Abstract
Assessment of groundwater chemistry of a region is important to determine its usability. The objective of this study is to understand the hydrochemistry and groundwater quality especially with respect to fluoride in the Perambalur District, Tamil Nadu, India where groundwater is used without treatment for drinking and agricultural purposes. Groundwater samples were collected from 44 locations once every three months between September 2015 and July 2018. Mixed Ca–Mg–Cl, Ca–HCO3, Na–Cl and mixed Ca–Na–HCO3 types were the predominant hydrochemical facies. Fissile hornblende biotite gneissic rocks had more fluoride concentration than the charnockite rocks in the study area. These weathered rocks leach high concentration of fluoride to groundwater. Ion exchange processes played a major role in fluoride dynamics in groundwater. Fluoride concentration was comparatively high in the wells where the groundwater level fluctuation is at deeper levels. Groundwater in most of the wells were suitable for drinking purpose based on the ions analysed, but few wells were unsuitable for drinking and irrigation purposes. Based on health risk assessment, children are likely to be more affected than infants and adults through intake of high fluoride groundwater from the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater quality varies due to rainfall, geology, hydrogeochemical processes, rock-sediment–water interaction, influence of anthropogenic sources, and from the impact of climate change. Understanding the variation in the concentration of dissolved constitutents in groundwater provides information on the origin, flow path, recharge mechanisms, geochemical reactions and also it helps to determine its suitability for specific purposes. Over-exploitation of groundwater can lead to negative impact on the groundwater quality, especially in the shallow aquifers (Khan et al. 2016; Pophare et al. 2014; Salameh 2008). Managing the groundwater resources from potential pollutants and contaminants is a difficult task and it requires long-term systematic monitoring of the water levels and quality as well as studying the stages of groundwater development in relation to the rate of replenishment. Though contamination of groundwater from human activities can be curtailed to a larger extent, geogenic contamination poses severe peril to freshwater demand and supply worldwide (Eawag 2015).
India, the world’s largest groundwater user is facing crisis due to uncontrolled exploitation of the resource established by drying of wells and rapid lowering of water levels. Groundwater use is largely in the agricultural sector (89%), followed by the domestic (9%) and industrial sectors (2%) (World Bank 2010). About 50% of urban and 85% rural water requirements are met by groundwater (CGWB 2011). Common anthropogenic pollutants causing problems in Indian groundwaters are salinity (Manivannan and Elango 2019; Gopal et al. 2020; Biplab et al. 2021), nitrate (Karunanithi et al. 2020; Abdur et al. 2021), chromium (Kanagaraj and Elango 2019; Christina et al. 2021), lead, cadmium and other trace metals (Sharma et al. 2019; Monika et al. 2021). Key geogenic pollutants are arsenic (Sridharan and Nathan 2018; Sumant et al. 2021), fluoride (Jagadeshan et al. 2015a; Raju 2017; Chetan and Surindra 2019), iron and salinity (Kumar et al. 2019; Paul et al. 2019; Gopal et al. 2020; Dinesh Kumar et al. 2022). Among these natural contaminants, arsenic and fluoride are global threat to human health (Eawag 2015). Arsenic is more common contaminant in groundwater of the north eastern part of India and in the alluvial plains of the Ganges and Brahmaputra river basins (Chakraborti et al. 2017a, b; Jain et al. 2018). Fluoride contamination is more prominent throughout the nation (Brindha et al. 2011, 2016; Kalpana et al. 2018; Kanagaraj and Elango 2019; Ajaykumar et al. 2020).
Since adequate drinking water is not supplied through pipes to home in rural parts of India, the large rural population use groundwater without treatment. Hence, the periodical assessment of groundwater quality and hydrogeochemistry of major ions is necessary. One such region is located in the western part of Perambalur district, Tamil Nadu, India. Based on the groundwater availability and the gross groundwater pumped for various uses this region is categorised as over-exploited (CGWB 2017). Of the 11 administrative revenue blocks in Perambalur district, 6 are over-exploited, 3 are semi-critical and 2 are safe based on the current groundwater extraction practises (TWAD 2018). Thus, it is well-established that groundwater forms the major source of water for the rural population of this area for domestic use including drinking and agricultural needs.
Compared to the available literature on the hydrogeochemistry of other parts of Tamil Nadu, this region has not been throughly investigated till now. Previously, only the general groundwater quality assessment (Kasthuri et al. 2007) and the suitability for agricultural use (Ahamed et al. 2013) has been carried out. The excessive groundwater abstraction together with decifit in rainfall, reduced recharge and subsequent decline in groundwater level led to the identification and demarcation of the groundwater potential zones in the area for future groundwater development activities (Anbarasu et al. 2019). Uranium in the groundwater was used the health risk assessment to understand the presence of uranium and distribution based on rock types (RamyaPriya et al. 2021). These studies however did not draw out an understanding of the hydrogeochemistry, and the presence of fluoride and its dynamics in groundwater. Considering that groundwater is used for drinking and irrgation uses, it is necessary to assess the possible impact of groundwater use on the human health and plant growth. Hence, the present study aims at quantifying the fluoride abundance in groundwater, understanding the sources, factors controlling its occurrence, release meachanisms and its movement in groundwater in the hard rock aquifers of upper part of the Chinnar river basin which forms a part of the Perambalur district, Tamil Nadu, India. Health risk assessment is also carried out to understand the potential health hazards due to ingestion of groundwater on the rural population. This study also assesses the general hydrogeochemistry and the suitablity of groundwater for drinking and agricultural uses based on the major ions.
Methodology
Field work
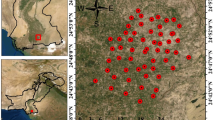
Initially, a detailed hydrogeological field investigation was carried out and information on the geological outcrop, depth of the wells, its dimensions, purpose of use etc. were noted. The electrical conductivity (EC) and groundwater level in the wells were also measured in the field. Based on these information, forty-four representative wells (Fig. 1) which were spread uniformly throughout the study area were selected for long-term monitoring. Totally 436 samples were collected from the 44 open wells (diameter from 4 to 10 m) once every three months between September 2015 and July 2018. Water level indicator (Solinist101) was used to measure the water levels. Temperature, pH and EC were measured in situ using a portable meter (Oakton Pcstestr 35). Sampling bottles were cleaned with dilute HCl and then washed thoroughly with distilled water before sample collection. Groundwater samples were filtered using millipore filter paper (0.45 µm) and filled in the sample collection bottles without air bubbles, properly labelled, stored in a cooler and brought to the laboratory for further analysis.
Laboratory methods
Carbonate and bicarbonate concentrations were measured immediately after sample collection by volumetric titration against diluted H2SO4 using the Aquamerck test kit (1.11009.0001). Calcium and magnesium were measured by volumetric titration. Sodium, potassium and nitrate concentrations were determined by a flame photometer. Sulphate concentration was measured by UV–VIS spectrophotometer (Systronics UV–VIS 118). Chloride concentration was measured by using titrando 905. Fluoride in groundwater was measured by a fluorimeter (Extech F700) which can measure from 0.1 to 10 mg/l. All analytical methods were followed as per standard procedures (APHA 2012). Verification of efficiency of the analysis was done by testing blanks and standards. Ion balance error was calculated and was within the ± 5%.
Exposure dose of fluoride
Fluoride intake water was calculated by using the given formula (USEPA 2011).
where, C is the measured fluoride concentration (mg/l), WI is the daily water intake (l/day) and BW is the body weight (kg). The water intake and body weight varies based on the different age groups. The water amount adopted for infants was 250 ml/day, for children was 1.5 l/day and for adults was 3 l/day (Planning Commission 2011). Infants, children and adult’s body weight were considered as 6.9, 18.7 and 57.5 kg, respectively (ICMR 2009).
Description of the study area
The study area is located in the hard rock terrain of Perambalur district, Tamil Nadu, India (Fig. 1a) and it covers about 220 km2. This region extends between the latitudes of 11° 8ʹ 16ʹʹ N and 11° 21ʹ 14ʹʹ N and longitudes of 78° 38ʹ 45ʹʹ E and 78° 57ʹ 35ʹʹ E. This region experiences semi-arid climate with temperature ranging from 20 to 42 °C. The average annual rainfall is about 900 mm with rainfall in the northeast monsoon, southwest monsoon and summer accounting for 60%, 25% and 15% of the rainfall respectively. The region is surrounded by the mountains of Eastern Ghats (Pachamalai) in the north, west and south, except for the eastern part. This area is characterized by discontinuous small with gentle uplands and the elevation ranges from 400 to 900 m msl. Subdendritic with trellis is the drainage pattern in this area (Fig. 1a). Agriculture is the primary activity in this area.
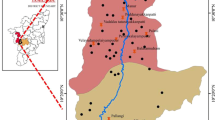
Geology and hydrology
The geology of the study area was demarcated based on the geological map obtained from the Geological Survey of India (GSI 1995) (Fig. 1b). This region comprises of fissile hornblende biotite gneisses and charnockite rocks. The gneissic and chanockite rocks are fractured over a thickness ranging mostly between 20 and 31 m and 18 and 25 m, respectively. The sand and silt occur in small quantity along the river course. Groundwater occurs in unconfined conditions in the weathered and fractured rocks. The aquifer thickness ranges between 15 and 35 m. Specific yield is between 80 and 210 lpm (litre per minute) and top soil thickness is from 0 to 6 m. Long-term fluctuation in groundwater table during the period from 1998 to 2007 indicates that there is no major change in its annual trend (CGWB, 2009).
Results and discussion
Statistical summary of the parameters analysed in groundwater are given in Table 1. The suitability of the groundwater for drinking purpose based on its properties are presented in Table 2.
Drinking water quality
Groundwater in this area is used for drinking and domestic purposes. Hence, the various hydrochemical parameters measured were compared with the prescribed values proposed for drinking water quality by the Bureau of Indian Standards (BIS 2012) and World Health Organisation (WHO 2011) (Table 2). Evaluation of water for drinking and domestic purposes depends on the parameters mentioned in the following sections.
Total dissolved salts and total hardness
The total dissolved salts (TDS) is the total amount of salts dissolved in water and comprises of major, minor ions and trace elements. TDS was calculated from the EC using the formula,
TDS was ranging between 148.5 and 2508 mg/l with an average of 959.3 mg/l (Table 1). Groundwater in this area was fresh based on the classification proposed by Freeze and Cherry (1979). BIS (2012) has proposed 500 mg/l and 2000 mg/l of TDS as the acceptable and permissible limit in drinking water. Groundwater in this area exceeds the maximum permissible limits for drinking water quality in 4% of the groundwater samples (Table 2). TDS decreases after the monsoon period due to dilution effect.
Calcium, magnesium and carbonate ions are the essential components determining the total hardness (TH) of groundwater. The TH in water was calculated from the given formula,
All values are expressed in mg/l. TH ranges from 30 to 789.1 mg/l with an average of 291 mg/l (Table 1). Groundwater of this area can be classified as moderately hard, hard and very hard water for drinking uses (Sawyer and McCarty 1978) (Fig. 2). About 16% wells were moderately hard, 34% wells were hard and 50% wells were very hard. About 71% of the samples exceed the acceptable limit and 2% of the samples exceed the permisbble limit (Table 2).
Cations and anions
In the study area, 52% of the samples exceeded the accepted limit for calcium (Table 2). The acceptable limit of magnesium is 30 mg/l in drinking water (BIS 2012) and its 13% of the groundwater samples exceeded the magnesium concentration in groundwater. Concentration of sodium exceeded the permissible limit (WHO 2011) in 12% of the samples and potassium exceeded the permissible limit (WHO 2011) in 2% of the groundwater samples. Carbonate content was low in groundwater. There were prescribed limits for carbonate and bicarbonate in drinking water. Chloride and sulphate also did not pose any threat to groundwater quality. About 24% of the groundwater samples exceeded the acceptable limit for chloride, but still were within the permissible limits. Sulphate in groundwater exceeded the acceptable limit in 2% samples (Table 2). However, it was within the permissible limit.
Minor ions
Nitrate concentration ranged up to 37 mg/l with an average of 10.8 mg/l in groundwater. There was no threat to groundwater quality and for drinking use based on nitrate (Tables 1, 2). Concentration of fluoride was ranging from 0.1 to 2.7 mg/l with average of 0.6 mg/l in the study area (Table 1). Table 3 shows that the fluoride concentration varied seasonally in the sampling locations.
The suitability of groundwater for drinking purpose in terms of TDS, TH, cations and anions was assessed as a combination of all these parameters. If all the parameters were within the permissible limits during three times of the four sampling times in a year, then the groundwater is considered as suitable for drinking. Based on the integration of all the parameters considered for drinking water quality analysis, 84% of the study area is suitable (Fig. 3).
Irrigation water quality
Groundwater in this area is widely used for irrigation purpose. Quality of groundwater for irrigation purpose was determined by various paramters as listed in following sections (Table 4).
Salinity and alkalinity hazard
High concentration of dissolved salts in groundwater can hinder soil permeability and may decrease the crop yield. The United States Salinity Laboratory (USSL) diagram (Richards 1954) classifies groundwater suitability for irrigation as two types: salinity hazard and sodium hazard. The salinity hazard is determined based on the EC and the sodium hazard is determined by using SAR equation. SAR determines the relative amount of sodium with respect of calcium and magnesium and is given as
where the values are expressed in meq/l. Salinity hazard is further classified into four classes: C1, C2, C3, C4 and sodium hazard i.e. SAR is classified into S1, S2, S3, S4. SAR is plotted against EC in the USSL diagram. EC ranged up to 3920 µS/cm and classification based on EC show 16% of the samples were good, 70% were suitable and 14% were doubtful in the study area (Fig. 4a, Table 4). SAR values were between 0.3 and 31 meq/l with average of 3.5 meq/l (Table 1). Based on SAR, 93% of the locations were excellent and 7% of the locations were good for irrigation purpose (Fig. 4a).
Sodium percent
Sodium is an essential component in groundwater, which at high concentrations is not favorable for plant growth. If sodium is excess in groundwater, it will reduce the permeability of the soil and can clog the soil layer. Based the sodium content, Wilcox (1955) proposed an index known as the sodium percentage (Na%) for classifying the water quality for irrigation use. It is given as,
All concentrations given in meq/l. Na% was found to range from 7 to 89 meq/l with an average of 40.7 meq/l. Wilcox plot between EC and Na% (Fig. 4b) indicates that 54% of the groundwater samples had excellent to good quality, 36% were good to suitable and 10% of the samples has doubtful to unacceptable water quality for irrigation (Table 4). Figure 4b shows that most of the groundwater in the area is suitable for irrigation purposes.
Residual sodium carbonate
Alkalinity hazard in groundwater can be understood from the residual sodium carbonate (RSC). When sodium concentration is high in groundwater, calcium and magnesium will precipitate more in the soil and cause soil infiltration problems. RSC is calculated as (Ragunath 1987),
All concentrations given in meq/l. RSC of groundwater was between − 10.4 and 6.7 meq/l with average of − 0.8 meq/l (Table 1). Based on RSC, 86% of the groundwater samples were good, 7% were suitable and 7% were unacceptable for irrigation purpose (Table 4).
Permeability index
Calcium, sodium, magnesium, and bicarbonate ions affect the soil permeability. Hence, based on the concentration of these ions, the permeability index (PI) was proposed by Doneen (1964) to determine the quality of water for irrigation and. PI is calculated based on the formula given below
All concentrations given in meq/l. PI in groundwater was ranging from 21.2 to 124.3 meq/l with average of 66.8 meq/l. The relation between PI and total concentration of ions in groundwater is plotted in Fig. 4c. The results show that 77% wells were good, 18% wells were within permissible limit, 5% wells were unsuitable for irrigation purpose (Table 4).
Kelly’s ratio
Kelle's ratio (KR) to evaluate water quality for irrigation purposes was suggested by Kelly (1957) and is calculated as given below
All concentrations given in meq/l. When sodium concentration is higher than the concentration of calcium and mangensium, it will affect the soil sailinity and is unsuitable for irrigation. So, if the KR is < 1, it is good for irrigation purposes. When KR is beyond 1, water is unacceptable for irrigation purpose. KR was found to range from 0.1 to 7.5 meq/l with an average of 0.9 meq/l (Table 1). About 18% of the groundwater samples were unsuitable for irrigation in the study area. Therefore, the results show that most of the groundwater wells were suitable for irrigation purpose.
Magnesium hazard
When exchangeable magnesium concentration is high in groundwater, it may cause infiltration issues. Szabolcs and Darab (1964) have proposed the following magnesium hazard (MH) index for irrigation purposes
All concentrations given in meq/l. If MH is greater than 50%, it is considerd as harmful and unsuitable for irrigation purpose. MH ranges from 13.4 to 48.8 meq/l with an average of 28.6 meq/l in groundwater (Table 1). Based on MH, all sampling locations are within the maximum permissible limit. Hence, groundwater can be used for irrigation purpose with respect to MH.
The above indices determine the irrigation water quality based on each index at a particular point in time. An overall irrigation water quality map was prepared based on the EC, SAR, Na%, RSC, PI, KR and MH. If the groundwater quality based on these indices were suitable three times out of the four sampling times in a year, the groundwater is considered suitable for irrigation. The irrigation water quality map prepared using this method is shown in Fig. 5. Based on the integration of all the irrigation water quality parameters, it was found that 93% of the study area has groundwater that can be utilised for irrigational purposes.
Hydrogeochemical facies
The pH of the groundwater was ranging between 6.6 and 9.5, which shows that it is slightly acidic to alkaline. EC in groundwater ranges from 232 to 3920 µS/cm with average of 1499 µS/cm (Table 1). Major ions of cations and anions in groundwater were in the order of sodium, calcium, magnesium and potassium, and bicarbonate, chloride and sulphate, respectively. Most of the groundwater samples exhibited mixed Ca–Mg–Cl, Ca–HCO3 type and Na–Cl type (Fig. 6a). Key processes contributing to groundwater geochemistry were identified using Gibbs plot (Gibbs 1970). Figure 6b shows that rock water interaction and evaportaion are the domiant processes controlling the chemical composition of the groundwater.
Sources of fluoride
Figure 7a indicates that about 10% of wells exceeded the permissible limit of 1.5 mg/l, 18% of groundwater wells were within the permissible range of 1–1.5 mg/l which are good for drinking purpose and 72% of groundwater wells were below the permissible limit of (< 1 mg/l) in the study area. The average concentration of fluoride in the groundwater at different locations is shown in Fig. 7b. The percentage of samples exceeding fluoride concentration during this study is given in Table 3. The fluoride concentration exceeds 1.5 mg/l in many wells during October and January months i.e. post-monsoon. After monsoon, the concentration of ions increases in specific regions due to dissolution and leaching of minerals by the groundwater.
The sampling wells were differentiated based on the geology in which they were located. Groundwater samples collected from wells located in the fissile hornblende biotite gneiss had higher fluoride concentration in comparison with the wells located in the charnockite. Table 5 shows the range of fluoride concentration in the samples located in the two different geology. Fluoride concentration from wells in the fissile hornblende biotite gneissic rock ranges between 0.1 and 2.7 with average of 0.6 mg/l. The concentration of fluoride from samples located in charnockite areas range from 0.1 to 1.3 mg/l with an average of 0.35 mg/l. The key source of fluoride is mainly from the biotite, hornblende and amphibole minerals from the granite, gneissic and charnockite rocks of this area. The mobilization of these minerals by hydrogeological processes had led to high fluoride in groundwater. Jagadeshan et al. (2015b) reported maximum fluoride concentration in charnockite and epidote hornblende biotite gneiss as 68 mg/kg and 97 mg/l in a nearby region. The interaction between groundwater and these fluoride bearing minerals over longer time period lead to the leaching and dissolution of these minerals in groundwater. About 2.9 and 1.1% of fluoride is present in hornblende and biotite minerals respectively (Jagadeshan et al. 2015a). The fissile hornblende gneissic rock is comparatively more weathered than the charnockites (Anbarasu et al. 2019) and hence the concentration of fluoride is higher in the former regions. Equations governing the weathering of these fluoride bearing minerals are given below
The correlation between fluoride, EC and major ions is used to understand the source i.e. the weathering of rocks (Fig. 8). In general, fluoride shows a positive correlation with EC, sodium and bicarbonate. The relationship between the concentration of fluoride and well depth is shown in the Fig. 9a. When the groundwater level in wells was in top soil (0.1–6 m bgl) and fractured rock (18.1–22 m bgl), the concentration of fluoride in most of the wells was less than 1 mg/l. When the groundwater level in wells was in the weathered zone (6.1–18 m bgl), the concentration of fluoride was more than 1.5 mg/l in most of the wells. As the leaching of weathered gneissic rock is contributing more fluoride to groundwater, the wells in this area have high concentration of fluoride in groundwater. Comparison of the fluoride concentration with the groundwater level in different rock types is shown in Fig. 9b. This figure also confirms that groundwater in the fissile hornblende gneissic areas have high fluoride. Also, the fluoride concentration, irrespective of shallow or deep groundwater table was low in the charnockite areas. However, in fissile hornblende gneiss such differentiation could not be made as both shallow and deep water levels showed fluoride concentrations at all ranges. A clear understanding of the relationship between fluoride concentration and groundwater level could not be made from Fig. 9b as they were collectively plotted. The concentration of fluoride in groundwater varies temporally in the different rock types. It is evident that the groundwater fluctuation depth plays an important role in determining the fluoride concentration (Brindha et al. 2011, 2016; Fantong et al. 2009; Jagadeshan et al. 2015b). Hence, all the sampling wells were individually plotted to identify the relationship.
The temporal relationship between fluoride and groundwater level is classified as type I wells and type II wells. The shallow groundwater table increases during the rainy season, decreasing the fluoride concentration in groundwater due to dilution. During summer, fluoride concentration increases as the groundwater level declines due to evaporation and no natural recharge. This is the characteristic if type I wells (Fig. 10a). In type II wells, during the rainy season, infiltration of rainwater flushes the precipitated fluoride salts from the unsaturated zone and hence increase in groundwater levels also increases the fluoride concentration. With prolonged infiltration, the fluoride concentration will decrease in the deep wells too (Fig. 10b). This relationship has been reported in several regions with high fluoride concentrations (Brindha et al. 2011, 2016).
Health risk analysis
Groundwater with fluoride concentration between 1 and 1.5 mg/l as suggested by BIS (2012) is considered to be permissible for drinking use. When fluoride concentration in drinking water was deficient (< 1 mg/l), it can cause dental caries, especially in children (Raju 2017). Fluoride concentration above 1.5 mg/l in drinking water cause dental and skeletal fluorosis (Mandinic et al. 2010; Perumal et al. 2013). Fluoride exposure dose for infants ranges from 0 to 0.1 mg/kg/day, for children it ranges from 0.01 to 0.22 mg/kg/day and for adults, it ranges from 0 to 0.14 mg/kg/day. The results show that fluoride exposure dose was minimum risk for infants and adults but maximum risk for children (Fig. 11). The minimal risk level from oral ingestion based on chronic-duration studies is given as 0.05 mg fluoride/kg/day (ATSDR 2003). About 9% of the groundwater samples exceed the minimum risk level for infants, 21% of the samples exceeded for children and 13% of the samples exceed for adults. The fluoride exposure dose calculated for infants, children and adults shows that children have a high exposure dose than infants and adults.
Conclusion
The present study focused on the fluoride contamination, its sources and the associated human health risk due to the ingestion of contaminated groundwater. Mixed Ca–Mg–Cl, Ca–HCO3, Na–Cl and mixed Ca–Na–HCO3 types were the dominant groundwater facies. Groundwater is generally slightly acidic to alkaline nature. The suitability of groundwater for drinking purpose was estimated by TDS, TH, major and minor ions, and 84% of the study area wells were good for drinking purpose. Except a few of well, about 93% of the groundwater wells were good for irrigation purpose based on integrating of Na%, SAR, RSC, KR, MH and PI values. 10% of groundwater samples were exceeding the permissible limit of fluoride and 31% of groundwater samples were within the permissible limit for drinking purposes based on BIS and WHO. Fluoride concentration was released more to groundwater due to recharge of rainwater and weathering processes during the monsoon and post-monsoon. Fluoride was showing positively good relation with EC, sodium and bicarbonate ion. Weathering of minerals, dissolution, evaporation and dilution were the major hydrogeochemical processes controlling high fluoride in groundwater. High fluoride concentration occurred mostly in regions with weathered fissile hornblende biotite gneissic rocks than in charnockite rocks. The health risk assessment indicates that children are affected more than infants and adults through intake of high fluoride groundwater. Suitable management of water resources especially to overcome fluoride contamination is required in this area.
References
Abdur R, Mondal NC, Tiwari KK (2021) Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi arid area of Rajasthan, India. Nat Sci Rep 11:9279
Ahamed AJ, Ananthakrishnan S, Loganathan K, Manikandan K (2013) Assessment of groundwater quality for irrigation use in Alathur Block, Perambalur District, Tamilnadu, South India. Appl Water Sci 3:763–771. https://doi.org/10.1007/s13201-013-0124-z
Ajaykumar K, Vasant V, Bhavana U, Rabindranath S (2020) An implication of boron and fluoride contamination and its exposure risk in groundwater resources in semi arid region, Western India. Environ Dev Sustain 22:7033–7056. https://doi.org/10.1007/s10668-019-00527-w
Anbarasu S, Brindha K, Elango L (2019) Multi-influencing factor method for delineation of groundwater potential zones using remote sensing and GIS techniques in the western part of Perambalur district, southern India. Earth Sci Inf 13:317–332. https://doi.org/10.1007/s12145-019-00426-8
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. APHA-AWWA-WEF, Washington, D.C.
ATSDR (2003) Toxicological profile for fluorides. hydrogen fluoride and fluorine. Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services, p 356
Biplab S, Aznarul I, Arijit M (2021) Seawater intrusion into groundwater and its impact on irrigation and agriculture: evidence from the coastal region of West Bengal, India. Region Stud Mar Sci 44:101751. https://doi.org/10.1016/j.rsma.2021.101751
BIS (2012) Indian standard drinking water specification. Second Revision ISO: 10500:2012, Bureau of Indian Standards, Drinking Water Sectional Committee, FAD 25. New Delhi, India
Brindha K, Rajesh R, Murugan R, Elango L (2011) Fluoride contamination in groundwater in parts of Nalgonda District, Andhra Pradesh, India. Environ Monit Assess 172:481–492. https://doi.org/10.1007/s10661-010-1348-0
Brindha K, Jagadeshan G, Kalpana L, Elango L (2016) Fluoride in weathered rock aquifers of southern India: managed aquifer recharge for mitigation. Environ Sci Pollut Res Int 23:8302–8316. https://doi.org/10.1007/s11356-016-6069-7
CGWB (Central Ground Water Board) (2009) Groundwater information. Perambalur district, Tamilnadu, pp 1–17
CGWB (2011) Dynamic ground water resources of India—2009. Central Ground Water Board, Ministry of Water Resources, Government of India, Faridabad, India. p 255
CGWB (2017) Dynamic ground water resources of India—2013. Central Ground Water Board, Ministry of Water Resources, River Development & Ganga Rejuvenation, Government of India, Faridabad, India, p 280
Chakraborti D, Das B, Rahman MM, Nayak B, Pal A, Sengupta MK, Ahamed S, Hossain MA, Chowdhury UK, Biswas BK, Saha KC, Dutta RN (2017a) Arsenic in groundwater of the Kolkata Municipal Corporation (KMC), India: critical review and modes of mitigation. Chemosphere 180:437–447. https://doi.org/10.1016/j.chemosphere.2017.04.051
Chakraborti D, Rahman MM, Das B, Chatterjee A, Das D, Nayak B, Pal A, Chowdhury UK, Ahmed S, Biswas BK, Sengupta MK, Hossain MA, Samanta G, Roy MM, Dutta RN, Saha KC, Mukherjee SC, Pati S, Kar PB, Mukherjee A, Kumar M (2017b) Groundwater arsenic contamination and its health effects in India. Hydrogeol J 25:1165–1181. https://doi.org/10.1007/s10040-017-1556-6
Chetan PS, Suthar S (2019) Assessment of human health risk associated with high groundwater fluoride intake in Southern Districts of Punjab, India. Expos Health 11:267–275
Christina RV, Divya Priya B, Hazi MA (2021) Assessment of groundwater quality and human health risk associated with chromium exposure in the industrial area of Ranipet, Tamil Nadu, India. J Water Sanit Hyg Dev 12(1):58–67. https://doi.org/10.2166/washdev.2021.260
Dinesh Kumar M, Nitin B, Kumar S (2022) Managing groundwater quality for drinking water security in India: emerging challenge. Drink Water Secur Rural India. https://doi.org/10.1007/978-981-16-9198-0_7
Doneen LD (1964) Water quality for agriculture. Department of Irrigation, University of California, Davis
Eawag (2015) Geogenic contamination handbook—addresseing arsenic and fluoride in drinking water. Swiss Fedral Institute of Aquatic Science and Technology (Eawag), Dubendorf
Fantong WY, Satake H, Ayonghe SN, Suh EC et al (2009) Geochemical provenance and spatial distribution of fluoride in groundwater of Mayo Tsanaga river basin, Far North Region, Cameroon: implications for incidence of fluorosis and optimal consumption dose. Environ Geochem Health 32:147–163
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall Inc, Englewood Cliffs
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gopal K, Mamta B, Ghose NC, Gokul P (2020) Groundwater salinity in northwestern region of India: a critical appraisal. Environ Process Manage. https://doi.org/10.1007/978-3-030-38152-319
Gopal K, Ajay K, Natarajan S, Prosun B, Sumant K, Narayan C, Surjeet S, Anupma S, Rao S, Sanjay M, Balwinder S, Rajesh V, Ramnik K (2022) Occurrence of potentially toxic trace elements in groundwater of the state of Punjab in Northern India. Groundw Sustain Dev 15:100655. https://doi.org/10.1016/j.gsd.2021.100655
GSI (1995) Geology map of Perambalur district, Tamil Nadu. Geological Survey of India, Government of India
ICMR (2009) Nutrient requirements and recommended dietary allowances for Indians. A report of the expert group of the Indian Council of Medical Research (ICMR), Hyderabad, India. p 334
Jagadeshan G, Kalpana L, Elango L (2015a) Hydrogeochemistry of high fluoride groundwater in hard rock aquifer in a part of Dharmapuri district, Tamil Nadu, India. Geochem Int 53:554–564. https://doi.org/10.1134/s0016702915060038
Jagadeshan G, Kalpana L, Elango L (2015b) Major ion signatures for identification of geochemical reactions responsible for release of fluoride from geogenic sources to groundwater and associated risk in Vaniyar River basin, Dharmapuri district, Tamil Nadu, India. Environ Earth Sci 74:2439–2450. https://doi.org/10.1007/s12665-015-4250-9
Jain CK, Sharma SK, Singh S (2018) Physico-chemical characteristics and hydrogeological mechanisms in groundwater with special reference to arsenic contamination in Barpeta District, Assam (India). Environ Monit Assess 190:417. https://doi.org/10.1007/s10661-018-6781-5
Kalpana L, Brindha K, Elango L (2018) FIMAR: a new fluoride index to mitigate geogenic contamination by managed aquifer recharge. Chemosphere 220:381–390. https://doi.org/10.1016/j.chemosphere.2018.12.084
Kanagaraj G, Elango L (2019) Chromium and fluoride contamination in groundwater around leather tanning industries in southern India: implications from stable isotopic ratio δ53Cr/δ52Cr, geochemical and geostatistical modelling. Chemosphere 220:943–953. https://doi.org/10.1016/j.chemosphere.2018.12.105
Karunanithi D, Aravinthasamy P, Roy D, Praveenkumar RM, Prasanth K, Selvapraveen S, Thowbeekrahman A, Subramani T, Srinivasamoorthy K (2020) Evaluation of non-carcinogenic risks due to fluoride and nitrate contaminations in a groundwater of an urban part (Coimbatore region) of south India. Environ Monit Assess. https://doi.org/10.1007/s10661-019-8059-y
Kasthuri R, Kalaivani D, Banumathi K, Shanmugapriya R (2007) Evaluation of water quality in A. Mettur area of Perambalur district. Indian J Environ Prot 27:1011–1014
Kelly WP (1957) Adsorbed sodium cation exchange capacity and percentage sodium sorption in alkali soils. Science 84:473–477
Khan MR, Koneshloo M, Knappett PSK, Ahmed KM, Bostick BC, Mailloux BJ, Mozumder RH, Zahid A, Harvey CF, van Geen A, Michael HA (2016) Megacity pumping and preferential flow threaten groundwater quality. Nat Commun 7:12833. https://doi.org/10.1038/ncomms12833
Kumar M, Nagdev R, Tripathi R, Singh VB, Ranjan P, Soheb M, Ramanathan AL (2019) Geospatial and multivariate analysis of trace metals in tubewell water using for drinking purpose in the upper Gangetic basin, India: heavy metal pollution index. Groundw Sustain Dev 8:122–133. https://doi.org/10.1016/j.gsd.2018.10.001
Mandinic Z, Curcic M, Antonijevic B, Carevic M, Mandic J, Djukic-Cosic D, Lekic CP (2010) Fluoride in drinking water and dental fluorosis. Sci Total Environ 408:3507–3512. https://doi.org/10.1016/j.scitotenv.2010.04.029
Manivannan V, Elango L (2019) Seawater intrusion and submarine groundwater discharge along the Indian coast. Environ Sci Pollut Res 26:31592–31608. https://doi.org/10.1007/s11356-019-06103-z
Monika M, Pankaj Kumar G, Anitha S, Barkha V, Pooja S, Richa K, Rajeev Pratap S (2021) A comprehensive study on aquatic chemistry health risk and remediation techniques of cadmium in groundwater. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151784
Paul R, Brindha K, Gowrisankar G, Tan ML, Singh MK (2019) Identification of hydrogeochemical processes controlling groundwater quality in Tripura, Northeast India using evaluation indices, GIS, and multivariate statistical methods. Environ Earth Sci 78:470. https://doi.org/10.1007/s12665-019-8479-6
Perumal E, Paul V, Govindarajan V, Panneerselvam L (2013) A brief review on experimental fluorosis. Toxicol Lett 223:236–251. https://doi.org/10.1016/j.toxlet.2013.09.005
Planning Commission (2011) Report of the working group on rural domestic water and sanitation. In: Twelfth five year plan- 2012–2017, Ministry of Drinking Water and Sanitation, Government of India, p 220
Pophare AM, Lamsoge BR, Katpatal YB, Nawale VP (2014) Impact of over- exploitation on groundwater quality: a case study from WR-2 Watershed, India. J Earth Syst Sci 123:1541–1566
Ragunath HM (1987) Ground water, 2nd edn. New Age International, New Delhi
Raju NJ (2017) Prevalence of fluorosis in the fluoride enriched groundwater in semi-arid parts of eastern India: geochemistry and health implications. Quatern Int 443:265–278. https://doi.org/10.1016/j.quaint.2016.05.028
RamyaPriya R, Manoj S, Elango L, Anbarasu S, Gowrisankar G (2021) Human health risk assessment using Monte Carlo simulations for groundwater with uranium in southern India. Ecotoxicol Environ Saf 226:112781
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. USDA handbook 60
Salameh E (2008) Over-exploitation of groundwater resources and their environmental and socio-economic implications: the case of Jordan. Water Int 33:55–68. https://doi.org/10.1080/02508060801927663
Sawyer CN, McCarty PL (1978) Chemistry of environmental engineering, 3rd ed. Series in Water Resources and Environmental Engineering, McGraw–Hill
Sharma S, Nagpal AK, Kaur I (2019) Appraisal of heavy metal contents in groundwater and associated health hazards posed to human population of Ropar wetland, Punjab, India and its environs. Chemosphere 227:179–190. https://doi.org/10.1016/j.chemosphere.2019.04.009
Sridharan M, Nathan DS (2018) Chemometric tool to study the mechanism of arsenic contamination in groundwater of Puducherry region, South East coast of India. Chemosphere 208:303–315. https://doi.org/10.1016/j.chemosphere.2018.05.083
Sumant K, Suneel Kumar J, Neeraj P, Surjeet S, Biswajit C, Ravi K, Vinoth K, Ankit S, Narayan C, Abhijit M, Prasant R, Vijeta S (2021) Hydrogeochemical evolution and groundwater recharge processes in arsenic enriched area in central Gangetic plain, India. Appl Geochem 131:105044. https://doi.org/10.1016/j.apgeochem.2021.105044
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th ISSS, Trans vol II, pp 802–812
TWAD (2018) Perambalur District Profile, Tamil Nadu Water Supply and Drainage Board. https://www.twadboard.tn.gov.in/content/perambalur-district. Accessed 25 Nov 2019
USEPA (2011) Exposure factors handbook: 2011 edition. U.S. Environmental Protection Agency, National Center for Environmental Assessment, Washington, DC
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Washington, DC Circular 969
World Bank (2010) Deep wells and prudence: towards pragmatic action for addressing groundwater overexploitation in India, p 97
Acknowledgements
The authors would like to thank the University Grants Commission, New Delhi, for the financial support for this work (F 17-68/2008 (SA-1)).
Funding
The first author thanks the University Grants Commission and Council of Scientific and Industrial Research - National Eligibility Test scheme (Sr.No.2121220427, dt: 23/12/2012) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a Topical Collection in Environmental Earth Sciences on ‘‘Recent Advances in Environmental Sustainability’’, guest edited by Peiyue Li.
Rights and permissions
About this article
Cite this article
Subramaniyan, A., Karthikeyan, B. & Lakshmanan, E. Geochemical evaluation of fluoride and groundwater quality for drinking and irrigation purposes in a weathered gneissic rock aquifer of southern penisular India. Environ Earth Sci 81, 273 (2022). https://doi.org/10.1007/s12665-022-10395-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10395-9