 for photon flux density.
Experimental design
Si, Cd and Zn treatments were conducted 3 weeks after rice sowing. Seven treatments with five replications were prepared for both rice varieties: (1) CK (no silicon added and no Cd/Zn stress); (2) Cd; (3) Zn; (4) Cd + Zn; (5) Cd + Si; (6) Zn + Si; and (7) Cd + Zn + Si. Si (1.5 mM) was added as potassium silicate (K2SiO3) solution, and Cd (50 μM) or Zn (200 μM) was added as CdCl2 or ZnSO4 to the solution when the 20-day-old seedlings were transplanted. In the non-Si treatment, KCl was used to replenish K. Randomized complete block design were used in this experiment for each variety. Roots and root systems were collected 7 days after the treatments to determine the root morphological traits, root activity, and root exudates. Root cells were also observed using transmission electron microscopy (TEM).
Measurement of root morphological traits
The treated root samples were collected, washed, and then scanned using WinRhizo Arabidopsis V2009c (Regent Instruments Inc., Chemin Sainte-Foy, Canada) to calculate length, volume, diameter and surface area.
Determination of root activity
Rice root activity rate were measured by assaying the oxidation of alpha naphthylamine (α-NA) (Zhang et al. 1994). In brief, 1.0 g of fresh root sample was placed into a 50 mL flask containing 25 mL of 50 µg mL−1 α-NA and incubated for 2 h. About 2 mL of the filtered aliquot was mixed with the addition of 1 mL NaNO3 (100 µg mL−1) and 1 mL 1 % sulphanilic acid. The oxidation activity in mixed solution was determined with a spectrophotometer at 485 nm.
Microscopy observation of root structure
Fresh root samples were collected after the treatments mentioned above. The samples were washed three times and the root tips were cut into 0.3–0.5 cm pieces. The pieces were fixed with 25 % glutaraldehyde at 4 °C for 2–4 h and soaked in a phosphate buffer (0.1 M, pH 7.2) for 10 min. The resulting samples were post-fixed in 1 % OsO4 for 30 min and washed with phosphate buffer. Subsequently, the samples were dehydrated with 50, 70, 80, 90, and 100 % acetone and soaked in isoamyl acetate for 5 min twice. After soaking in the solution, the samples were embedded in Epon 812 and cut using a microtome (Leica, Germany). Uranyl acetate and lead citrate were used to stain the samples and then examined using a Tecnai 12 TEMat an accelerating voltage of 100 kV (FEI, Netherlands).
Root exudates analysis
Collection of root exudates was conducted following the method of Shen et al. (2004) and Huang et al. (2016) with some modifications. Seedling samples were transferred to 500 mL of 0.5 mM CaCl2 solution, keeping the roots in the dark with black plastic bags. The seedlings were incubated in 500 mL beakers and illuminated at a photon flux density with 200 μmol m−2 s−1 at 25 ± 1 °C for 5 h. Root exudates were collected and then filtered using 0.22 μm pore size nylon filters to remove root residues. The collected root exudate samples were purified with 5 g of cation exchange resin [001 × 7(732), ZhanYun Chemistry Co. Limited, Shanghai, China)] and 5 g anion exchange resin [201 × 7(717), ZhanYun Chemistry Co. Limited, Shanghai, China]. The anion exchange resin was eluted using 10 mL of 1 M HCl to collect the organic acid. The eluant was concentrated to dryness at 40 °C, and the residue was dissolved in 5 mL of deionized water. The samples were filtered using nylon filters with 0.45 μm pore size. After filtration, the exudates were stored at −20 °C for further analyses.
Soluble sugar and total amino acid contents of root exudates were determined using anthrone colorimetry and ninhydrin reactions method (Yan et al. 2007), respectively. To determine soluble sugar and total amino acid concentration, 100 μL of concentrated root exudates were sampled. Taking glucose as a standard sample, 9,10-dihydro-9-oxoanthracene was used to determine soluble sugar content at 620 nm. After reaction with 1,2,3-indanetrione monohydrate, total amino acid were detected at 580 nm with leucine as standard.
Organic acid content was measured using the method described by Wang and Zhou (2006). Five kinds of organic acids (oxalic, tartaric, maleic, fumaric and acetic acids) were identified using HPLC (Agilent 1100, USA). The working conditions were: chromatographic column: Bio-Rad Aminex HPX-87H, mobile phase: 5 mM H2SO4, temperature of column: 50 °C, velocity of flow: 0.5 mL min−1, detector wavelength: 210 nm, and injection volume: 10 μL.
Statistical analysis
The data from root morphology and activity, the contents of root exudates were expressed as the mean ± SE. One-way ANOVA was performed to test the significance of the observed differences using SPSS 16.0. Differences between the parameters were evaluated using the Duncan’s method, and P ≤ 0.05 was considered to be statistically significant.
Results
Influence of heavy metals and/or Si on root morphology
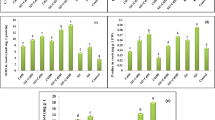
Cd and/or Zn stress significantly inhibited root growth, while this situation was improved by Si addition (Fig. 1). The size of rice roots with Cd and/or Zn stress was small, but they remained large in the Si-treated plants. As shown in Table 1, Cd and/or Zn treatments resulted in a significant reduction of length, diameter, volume and surface area of roots for both varieties; however, silicon supply alleviated these negative effects. For Feng-Hua-Zhan, root volume and average root diameter of Si-treated plants were significantly increased compared with heavy metal treatments. Similarly, for Hua-Hang-Si-Miao, Si addition increased total root length. Moreover, Cd and/or Zn stress also reduced the root activity of both rice varieties. Si supply significantly increased the root activity of Feng-Hua-Zhan, but had no effect on root activity of Hua-Hang-Si-Miao.
Influence of heavy metals and/or Si on root ultrastructure
Under non-stressed conditions, root cortex of both cultivars has fine cell structure with well-shaped cytolemma and vacuoles (Fig. 2). In Cd and Cd + Zn treated plants, the whole cortex cells were deformed with swollen and disordered vacuoles, which led to cytoplasm leakage. In Zn treatment, cell arrangement was disordered both for Feng-Hua-Zhan and Hua-Hang-Si-Miao.
By contrast, for Feng-Hua-Zhan, Cd + Si and Cd + Zn + Si treated plants maintained good cell shapes, although some of them were misshaped. There was no obvious difference between Zn + Si and Zn stress. Compared with Cd treatment, cytolemma and vacuoles were closely arranged in Cd + Si treatment (Fig. 2). The cortical cells were breached upon addition of Cd and/or Zn, while Si treatment improved the structure of cytolemma and vacuole, maintaining the cell integrity.
Influence of Si application on rice root exudates under Cd and/or Zn stress
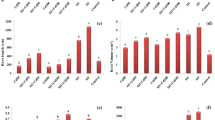
In comparison with control (CK), the contents of soluble sugar and amino acids in root exudates were significantly increased after Cd and/or Zn treatment, but reduced by Si application and they were close to the content level of control (CK) (Fig. 3). However, compared with individual Cd or Zn stress, combined Cd and Zn stress (Cd + Zn) significantly decreased soluble sugar and amino acids levels of root exudates.
Effects of 1.5 mM Si treatment on soluble sugar (a) and amino acid (b) concentrations in root exudates under Cd and/or Zn stress. Data are expressed as mean ± SE (n = 4). Different letters on bars show significant differences at 0.05 level of probability according to Duncan’s multiple comparison test among treatments of Cd, Zn, and Si
Effect of Si on the organic acid contents of root exudates under heavy metal stress is presented in Fig. 4. Concentration of oxalic acid was significantly reduced under single and combined Cd and Zn stress, but increased in Si-treated groups. For Feng-Hua-Zhan, the contents of oxalic and tartaric acid were the highest, and fumaric acid was the lowest. Meanwhile, the concentrations of oxalic, fumaric and acetic acids were significantly reduced after Cd and/or Zn stress, but increased by Si addition. For Hua-Hang-Si-Miao, oxalic acid and fumaric acid levels of root exudates decreased under heavy metal stress, but increased with the addition of Si. However, the concentrations of acetic, tartaric and maleic acid were opposite.
Discussion
Effects of Si application on root morphology of rice under heavy metal stress
Our results showed that heavy metals (Cd and Zn) negatively influenced the root morphology of both rice varieties, but the addition of Si-alleviated metal toxicity and then improved root traits (Fig. 1; Table 1). This observation was similar to the previous study by Huang et al. (2015). Si induces crops secreting secondary metabolites, which can alleviate heavy metals toxicities and then improve the growth of roots. Keller et al. (2015) reported that Si application could alleviate Cu toxicity and increased root length, photosynthetic pigment, macro elements, organic acids and amino acids concentrations. Nwugo and Huerta (2008a, b) found that Si treatment enhanced maize resistance against Cd leading to improvement of shoot and root traits. In this study, the root traits and morphology of both varieties were improved by Si treatment in all cases, revealing the important role of Si in mitigating metal stress in rice roots.
Effects of Si application on root ultrastructure of rice under heavy metal stress
Cd has been found to negatively affect cell ultrastructure in chloroplasts of bundle sheath cells (Dalla Vecchia et al. 2005). Daud et al. (2013) found that high Cd concentration (100 μM) altered cell membranous structures, including nucleus, vacuoles, and mitochondria, and decreased the nuclear size. Moreover, the number of lipid bodies increased, but nucleoli and chloroplasts were misshaped in Cd-treated cells (Daud et al. 2015). Samardjieva et al. (2015) reported that Zn stress damaged normal cell structures, such as nucleus, cytolemma, and vacuoles, and they demonstrated that the presence of Zn in the vacuoles of cortical parenchyma, starch sheath, and tonoplast of mesophyll cells, negatively influences the ultrastructure of plants. In our study, TEM observation showed that Cd or Zn stress resulted in serious destruction of rice root cortex, whereas Si treatment improved integrity of cell structure (Fig. 2). These observations were similar to those of Vaculik et al. (2015), who found that Si could improve thylakoid formation in the chloroplasts of bundle sheath cells in Cd-treated maize plants.
Our results showed that Si has significant positive effects in improving root morphological traits ultrastructure properties under Cd and/or Zn stress (Fig. 2). These improvements could be attributed to the precipitation of Si and heavy metals, which have been reported by other studies (Neumann and Zur Nieden 2001; Da Cunha and do Nascimento 2009; Van Bockhaven et al. 2013). Among these studies, Da Cunha and do Nascimento (2009) reported that silica is found in many parts of root cell wall, where Si can co-precipitate with Cd and Zn. Neumann and Zur Nieden (2001) demonstrated that Si and Zn can form as zinc-silicate, which may play role in ameliorating Zn toxicity in Cardaminopsis. In this study, the cytolemma and vacuoles were damaged after heavy metal treatment, but relieved by Si treatment. This observation suggested that Si can strengthen the cell wall to prevent the rupture of cytolemma and vacuoles under heavy metal stress.
Effects of Si application on root exudates of rice under heavy metal stress
In all cases, the concentrations of soluble sugar and amino acid in root exudates were increased under Cd and/or Zn treatment, but decreased by Si addition (Fig. 3), which verified our hypothesis that Si-mediated metal tolerance is related to root secretion. Similar results were obtained by Sharma (2006), who reported that amino acids, such as histidine and amino acid-derived molecules like phytochelatins and glutathione, affected metal binding and increased the adaptability of plants to heavy metal stress.
Root exudates, including organic acids, positively affect nutrient absorption, microorganism distribution, and stress adaptation (de Weert et al. 2002; Rudrappa et al. 2008; Ding et al. 2014). In this work, five organic acids (i.e., oxalic, acetic, tartaric, maleic, and fumaric acids) were identified in root exudates, some of which differed in both varieties (Fig. 4). Under heavy metal stress, acetic, tartaric, and maleic acid levels decreased in Feng-Hua-Zhan, but increased in the Hua-Hang-Si-Miao. These observations showed that organic acids played different roles in different rice varieties when responding to heavy metal stress. Similar results were reported, and the increase in some organic acids, such as citric, lactic, and acetic acid, was found to enhance the resistance of Cd (Xie et al. 2013; Ehsan et al. 2014). In addition, Johansson et al. (2008) found that oxalate and total organic acids in exudates increased upon Cd and Pb treatment, resulting in increased plant resistance to Cd. Sun et al. (2013) demonstrated that citric acid can reduce the negative effects caused by Cd by increasing plant biomass and photosynthesis, as well as reducing oxidative stress. Ehsan et al. (2014) reported that the root exudates of Cd-tolerant plants have higher concentration of citric acid concentrations than those of Cd-sensitive plants under Cd treatment, suggesting that citric acid is crucial in alleviating Cd toxicity.
In particular, oxalic and fumaric acids of root exudates decreased under Cd and/or Zn stress, but increased with Si treatment for both varieties (Fig. 4), suggesting that Si may stimulate the production of some specific organic acids, which may chelate metals, and therefore reduce their uptake. Our previous finding showed that Si addition significantly reduce Cd and Zn concentration in shoots and roots for both Feng-Hua-Zhan and Hua-Hang-Si-Miao varieties (Wen et al. 2011). Similarly, Kidd et al. (2001) found that Si application increase the release of flavonoid-type phenolics in Ai-stressed maize roots, indicating that phenolics may play a role in Si-mediated mitigation of Al toxicity.
Based on the above discussion, Si may induce resistance of rice against heavy metals (Cd and Zn) by secreting organic acids, which alter the uptake or distribution of heavy metals in rice. Moreover, the secretion might chelate heavy metal in soil; thereby reduce the transportation of heavy metals from contaminated soil to rice plants. Furthermore, Si could also strengthen cell wall integrity, and therefore reduce the accumulation metal levels in the cytosol.
Conclusions
This study showed single and combined Cd and Zn stress negatively influenced rice root morphological traits, cell structure. However, Si supply improved root morphological traits and maintained root cells integrity under Cd and/or Zn stress condition. Moreover, oxalic acid concentration in root exudates was significantly increased in Si-supplied treatments for both rice cultivars, which suggested that oxalic acid secretion plays a role in Si-mediated alleviation of heavy metal stress.
Author contribution statement
Xueying Fan analyzed the data and wrote the draft. Xiaohui Wen conducted the experiments. Fei Huang and Yixia Cai analyzed the data and revised the manuscript. Kunzheng Cai planned the experiments and revised the manuscript.
References
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Safe 119:186–197
Da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323–330
Dalla Vecchia F, La Rocca N, Moro I, De Faveri S, Andreoli C, Rascio N (2005) Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci 168:329–338
Daud MK, Ali S, Variath MT, Zhu SJ (2013) Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere 93:2593–2602
Daud MK, Quiling H, Lei M, Ali B, Zhu SJ (2015) Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere 120:309–320
de Weert S, Vermeiren H, Mulders IH, Kuiper I, Hendrickx N, Bloemberg GV, Vanderleyden J, De Mot R, Lugtenberg BJ (2002) Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant Microbe Interact 15:1173–1180
Ding YZ, Song ZG, Feng RW, Guo JK (2014) Interaction of organic acids and pH on multi-heavy metal extraction from alkaline and acid mine soils. Int J Environ Sci Te 11:33–42
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Safe 106:164–172
Gu H, Zhan S, Wang S, Tang Y, Chaney RL, Fang X, Cai X, Qiu R (2012) Silicon-mediated amelioration of zinc toxicity in rice (Oryza sativa L.) seedlings. Plant Soil 350:193–204
Huang B, Xin J, Dai H, Liu A, Zhou W, Yi Y, Liao K (2015) Root morphological responses of three hot pepper cultivars to Cd exposure and their correlations with Cd accumulation. Environ Sci Pollut R 22:1151–1159
Huang G, Guo G, Yao S, Zhang N, Hu H (2016) Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress. Int J Phytoremediation 18:33–40
Johansson EM, Fransson PMA, Finlay RD, van Hees PAW (2008) Quantitative analysis of root and ectomycorrhizal exudates as a response to Pb, Cd and as stress. Plant Soil 313:39–54
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 241:847–860
Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Lu H, Zhuang P, Li Z, Tai Y, Zou B, Li Y, McBride MB (2014) Contrasting effects of silicates on cadmium uptake by three dicotyledonous crops grown in contaminated soil. Environ Sci Pollut R 21:9921–9930
Lukačová Z, Švubová R, Kohanová Lux AJ (2013) Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul 70:89–103
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18
Ma JF, Ryan PR, Delhaize E (2001) Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, New York
Ming H, Naidu R, Sarkar B, Lamb DT, Liu Y, Megharaj M, Sparks D (2016) Competitive sorption of cadmium and zinc in contrasting soils. Geoderma 268:60–68
Neumann D, Zur Nieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685–692
Nwugo CC, Huerta AJ (2008a) Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 311:73–86
Nwugo CC, Huerta AJ (2008b) Silicon-induced cadmium resistance in rice (Oryza sativa). J Plant Nutr Soil Sci 171:841–848
Patrícia Vieira Da Cunha K, Williams Araújo Do Nascimento C, José Da Silva A (2008) Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on a contaminated soil. J Plant Nutr Soil Sci 171:849–853
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut R 23:1414–1427
Rudrappa T, Czymmek KJ, Pare PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547–1556
Samardjieva KA, Tavares F, Pissarra J (2015) Histological and ultrastructural evidence for zinc sequestration in Solanum nigrum L. Protoplasma 252:345–357
Sasaki A, Yamaji N, Ma JF (2014) Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot 65:6013–6021
Satoh-Nagasawa N, Mori M, Nakazawa N, Kawamoto T, Nagato Y, Sakurai K, Takahashi H, Watanabe A, Akagi H (2012) Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol 53:213–224
Schutzendubel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol 127:887–898
Sharma SS (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Shen H, Yan X, Cai K, Matsumoto H (2004) Differential Al resistance and citrate secretion in the tap and basal roots of common bean seedlings. Physiol Plant 121:595–603
Shen X, Xiao X, Dong Z, Chen Y (2014) Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol Plant 36:3063–3069
Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y (2009) Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater 172:74–83
Sun J, Cui J, Luo C, Gao L, Chen Y, Shen Z (2013) Contribution of cell walls, nonprotein thiols, and organic acids to cadmium resistance in two cabbage varieties. Arch Environ Con Toxicol 64:243–252
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanshi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ 35:1948–1957
Takahashi R, Ishimaru Y, ShimoH Bashir K, Senoura T, Sugimoto K, Ono K, Suzui N, Kawachi N, Ishii S, Yin Y, Fujimaki S, Yano M, Nishizawa NK, Nakanishi H (2014) From laboratory to field: OsNRAMP5-knockdown rice is a promising candidate for cd phytoremediation in paddy fields. PLoS One 9:e98816
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012) Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol Plant 34:279–289
Vaculik M, Landberg T, Greger M, Luxova M, Stolarikova M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot 110:433–443
Vaculik M, Pavlovic A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol Environ Safe 120:66–73
Van Bockhaven J, De Vleesschauwer D, Hofte M (2013) Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot 64:1281–1293
Wang P, Zhou R (2006) Determination of organic acids exuded from plant roots by high performance liquid chromatography. Chin J Chromatogr 24:239–242
Wen XH, Cai KZ, Ge SB, Chen GW, Liu Y (2011) Effects of silicon on plant growth and heavy metal absorption in rice seedlings under Cd and Zn stress. Acta Agricult Boreali Sin 26:153–158
Xie X, Weiss DJ, Weng B, Liu J, Lu H, Yan C (2013) The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata (S., L.) Yong) roots. Environ Sci Pollut R 20:997–1008
Yan W, Shi W, Li B, Zhang M (2007) Overexpression of a foreign Bt gene in cotton affects the low-molecular-weight components in root exudates. Pedosphere 17:324–330
Zhang X, Tan G, Huang Y (1994) Experimental technology of plant physiology. Liaoning Science and Technology Press, Shenyang
Zhang Q, Yan C, Liu J, Lu H, Wang W, Du J, Duan H (2013) Silicon alleviates cadmium toxicity in Avicennia marina (Forsk.) Vierh. Seedlings in relation to root anatomy and radial oxygen loss. Mar Pollut Bull 76:187–193
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31370456, 41501338), and Science and Technology Program of Guangdong Province (2013B020310010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J Zwiazek.
Rights and permissions
About this article
Cite this article
Fan, X., Wen, X., Huang, F. et al. Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol Plant 38, 197 (2016). https://doi.org/10.1007/s11738-016-2221-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2221-8