Abstract
Groundwater resource in alluvial aquifers is significantly essential for the sustainable development of arid regions worldwide. Forty-eight phreatic groundwaters were sampled and analyzed to elucidate the hydrochemical features, genesis and constraints on domestic and irrigational water supply in a hyper-arid alluvial fan on Tibetan plateau. The human health risk assessment and entropy-weighted water quality index (EWQI) along with drinking water guidelines were introduced to assess groundwater quality for domestic purpose, and the irrigation suitability was discussed in terms of the potential soil permeability damage and salinity hazard. The results indicated groundwater in the Golmud alluvial fan plain is slightly alkaline water and dominated in the hydrochemical faces of mixed Cl–Mg–Ca type, and followed Cl–Na type and mixed HCO3–Na–Ca type. Groundwater nitrite (~ 6.25%) and ammonia (~ 2.08%) exceed the drinking water standards in the farmlands and human settlements at the alluvial fan front, but would not threaten human health. The geogenic toxic elements of fluoride and boron would potentially pose health risks to the populations of infants, children and adult males at some sporadic locations. The risks are mainly attributed to the aqueous fluoride. Groundwaters in the alluvial fan plain are almost suitable for domestic purpose according to the EWQI appraisal results (35–151), and have suitable quality for irrigation regardless of sodium adsorption ratio, percentage sodium and permeability index. But, salinity hazard is widespread and should be concerned. Groundwater chemistry is basically governed by the natural rock–water interactions and influenced by agricultural practices and urban domestic effluents at the alluvial fan front. These anthropogenic contamination inputs resulted in the excessive nitrogen and elevated salinity of groundwater. Measures should be taken to protect groundwater resources in alluvial aquifers out of anthropogenic contamination. Geogenic fluoride is also needed to pay attention in the drinking water supply.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is one of the major water sources for various usage in many regions over the world (Adimalla 2019; Singha et al. 2021; Xu et al. 2019a). It is estimated that groundwater serves as the primary water resource for approximately 50% of agricultural production, 40% of the domestic usage of global population, 40% of industrial consumption (Dash et al. 2019). This is much more significant in arid and semiarid regions due to the rare precipitation and scarcity of surface water (Gu et al. 2018; Hao et al. 2020; Xiao et al. 2021c; Zhang et al. 2021b). The availability of fresh groundwater resource is essential for the sustainable development of social economy and ecological environment in arid and semiarid regions (Xiao et al. 2018). Hydrochemistry is one of the vital properties determining the availability of groundwater resources. Comprehensively understanding the hydrochemical quality and formation mechanisms of groundwater is the premise for realizing rational management and sustainable utilization of water resource in water-scarce regions (Gao et al. 2019, 2020).
Naturally, the hydrochemcal composition of groundwater is governed/influenced by many factors during the water circulation beneath the ground surface (Sheikhy Narany et al. 2018). Recharge water is the initial factor and determines the fundamental chemical composition of groundwater. The new inputs of groundwater chemical components along the flow path are predominantly contributed by rock–water interaction in aquifers, which is greatly up to the lithology along the flow path, residence time, aquifer hydrodynamical features (Kumar and Kuriachan 2020; Venkatramanan et al. 2017). The geogenic toxic elements such as As, I, F in groundwater are commonly originated from this process (Wang et al. 2020). In addition, aquifers are not isolated systems and widely interacted with other adjacent aquifers, rivers, lakes and seas (Zhang et al. 2021a). As a result, the hydrochemistry of groundwater would be affected via direct external substances inputs or indirect redox state/acid–base state change. For instance, the rich ions would be brought into the aquifers by the interaction between groundwater and sea water, leading to the salinity of groundwater (Chowdhury et al. 2018). Evaporation is another critical factor regulating groundwater hydrochemistry in nature and is much more significant in arid and semiarid regions (Tweed et al. 2011).
Besides the natural mechanisms, anthropogenic disturbance has been becoming a more and more crucial factor affecting groundwater chemistry (Gao et al. 2021; Zhang et al. 2021c). This disturbance can be across all human activities, such as agricultural practices, industrialization, urbanization, mining and groundwater exploitation (Mal and Adhikari 2021; Yan et al. 2021; Yuan et al. 2017). External chemical substances could be directly inputted in the aquifers and deteriorate the quality of groundwater (Zhu et al. 2020). This is commonly observed in the agricultural lands and human settlements where with high loads of pollutants on ground surface. But not all these cases could find groundwater pollution (Xiao et al. 2017a). The disturbance of anthropogenic contamination to aquifers is significantly regulated by the geological/hydrogeological conditions, as a result, the anthropogenic influences on groundwater chemistry become more complex. In addition, the disturbance of human activities like groundwater over-exploitation on groundwater hydrodynamical condition could also affect the quality of groundwater through indirectly regulating hydrochemical balance or driving the movement of contaminants (Luo et al. 2021; Peng et al. 2018; Rao et al. 2017; Wang et al. 2018; Xiao et al. 2021b; Yin et al. 2020).
Alluvial fan plains are the crucial geomorphological units for human society where developed many cities around the world (Li et al. 2008). Groundwater in alluvial fan plains is significantly important water resource for various aspects of social development in arid and semiarid regions over the world due to the abundance and easy access of groundwater resources there (Blackburn et al. 2021; Hao et al. 2014; Jean-Baptiste et al. 2020; Xu et al. 2019d; Zhang et al. 2021a). Additionally, the contamination from anthropogenic practices are great threats to the quality of alluvial groundwaters due to the high pollution loads of ground surface and vulnerability of vadose zone (Yin et al. 2019; Zhang et al. 2017). Consequently, groundwater in alluvial fan plains has been undergone severe disturbances and threats from human community in both quantity and quality aspects (Gu et al. 2017b; Hussain et al. 2017; Kumar Joshi et al. 2021; Li et al. 2016). The natural mechanisms coupled with these intense anthropogenic disturbances make the groundwater chemistry complex and significantly degraded. Therefore, special attentions should be paid to the hydrochemical genesis and quality of groundwater to support the sustainable development of water scarce alluvial fans.
The present study focuses on groundwater chemistry in hyper-arid alluvial fan plains and its constraints and implications to sustainable development of water-scarce regions. The prime aims of this study are to (1) characterize the physiochemical features of groundwater; (2) reveal the groundwater quality for domestic purposes and potential health risks; (3) assess the suitability of groundwater for agricultural irrigation; and (4) get insights into the formation mechanisms governing groundwater chemistry in alluvial fan plains that undergone intense anthropogenic disturbances. This research will definitely improve the knowledge of groundwater chemistry and its constraints to water supply, and be helpful for realizing the sustainable groundwater development in arid regions over the world.
Study area
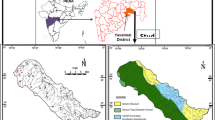
The study area, Golmud alluvial fan plain, is located in the south margin of the Qaidam Basin on Tibetan plateau (Fig. 1). This alluvial fan plain extends from 36°8′12″ N to 36°30′19″ N for latitude and between 94°33′23″ E and 95°6′25″ E for longitude, encompassing an area of 1665 km2. It belongs to a typical continental plateau climate and is featured by very low precipitation but intense evaporation. Precipitation has significant spatial difference with more than 200 mm per year in the southern mountainous area but less than 50 mm per year in the northern plain. More than 70% of precipitation occurs in the wet season spanning May to September (Huang et al. 2020). Converse to the low precipitation, the annual potential evaporation is extremely high in the plain and ranges from 2000 to 3000 mm (Yang and Wang 2020).
The study area belongs to the Golmud watershed and is formed by the second largest river, Golmud River, in Qaidam Basin. This alluvial fan plain is covered by Gobi desert in the middle–upper area and desert oasis in the lower part. Golmud city, the third largest city on Tibetan plateau, is located at the northeast of the Golmud alluvial fan. Farmlands are mainly distributed in the northwest and north of the alluvial fan. The lithology of aquifer varies from coarse-grained pebbly gravel in the south to fine sand and silt in the north with the permeability in the range of 8–60 m/day (Xiao et al. 2018). Groundwater is predominantly unconfined in the alluvial fan and becomes semi-confined and confined in the front. The depth of groundwater has a large variation in spatial and is more than 100 m in the mountain pass but 0 m (naturally overflow as descending springs) at the front of alluvial fan. The hyper-arid climate makes aquifers in the plain hard to be recharged by local precipitation. Groundwater is mainly recharged by the seepage of Golmud river in the middle–upper area of the alluvial fan, and discharged via lateral outflow, spring and evaporation. Additionally, human exploitation has become the important form of groundwater discharge in the study area. The social-economic development of this region relies heavily on groundwater resources. Almost all domestic water source is exploited from aquifers of the alluvial fan. It can be said that groundwater resource is significantly essential for the sustainable development of the whole region including the Golmud city (Huang et al. 2020).
Materials and methods
Sampling and analytical techniques
A total of 48 groundwater samples were collected from the Golmud alluvial fan plain (Fig. 1). The sampled groundwaters are obtained from the phreatic boreholes and wells. All boreholes and wells were pumped for more than 30 min to remove the stagnant water there. Sampling strategies were performed after the target water’s in situ physicochemical parameters (such as pH and electrical conductivity) becoming stable. Groundwater samples were collected using the high-density polyethylene bottles (Xu et al. 2019c) that had been thoroughly washed by the target water for more than three times. Portable incubators were used to store the sampled groundwaters and keep them in a 4 ℃ condition (Xu et al. 2019b). Samples were sent to laboratory for hydrochemical analysis soon after the sampling practice.
In situ physicochemical parameters like pH and electrical conductivity (EC) were determined at field using a portable parameter device (HANNA HI98130, Italy). Other hydrochemcial parameters were measured in the Laboratory of Groundwater Sciences and Engineering in the Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences (Shijiazhuang, China). The total dissolved solids (TDS) and HCO3− were determined by gravimetric analysis and acid–base titration, respectively. Anions (SO42−, Cl−, NO2−, NO3−, F−, I−) and Ammonia (NH4+) were measured by ion chromatography (Shimadzu LC-10ADvp, Kyoto, Japan). Cation ions including Ca2+, Mg2+, Na+, K+ and trace elements (such as Cu, Zn, Al, B) were determined with the aid of inductively coupled plasma-maa spectrometry (Agilent 7500ce ICP-MS, Tokyo, Japan). The analysis accuracy was checked by the ionic charge balance error, which can be determined by Eq. (1) (Adimalla 2021). All groundwater samples were observed with the ionic charge balance error percentage in the range between − 5% and + 5%, demonstrating reliable analysis results:
where \(ce_{i}^{ + }\) and \(ce_{i}^{ - }\) are in milliequivalents per liter and represent the charge concentration of a cation and anion, respectively.
Human health risk assessment
Toxic elements in drinking water would pose potential health risks to human beings. Human Health Risk Assessment model (HHRA model) that proposed by the United States Environmental Protection Agency (USEPA 1989) is a useful and widely applied approach to quantitatively evaluate the potential health risks of aqueous toxic elements to water consumers. Populations of different age and gender have different toleration and vulnerability to toxic elements in drinking water. In present study, four population groups, i.e. infants (0–6 months), children (7 months to 17 years), adult females and males (> 18 years), were divided to determine the potential health threats of toxic elements in supplying groundwater resource for various population. The assessment should follow the procedure below.
The chronic daily intake dose of groundwater toxic elements via oral intake can be estimated as follows (Zhang et al. 2020a):
where CDIi denotes the chronic daily intake dose of toxic element i; Ci is the concentration of toxic element i in groundwater; IR signifies the water oral intake rate; EF, ED, AT indicate the exposure frequency, duration and average exposure time, respectively. BW refers to the average body weight of various population.
The hazard quotient (HQ) is identified using the following equation:
where HQi signifies the hazard quotient, namely the health risk, of toxic element i; RfDi expresses the toxic element i’s reference dose via drinking water intake pathway.
The total probabilistic health risk (HI) is synthetically determined as follows:
Parameters involved in the assessment of this study are presented in Table 1.
Entropy-weighted water quality index
Entropy-weighted water quality index (EWQI) is an advanced approach to evaluate groundwater quality appropriateness for domestic purpose and has been widely used worldwide (Adimalla 2021; Siddique et al. 2020; Subba Rao et al. 2019; Wu et al. 2018). The following equations are used to estimate the entropy-weight and perform the EWQI appraisal.
First, constructing the eigenvalue matrix Y (Eq. (6)) involves the normalized physicochemical data, which are obtained by Eq. (7):
where m and n signify the number of sample and index, respectively; xij represents the value of physicochemical parameter j of groundwater sample i; (xij)max and (xij)min are the maximum and minimum of physicochemical parameters of groundwater samples, respectively.
The second step is computing the entropy weight of each physicochemical parameter as below:
where Pij signifies the ratio of parameter j for groundwater sample i; ej denotes the information entropy of physicochemical parameter j; wj is the entropy weight of parameter j.
Then Eq. (10) is used to compute the quality rating scale (qj) for each groundwater sample:
where Cj represents the physicochemical parameter value of each groundwater sample; Sj signifies the permissible limit of physicochemical parameter given by World Health Organization or national standard.
At last, the EWQI value is obtained with the aid of following formula:
Groundwater quality can be classified into five categories based on the above EWQI value, i.e. excellent (< 50), good (50–100), medium (100–150), poor (150–200) and extremely poor (> 200).
Groundwater quality criteria for agricultural irrigation
The quality of water for irrigation is essential for agricultural practice and determines the crops’ production in a certain degree (Luo et al. 2021; Xiao et al. 2020). Soil permeability is one of the most important factors that should be considered for sustainable development of agricultural practice. Poor quality of irrigation water can pose negative effects on soil structure, and finally lead soil to be compact and dry (Aravinthasamy et al. 2020). Additionally, the high salt content of irrigation water would also negatively affect the growth of plants by reducing its capacity of water and nutrient uptake (Marghade et al. 2021). These potential effects of irrigation water on soil can be evaluated with the aid of salinity (EC), sodium adsorption ratio (SAR), percentage sodium (%Na) and permeability index (PI). The SAR, %Na, PI can be determined as below, and all ions used in these equations are with the unit of meq/L:
Results and discussion
Physicochemical characteristics of groundwater
The physicochemical composition of groundwater collected from the Golmud alluvial fan plain is statistically summarized in Table 2. The desirable limits of each physicochemical parameter suggested by Chinese guideline (GAQS 2017) and World Health Organization guideline (WHO 2017) are also listed in Table 2 for comparison.
The measure pH of sampled groundwaters ranged from 7.42 to 8.56 with an average of 8.31, indicating slightly alkaline nature of groundwater in the study area. Most of groundwater samples had the pH value within the desirable limit range of 6.5–8.5 recommended by Chinese Guideline (GAQS 2017). Only 10.42% of sampled groundwaters were found to have the pH value slightly beyond the recommended limit and with the maximum pH of 8.56. The TDS were observed with a relatively large variation from 236 to 1560 mg/L with a mean of 685 mg/L. Approximately 87.5% of the total groundwater samples had the TDS within the desirable limit of 1000 mg/L given by Chinese Guideline (GAQS 2017), and the rest were slightly beyond this desirable limit with the maximum of 1560 mg/L. The total hardness (TH) of sampled groundwaters varied from 115 to 546 mg/L and averaged at 301 mg/L. Groundwater samples had the TH value dominantly below the desirable limit of 450 mg/L, and only 6.25% of the total samples were observed slightly exceeding this limit. As shown in the integrated water quality diagram based on TDS and TH (Fig. 2), groundwater in the Golmud alluvial fan plain were predominantly moderately hard fresh water. A small portion of sampled groundwaters situated in the soft fresh, hard fresh, moderately hard brackish, and hard brackish category.
Among the cations, Na+ was the dominant ion with the concentration ranging from 77.27 to 482.20 mg/L with an average of 139.34 mg/L. The concentration of Ca2+ and Mg2+ varied from 14.83 to 101.10 mg/L with a mean of 51.00 mg/L and between 18.86 and 75.76 mg/L with an average of 41.61 mg/L, respectively, and ranked the second and third abundance in the cations. K+ was the least abundant major cation in the study area and varied from 2.00 to 12.57 mg/L, averaging at 5.85 mg/L. For the major anions, all ions were with a relatively large variation in concentration. The concentration of HCO3−, SO42−, Cl− ranged from 112.28 to 457.44 mg/L, between 28.82 and 304.99 mg/L and from 83.67 to 577.88 mg/L. The order of mean concentration of these three major anions was HCO3− (246.65 mg/L) > Cl− (174.03 mg/L) > SO42− (123.58 mg/L) (Table 2). Overall, groundwaters in the Golmud alluvial fan plain were dominantly mixed Cl–Mg–Ca type, and followed by Cl–Na type and mixed HCO3–Na–Ca type (Fig. 3).
The common minor elements including nitrogen, F, I, As and toxic metals, etc. were also detected. Beside nitrogen, F, I, Cu, Zn, Al and B, other detected minor elements were observed in very low concentrations and even below the detection limits. As shown in Table 2, groundwater nitrogen was in the range of 0.13–24.44 mg/L for NO3−, 0.003–0.174 mg/L for NO2− and 0.01–0.76 mg/L for NH4+. All sampled groundwaters had the NO3− concentration below the desirable limit of 50 mg/L recommended by WHO guideline (WHO 2017). While a small portion of the groundwater samples was found exceeding the drinking water standard of NO2− (~ 6.25%) and NH4+ (~ 2.08%). The maximum concentration of NO2− and NH4+ were more than 8 times and 3 times of the desirable limits, respectively. Overall, the mean concentration of nitrogen was relatively low with the value of 2.11, 0.010 and 0.03 mg/L, respectively. Thus, nitrogen contamination is not serious over the whole region, but the high NO2− and NH4+ contaminant in groundwater should be concerned. The concentration of F− in groundwater samples varied from 0.18 to 1.01 mg/L with a mean of 0.006 mg/L. Only one sample (accounting 2.08% of the total groundwater samples) was found slightly beyond the desirable limit of 1.0 mg/L. The B concentration was found in the range of 0.426–1.179 mg/L with an average of 0.811 mg/L. Approximately 86.49% of the sampled groundwaters had the B element exceeding the desirable limit of 0.5 mg/L. The other four elements (I, Cu, Zn, Al) were in relatively low concentration and all within the drinking water standard. Therefore, the exceeding elements of NO2−, NH4+, F−, B should be paid attention in terms of their potential threats to the safety of water supply.
Groundwater quality assessment
Domestic purpose
As mentioned above, the minor elements including NO2−, NH4+, F−, B were found with relatively high concentrations in some sampled groundwaters and exceeding the recommended drinking water limits of Chinese guideline (GAQS 2017). The high contents of these elements could deteriorate groundwater quality and ultimately threaten the health of water consumers. The potential health risks of aforementioned exceeding elements were revealed with the aid of human health risk assessment model (HHRA model).
According to the risk appraisal result, the HI values ranged from 3.31 × 10–3 to 1.99 × 100 for infants, from 2.04 × 10–3 to 1.23 × 100 for children, from 1.47 × 10–3 to 0.88 × 100 for adult females and from 1.75 × 10–3 to 1.05 × 100 for adult males. Generally, the HI value less than 1 indicates low health risk. As can be seen from Fig. 4, most sampled groundwaters had the HI value below 1, implying the potential health threats posed by aforementioned contaminants in these groundwaters were negligible. However, 31.25, 8.33 and 2.08% of the total groundwater samples were found with the HI value greater than 1 for infants, children and adult males, respectively, suggesting existing potential health risk for these three populations. The infants were more prone to the negative health effects of exceeding contaminants in groundwater than other populations. According to the human health risk classification, an HI value below 1, between 1 and 4, greater than 4 is regarded as low risk, medium risk, high risk, respectively. Thus, the aforementioned health risks to infants, children and adult males were all medium risk, and that to children and adult males were very slight.
For further illustrating the responsible elements posing the health risk, the hazard quotient (HQ) of each contaminant was statistically presented in Fig. 5. It can be clearly seen that the HQ of NO2− and NH4+ contaminants were far below the permissible limit of 1, indicating very low health risk and can be ignored. The HQ value of B was in the range of 0.24–0.65 for infants, 0.15–0.40 for children, 0.10–0.29 for adult females and 0.12–0.34 for adult males. All groundwater samples had the HQ of B below 1 for various populations, although the B element was found widely exceeding the drinking water standard (Table 2). This suggests that the risk of B element in groundwater can be ignored. For F− contaminant, only 5 samples (accounting 10.42% of the total groundwater samples) were found had the HQ value slightly exceeding the permissible limit of 1 for infants. The HQ of F− contaminant for other three populations were all below 1, demonstrating negligible health threats. Totally, the aforementioned potential health threats in the study area were dominantly contributed by F− contaminant and flowed by B element. The contribution of NO2− and NH4+ contaminants can be ignored. Overall, the non-carcinogenic risk posed by F− and B should be concerned, especially for infants.
To comprehensively understand the quality of groundwater for drinking purpose, the entropy-weighted water quality index (EWQI) approach was introduced to evaluate the overall hydrochemical quality of sampled groundwaters. All the physicochemical parameters listed in Table 2 were involved in the EWQI appraisal. The assessment results demonstrated that the EWQI value of sampled groundwaters ranged from 35 to 151 (Fig. 6), implying the quality of groundwater in the study area had a large variation from excellent quality (Rank 1) to poor quality (Rank 4). According to the contribution percentage of various parameters to the EWQI value, B ranked the first (mean: 40.44%, median: 49.54%), and followed by Mg (mean: 12.76%, median: 10.60%), TDS (mean: 11.51%, median: 9.72%). Other parameters had relatively low contribution with both the mean and median contribution percentage below 10%. Thus, the difference of groundwater overall quality was mainly made by the B, Mg and salinity (TDS).
Specifically, more than half of the sampled groundwaters (about 52.08% of the groundwater samples) were with the EWQI value below 100, indicating excellent to good quality (Rank 1 to Rank 2) for drinking purpose. Only one sample (2.08%) was found with the EWQI value beyond 150 and situated in the poor quality category (Rank 4). Other samples had the EWQI value ranging between 100 and 150, belonging to the medium water quality category (Rank 3). Generally, groundwater with the EWQI value below 100 (Rank 1 to Rank 2, i.e. excellent and good water quality categories), between 100 and 150 (Rank 3, i.e. medium water quality category), greater than 150 (Rank 4 to Rank 5, i.e. poor and extremely poor water quality categories) is suitable for drinking purpose, only domestic purpose, totally non-drinking purpose, respectively (Adimalla et al. 2020; Subba Rao et al. 2019). Most of the sampled groundwaters are suitable for direct drinking purpose/domestic purpose, and the only sample belonging to poor quality category is just slightly exceeding the upper limit (EWQI value of 150) of medium water quality category. Thus, groundwater in the study area had a relatively good quality, while differential water supply should be performed according to the groundwater quality rank. Groundwater with excellent and good water quality can be allocated for direct drinking usage, and that with medium quality should be used for domestic purpose besides direct drinking.
Irrigation purpose
The sodium adsorption ratio (SAR) is a robust tool combing Na+, Ca2+ and Mg2+ to assess the suitability of water quality for irrigation purpose. It can get insights into the potential damage of soil structure by aqueous Na+ replacing adsorbed Ca2+ and Mg2+ (Kumari and Rai 2020), which is usually called sodium hazard. In present study area, the SAR of groundwater varied from 2.14 to 13.52 with an average of 3.59. Generally, irrigation water quality can be classified into 4 categories based on the SAR. The quality of water that with the SAR of < 10, 10–18, 18–26, > 26 is defined as excellent, good, doubtful, not suitable, respectively, for sustainable irrigation. Thus, all groundwaters in the study area belonged to the excellent and good water quality categories and were suitable for irrigation purpose in terms of the SAR appraisal results.
However, sodium hazard can also occur if irrigation water has relatively high salinity (EC) although SAR is low (Adimalla and Venkatayogi 2018; Aravinthasamy et al. 2020). According to the Wilcox diagram composed by salinity and SAR (USSL 1954), the sampled groundwaters were predominantly situated in the S1 category (Fig. 7) and suggested low sodium hazard. Approximately 10.42% of the total groundwater samples belonged to the medium category (S2) of sodium hazard, and 2.08% of samples were classified in the high sodium hazard category (S3). It can also be seen from Fig. 7, majority of sampled groundwaters plotted in the high salinity hazard category (C3). Thus, special attentions should be paid to the potential salinity hazard in long-term agricultural practice. Drainage channel is a good agricultural management option to control the salinity (El-Ghannam et al. 2021; Wen et al. 2020).
Percentage sodium (%Na) is another effective parameter to illustrate the potential hazard of sodium to soil structure (Qu et al. 2021). The %Na value of sampled groundwaters in the study area varied between 37.67 and 80.55%. Generally, water with the %Na value below 60% is suitable for long-term agricultural irrigation (Aravinthasamy et al. 2020). Accordingly, majority of groundwaters (more than 85% of the total groundwater samples with the %Na less than 60%) in the study area is suitable severing as irrigation water and would not pose sodium hazard to soil in the long-term irrigation practice. As shown in USSL diagram combining of EC and %Na (Fig. 8), most of sampled groundwaters plotted in the categories of excellent to good, good to permissible, and permissible to suitable quality, indicating suitable for irrigation in terms of the combined effects of salinity and %Na. Only one sample (accounting 2.08% of the total samples) was observed situating in the doubtful to unsuitable quality category, and should be concerned to its potential negative effects.
Permeability index (PI) is another useful parameter to evaluate the suitability of water for agricultural irrigation purpose. According to the assessment results, the value of PI ranged from 51.48 to 95.89 for sampled groundwaters in the present alluvial fan plain. Three irrigation water quality categories can be divided based on the relationship between PI and the total concentration of ions, namely Class-I (good), Class-II (doubtful) and Class-III (unsuitable). As demonstrated in Fig. 9, majority of the sampled groundwaters (93.75%) belonged to the Class-I category of irrigation water quality, suggesting suitable for irrigation purpose and would not affect the permeability of soil during the long-term agricultural practice. While about 2.08 and 4.16% of the sampled groundwaters were found situating in the water quality categories of Class-II and Class-III, implying doubtful to unsuitable water quality for irrigation purpose.
Groundwater chemistry formation and implication for sustainable development
Identifying the origin and controlling mechanisms of groundwater hydrochemistry is the main task of hydrogeology research and essential for the sustainable development and management of groundwater resource (Hao et al. 2020; Xiao et al. 2017b).
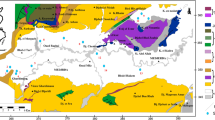
Naturally, the hydrochemical components of groundwater is mainly originated/controlled by precipitation, rock–water interaction and evaporation (Zhang et al. 2021d), which can be visually revealed by the relation of between the weight ratio of Na+/(Na+ + Ca2+) versus TDS and Cl−/(Cl− + HCO3−) versus TDS (Gibbs 1970). As demonstrated in Fig. 10, all sampled groundwaters were observed plotting in the rock dominance, indicating the hydrochemcal composition of groundwater was governed by water–rock interactions in nature. It can be also seen that almost no sample situated in the evaporation dominance, suggesting that the influence of evaporation was very limited and can be ignored. This was ascribed to the large depth of groundwater level in the middle–upper area of the alluvial fan plain and the relatively fast flowing/circulating rate in the aquifer of whole alluvial fan plain.
A lot of rocks may involve in the water–rock interactions and contribute to the hydrochemical components of groundwater. The end-member diagrams constructed by Na-normalized Ca2+ versus Mg2+ and Na-normalized Ca2+ versus HCO3− are effective and widely used tools to illustrate the contributed rocks. As presented in Fig. 11, most of sampled groundwaters situated in the silicates dominance of both the two end-member diagrams, suggesting silicates weathering was the predominant source of groundwater components in the study area. This was coincided with the hydrogeological background of the study area. In the mountainous headwater areas and alluvial fans, many favorably dissolvable minerals of rocks had been dissolved and moved away in the long geological history and only the non-favorable ones were left over. Thus, the involved rocks in the mountainous headwater areas and alluvial fans were mainly those non-favorably dissolvable ones like silicates. In addition, a few of groundwater samples were observed plotting in the transition dominance from silicates to evaporites. This was due to the existence of evaporites at some local area of aquifers in the alluvial fan plain, which was formed during the relatively weak surface hydrodynamic environment in the geological history and deposited some evaporite minerals in the aquifers of local areas. The significant correlations between Cl− and Na+, SO42− and Ca2+, SO42− and Na+, SO42− and Mg2+ (Table 3) confirmed the potential contribution of the evaporates (halite, sulfates) dissolution to groundwater components (Xu et al. 2021a, b). While the limited number of sampled groundwaters showing a trend from the silicates dominance to evaporites dominance proved the influence of evaporites dissolution on groundwater chemistry was only at local area. Additionally, no groundwaters situated in the carbonates dominance, indicating almost no contribution from carbonates dissolution to groundwater chemistry. This is evidenced by the correlation matrix which showed no significant correlation between HCO3− and Ca2+, Mg2+. Overall, groundwater chemistry in the study area was dominantly governed by silicates weathering and sporadically influenced by evaporites (halite, sulfates, etc.) dissolution, but without significant evaporation effect.
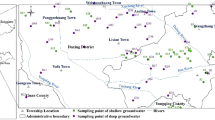
Human activity has become one of the most important factors influencing groundwater chemistry, and even the dominate mechanism regulating the hydrochemical composition of groundwater across the world (Adimalla 2020; Gao et al. 2020; Hussain et al. 2017; Marghade et al. 2021; Siddique et al. 2020; Zhang et al. 2021d). Nitrogen is an important indicator of contamination from human community, which has been widely used to indicate the anthropogenic inputs of pollutant from agricultural practice, domestic effluents and so on (Adimalla 2020; Gu et al. 2018, 2017a; Li et al. 2020; Zhang et al. 2019). As mentioned before, 2.08% (i.e. groundwater sample of P38) and 6.25% (i.e. groundwater sample of P23, P37, P38) of sampled groundwaters were found with the NH4+ and NO2− beyond the permissible limits of drinking water guideline. Generally, the high nitrogen in water bodies originates from the anthropogenic sources of agriculture fertilizer, livestock manure, domestic lives, etc. Thus, groundwater in these three locations was influenced by anthropogenic contaminant inputs. As shown in Fig. 12e, f, these high-nitrogen groundwaters (P23, P37, P38) were situated in the agricultural lands (P37 and P38) and urban settlement (P23), confirming the anthropogenic influences on groundwater chemistry. Although NO3− in groundwater was not exceeding the permissible limit of drinking standard, one sample (P42) was found with the NO3− concentration of 24.44 mg/L, more than 2 times of the natural baseline of 10 mg/L (Xiao et al. 2017a), implying the anthropogenic contaminant inputs.
As demonstrated in Fig. 12, the areas that with high nitrogen content also had relatively high concentration of TDS and major ions (such as Cl−, Na+ and Ca2+). This was confirmed by the correlation matrix (Table 3). Specifically, the high nitrogen groundwaters were predominantly distributed in the northwestern part (Fig. 12e, f) and northeastern part (Fig. 12f, h) of the study area. This was coincided with the spatial distribution of exceeding TDS and major ions like Cl−, Na+ and Ca2+ (Fig. 12), suggesting agricultural and domestic inputs of contamination had significant influences on the hydrochemical composition of groundwater in the northwestern part (agricultural lands) and northeastern part (population settlement) of the study area. While the high-F− groundwater was only found at location of P15 with the concentration of 1.01 mg/L, which was just slightly beyond the permissible limit of 1.0 mg/L. The relatively high B groundwaters were widespread in the alluvial fan. This distribution of both high-F− and high-B groundwater did not show relation with that of anthropogenic influences in spatial. Additionally, the correlation matrix also presented no significant relation between all other elements and F−, B. All the above implied the natural origins of F− and B in groundwater. This is coincided with many previous reports in other regions (Xiao et al. 2021a; Zango et al. 2019; Zhang et al. 2020b).
Overall, groundwater chemistry was dominantly governed by natural mechanisms of rock–water interaction (including predominant silicates weathering and partly evaporites dissolution) in the Golmud alluvial fan plain. Anthropogenic contamination inputs (agricultural fertilizer and urban domestic effluents) were responsible for the high nitrogen content and elevated major ions of groundwater in the study area. While the exceeding F− and B in groundwater were originated from the natural sources rather than human community. Although the inputs of anthropogenic contaminants did not affect the domestic usage of groundwater resources, the irrigation quality of groundwater had been deteriorated due to the elevated salinity by anthropogenic factors. Thus, measures should be taken to protect groundwater out of the influences of anthropogenic contaminants from agricultural practices and urban domestic effluents. Additionally, the potential health risk of sporadic groundwaters to infants should also be concerned.
Summary and conclusions
The present paper takes the Golmud alluvial fan plain on Tibetan Plateau as an example to present the hydrogeochemical status and formation mechanisms of groundwater resource in alluvial aquifers that undergone intense disturbances from human community and its constraints on the sustainable water development of hyper-arid regions. The main findings are as below:
Groundwater in the Golmud alluvial fan plain is slightly alkaline and has the hydrochemical faces dominantly in mixed Cl–Mg–Ca type and a few in Cl–Na type and mixed HCO3–Na–Ca type. Toxic elements of NO2−, NH4+, F− and B are beyond the permissible limits of drinking water at some locations and potentially pose medium overall noncarcinogenic risks to the populations of infants, children and adult males. These health risks are mainly attributed to the toxic F− in groundwater, but only exists at a few sites. Groundwater in the alluvial fan plain has the quality ranging from excellent to medium categories with the EWQI in the range of 35–151, and is almost suitable for domestic purposes. Groundwaters are predominantly suitable for irrigation purpose without sodium hazard based on the sodium adsorption ratio percentage sodium and permeability index. While the potential salinity hazard is widely distributed in the plain according to the Wilcox diagram, and rational measures should be taken to reduce or even avoid this hazard for realizing sustainable agricultural irrigation.
The hydrochemical composition of groundwater is governed by both natural and anthropogenic factors. Rock–water interactions of silicates weathering and evaporites dissolution are principally contributed the fundamental chemical components including the major ions and exceeding F− and B in groundwater. Anthropogenic inputs of agricultural and domestic contamination into aquifers have induced significant influences on groundwater chemistry in the farmlands and human settlements at the alluvial fan front, and are responsible for the excessive nitrogen (NO2− and NH4+) and elevated salinity there. For sustainable groundwater development, greater efforts should be done to protect groundwater out of anthropogenic contaminations.
Data availability
The data used to support the findings of the present study will be provided upon request by the corresponding author.
References
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health 11:109–123. https://doi.org/10.1007/s12403-018-0288-8
Adimalla N (2020) Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum Ecol Risk Assess 26:310–334. https://doi.org/10.1080/10807039.2018.1508329
Adimalla N (2021) Application of the entropy weighted water quality index (EWQI) and the pollution index of groundwater (PIG) to assess groundwater quality for drinking purposes: a case study in a rural area of Telangana State, India. Arch Environ Contam Toxicol 80:31–40. https://doi.org/10.1007/s00244-020-00800-4
Adimalla N, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl Water Sci 8:44. https://doi.org/10.1007/s13201-018-0682-1
Adimalla N, Qian H, Li P (2020) Entropy water quality index and probabilistic health risk assessment from geochemistry of groundwaters in hard rock terrain of Nanganur County, South India. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125544
Aravinthasamy P, Karunanidhi D, Subba Rao N, Subramani T, Srinivasamoorthy K (2020) Irrigation risk assessment of groundwater in a non-perennial river basin of South India: implication from irrigation water quality index (IWQI) and geographical information system (GIS) approaches. Arab J Geosci 13:1125. https://doi.org/10.1007/s12517-020-06103-1
Blackburn J, Comte J-C, Foster G, Gibbins C (2021) Hydrogeological controls on the flow regime of an ephemeral temperate stream flowing across an alluvial fan. J Hydrol 595:125994. https://doi.org/10.1016/j.jhydrol.2021.125994
Chowdhury AH, Scanlon BR, Reedy RC, Young S (2018) Fingerprinting groundwater salinity sources in the Gulf Coast Aquifer System, USA. Hydrogeol J 26:197–213. https://doi.org/10.1007/s10040-017-1619-8
Dash CJ, Sarangi A, Singh DK, Adhikary PP (2019) Numerical simulation to assess potential groundwater recharge and net groundwater use in a semi-arid region. Environ Monit Assess 191:371. https://doi.org/10.1007/s10661-019-7508-y
El-Ghannam MK, Aiad MA, Abdallah AM (2021) Irrigation efficiency, drain outflow and yield responses to drain depth in the Nile delta clay soil, Egypt. Agric Water Manag 246:106674. https://doi.org/10.1016/j.agwat.2020.106674
Gao Z, Wang Z, Wang S, Wu X, An Y, Wang W, Liu J (2019) Factors that influence the chemical composition and evolution of shallow groundwater in an arid region: a case study from the middle reaches of the Heihe River, China. Environ Earth Sci 78:390. https://doi.org/10.1007/s12665-019-8391-0
Gao X, Li X, Wang W, Li C (2020) Human activity and hydrogeochemical processes relating to groundwater quality degradation in the Yuncheng Basin, Northern China. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17030867
Gao Z, Han C, Xu Y, Zhao Z, Luo Z, Liu J (2021) Assessment of the water quality of groundwater in Bohai Rim and the controlling factors—a case study of northern Shandong Peninsula, north China. Environ Pollut 285:117482. https://doi.org/10.1016/j.envpol.2021.117482
GAQS (2017) Standards for groundwater quality (GB/T 14848–2017). General Administration of Quality Supervision, Beijing
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gu X, Xiao Y, Yin S, Pan X, Niu Y, Shao J, Cui Y, Zhang Q, Hao Q (2017a) Natural and anthropogenic factors affecting the shallow groundwater quality in a typical irrigation area with reclaimed water, North China Plain. Environ Monit Assess. https://doi.org/10.1007/s10661-017-6229-3
Gu X, Xiao Y, Yin S, Shao J, Pan X, Niu Y, Huang J (2017b) Groundwater level response to hydrogeological factors in a semi-arid basin of Beijing, China. J Water Supply Res Technol AQUA 66:266–278. https://doi.org/10.2166/aqua.2017.093
Gu X, Xiao Y, Yin S, Hao Q, Liu H, Hao Z, Meng G, Pei Q, Yan H (2018) Hydrogeochemical characterization and quality assessment of groundwater in a long-term reclaimed water irrigation area, North China Plain. Water. https://doi.org/10.3390/w10091209
Hao Q, Shao J, Cui Y, Xie Z (2014) Applicability of artificial recharge of groundwater in the Yongding River alluvial fan in Beijing through numerical simulation. J Earth Sci 25:575–586. https://doi.org/10.1007/s12583-014-0442-6
Hao Q, Xiao Y, Chen K, Zhu Y, Li J (2020) Comprehensive understanding of groundwater geochemistry and suitability for sustainable drinking purposes in confined aquifers of the Wuyi Region, Central North China. Plain Water. https://doi.org/10.3390/w12113052
Huang J, Wang W, Cui X, Wang D, Liu W, Liu X, Wang S (2020) Environmental risk-based hydroeconomic evaluation for alluvial aquifer management in arid river basin. Sci Total Environ 711:134655. https://doi.org/10.1016/j.scitotenv.2019.134655
Hussain Y, Ullah SF, Hussain MB, Aslam AQ, Akhter G, Martinez-Carvajal H, Cárdenas-Soto M (2017) Modelling the vulnerability of groundwater to contamination in an unconfined alluvial aquifer in Pakistan. Environ Earth Sci 76:84. https://doi.org/10.1007/s12665-017-6391-5
Jean-Baptiste J, Le Gal La Salle C, Verdoux P (2020) Use of mixing models to explain groundwater quality time and space variation in a narrowed fluctuating alluvial aquifer. Appl Geochem 121:104700. https://doi.org/10.1016/j.apgeochem.2020.104700
Kumar PJS, Kuriachan L (2020) Chemometric appraisal of groundwater quality for domestic, irrigation and industrial purposes in Lower Bhavani River basin, Tamil Nadu, India. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1770241
Kumar Joshi S, Gupta S, Sinha R, Logan Densmore A, Prakash Rai S, Shekhar S, Mason PJ, Dijk WMV (2021) Strongly heterogeneous patterns of groundwater depletion in Northwestern India. J Hydrol 598:126492. https://doi.org/10.1016/j.jhydrol.2021.126492
Kumari M, Rai SC (2020) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes using water quality index in semi arid region of India. J Geol Soc India 95:159–168. https://doi.org/10.1007/s12594-020-1405-4
Li F, Pan G, Tang C, Zhang Q, Yu J (2008) Recharge source and hydrogeochemical evolution of shallow groundwater in a complex alluvial fan system, southwest of North China Plain. Environ Geol 55:1109–1122. https://doi.org/10.1007/s00254-007-1059-1
Li Y, Zhang Z, Fei Y, Chen H, Qian Y, Dun Y (2016) Investigation of quality and pollution characteristics of groundwater in the Hutuo River Alluvial Plain, North China Plain. Environ Earth Sci 75:1–10. https://doi.org/10.1007/s12665-016-5366-2
Li J, Shi Z, Wang G, Liu F (2020) Evaluating spatiotemporal variations of groundwater quality in Northeast Beijing by self-organizing map. Water. https://doi.org/10.3390/w12051382
Luo Y, Xiao Y, Hao Q, Zhang Y, Zhao Z, Wang S, Dong G (2021) Groundwater geochemical signatures and implication for sustainable development in a typical endorheic watershed on Tibetan plateau. Environ Sci Pollut Res 28:48312–48329. https://doi.org/10.1007/s11356-021-14018-x
Mal U, Adhikari K (2021) Groundwater quality and hydrological stress induced by Lower Gondwana open cast coal mine. J Earth Syst Sci 130:32. https://doi.org/10.1007/s12040-020-01486-x
Marghade D, Malpe DB, Duraisamy K, Patil PD, Li P (2021) Hydrogeochemical evaluation, suitability, and health risk assessment of groundwater in the watershed of Godavari basin, Maharashtra, Central India. Environ Sci Pollut Res 28:18471–18494. https://doi.org/10.1007/s11356-020-10032-7
Peng C, He J-T, Wang M-l, Zhang Z-g, Wang L (2018) Identifying and assessing human activity impacts on groundwater quality through hydrogeochemical anomalies and NO3−, NH4+, and COD contamination: a case study of the Liujiang River Basin, Hebei Province, P.R. China. Environ Sci Pollut Res 25:3539–3556. https://doi.org/10.1007/s11356-017-0497-x
Qu S, Shi Z, Liang X, Wang G, Han J (2021) Multiple factors control groundwater chemistry and quality of multi-layer groundwater system in Northwest China coalfield—using self-organizing maps (SOM). J Geochem Explor 227:106795. https://doi.org/10.1016/j.gexplo.2021.106795
Rahman M, Tushar MAN, Zahid A, Ahmed KMU, Siddique MAM, Mustafa MG (2021) Spatiotemporal distribution of boron in the groundwater and human health risk assessment from the coastal region of Bangladesh. Environ Sci Pollut Res 28:21964–21977. https://doi.org/10.1007/s11356-020-11682-3
Rao MS, Krishan G, Kumar CP, Purushothaman P, Kumar S (2017) Observing changes in groundwater resource using hydro-chemical and isotopic parameters: a case study from Bist Doab, Punjab. Environ Earth Sci 76:175. https://doi.org/10.1007/s12665-017-6492-1
Sheikhy Narany T, Sefie A, Aris AZ (2018) The long-term impacts of anthropogenic and natural processes on groundwater deterioration in a multilayered aquifer. Sci Total Environ 630:931–942. https://doi.org/10.1016/j.scitotenv.2018.02.190
Siddique J, Menggui J, Shah MH, Shahab A, Rehman F, Rasool U (2020) Integrated approach to hydrogeochemical appraisal and quality assessment of groundwater from Sargodha District, Pakistan. Geofluids 2020:6621038. https://doi.org/10.1155/2020/6621038
Singha S, Pasupuleti S, Singha SS, Singh R, Kumar S (2021) Prediction of groundwater quality using efficient machine learning technique. Chemosphere 276:130265. https://doi.org/10.1016/j.chemosphere.2021.130265
Subba Rao N, Sunitha B, Adimalla N, Chaudhary M (2019) Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42:579–599. https://doi.org/10.1007/s10653-019-00393-5
Tweed S, Leblanc M, Cartwright I, Favreau G, Leduc C (2011) Arid zone groundwater recharge and salinisation processes; an example from the Lake Eyre Basin, Australia. J Hydrol 408:257–275. https://doi.org/10.1016/j.jhydrol.2011.08.008
USEPA (1989) Risk assessment guidance for superfund, Volume I: human health evaluation manual (Part A). Interim final. (EPA/540/1‐89/002)
USSL (1954) Diagnosis and improvement of saline and alkali soils. Agriculture handbook
Venkatramanan S, Chung SY, Selvam S, Lee SY, Elzain HE (2017) Factors controlling groundwater quality in the Yeonjegu District of Busan City, Korea, using the hydrogeochemical processes and fuzzy GIS. Environ Sci Pollut Res 24:23679–23693. https://doi.org/10.1007/s11356-017-9990-5
Wang Y, Zheng C, Ma R (2018) Review: safe and sustainable groundwater supply in China. Hydrogeol J 26:1301–1324. https://doi.org/10.1007/s10040-018-1795-1
Wang Y, Li J, Ma T, Xie X, Deng Y, Gan Y (2020) Genesis of geogenic contaminated groundwater: As, F and I. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2020.1807452
Wen Y, Shang S, Rahman KU, Xia Y, Ren D (2020) A semi-distributed drainage model for monthly drainage water and salinity simulation in a large irrigation district in arid region. Agric Water Manag 230:105962. https://doi.org/10.1016/j.agwat.2019.105962
WHO (2017) Guidelines for drinking-water quality, 4th edition incorporating the first addendum. World Health Organization
Wu C, Wu X, Zhu G, Qian C, Mu W-P, Zhang Y-z (2018) Influence of a power plant in Ezhou City on the groundwater environment in the nearby area. Environ Earth Sci 77:503. https://doi.org/10.1007/s12665-018-7674-1
Xiao Y, Gu X, Yin S, Pan X, Shao J, Cui Y (2017a) Investigation of geochemical characteristics and controlling processes of groundwater in a typical long-term reclaimed water use area. Water 9:800
Xiao Y, Shao J, Cui Y, Zhang G, Zhang Q (2017b) Groundwater circulation and hydrogeochemical evolution in Nomhon of Qaidam Basin, northwest China. J Earth Syst Sci. https://doi.org/10.1007/s12040-017-0800-8
Xiao Y, Shao J, Frape S, Cui Y, Dang X, Wang S, Ji Y (2018) Groundwater origin, flow regime and geochemical evolution in arid endorheic watersheds: a case study from the Qaidam Basin, northwestern China. Hydrol Earth Syst Sci 22:4381–4400. https://doi.org/10.5194/hess-22-4381-2018
Xiao Y, Yin S, Hao Q, Gu X, Pei Q, Zhang Y (2020) Hydrogeochemical appraisal of groundwater quality and health risk in a near-suburb area of North China. J Water Supply Res Technol AQUA 69:55–69. https://doi.org/10.2166/aqua.2019.101
Xiao Y, Hao Q, Zhang Y, Zhu Y, Yin S, Qin L, Li X (2021a) Investigating sources, driving forces and potential health risks of nitrate and fluoride in groundwater of a typical alluvial fan plain. Sci Total Environ 802:149909. https://doi.org/10.1016/j.scitotenv.2021.149909
Xiao Y, Liu K, Hao Q, Li J, Zhang Y, Cui W, Limao q, Pei Q, (2021b) Hydrogeochemical features and genesis of confined groundwater and health perspectives for sustainable development in Urban Hengshui, North China Plain. J Chem 2021:5578192. https://doi.org/10.1155/2021/5578192
Xiao Y, Xiao D, Hao Q, Liu K, Wang R, Huang X, Liao X, Zhang Y (2021c) Accessible phreatic groundwater resource in the Central Shijiazhuang of North China Plain: perspective from the hydrogeochemical constraints. Front Environ Sci. https://doi.org/10.3389/fenvs.2021.747097
Xu P, Feng W, Qian H, Zhang Q (2019a) Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16:1492
Xu P, Li M, Qian H, Zhang Q, Liu F, Hou K (2019b) Hydrochemistry and geothermometry of geothermal water in the central Guanzhong Basin, China: a case study in Xi’an. Environ Earth Sci 78:87. https://doi.org/10.1007/s12665-019-8099-1
Xu P, Zhang Q, Qian H, Li M, Hou K (2019c) Characterization of geothermal water in the piedmont region of Qinling Mountains and Lantian-Bahe Group in Guanzhong Basin, China. Environ Earth Sci 78:442. https://doi.org/10.1007/s12665-019-8418-6
Xu W, Zhu P, Yang F (2019d) Evaluation of groundwater recharge sources based on environmental tracers in an arid alluvial fan, NW China. J Radioanal Nucl Chem 319:123–133. https://doi.org/10.1007/s10967-018-6272-8
Xu P, Zhang Q, Qian H, Guo M, Yang F (2021a) Exploring the geochemical mechanism for the saturated permeability change of remolded loess. Eng Geol 284:105927. https://doi.org/10.1016/j.enggeo.2020.105927
Xu P, Zhang Q, Qian H, Yang F, Zheng L (2021b) Investigating the mechanism of pH effect on saturated permeability of remolded loess. Eng Geol 284:105978. https://doi.org/10.1016/j.enggeo.2020.105978
Yan J, Chen J, Zhang W (2021) Study on the groundwater quality and its influencing factor in Songyuan City, Northeast China, using integrated hydrogeochemical method. Sci Total Environ 773:144958. https://doi.org/10.1016/j.scitotenv.2021.144958
Yang N, Wang G (2020) Moisture sources and climate evolution during the last 30 kyr in northeastern Tibetan Plateau: insights from groundwater isotopes (2H, 18O, 3H and 14C) and water vapour trajectories modeling. Quatern Sci Rev 242:106426. https://doi.org/10.1016/j.quascirev.2020.106426
Yin S, Xiao Y, Gu X, Hao Q, Liu H, Hao Z, Meng G, Pan X, Pei Q (2019) Geostatistical analysis of hydrochemical variations and nitrate pollution causes of groundwater in an alluvial fan plain. Acta Geophys 67:1191–1203. https://doi.org/10.1007/s11600-019-00302-5
Yin S, Xiao Y, Han P, Hao Q, Gu X, Men B, Huang L (2020) Investigation of groundwater contamination and health implications in a typical semiarid basin of North China. Water 12:1137. https://doi.org/10.3390/w12041137
Yuan R, Wang S, Wang P, Song X, Tang C (2017) Changes in flow and chemistry of groundwater heavily affected by human impacts in the Baiyangdian catchment of the North China Plain. Environ Earth Sci 76:571. https://doi.org/10.1007/s12665-017-6918-9
Zango MS, Sunkari ED, Abu M, Lermi A (2019) Hydrogeochemical controls and human health risk assessment of groundwater fluoride and boron in the semi-arid North East region of Ghana. J Geochem Explor 207:1–21. https://doi.org/10.1016/j.gexplo.2019.106363
Zhang Q, Wang H, Wang Y, Yang M, Zhu L (2017) Groundwater quality assessment and pollution source apportionment in an intensely exploited region of northern China. Environ Sci Pollut Res 24:16639–16650. https://doi.org/10.1007/s11356-017-9114-2
Zhang Q, Xu P, Qian H (2019) Assessment of groundwater quality and human health risk (HHR) evaluation of nitrate in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16:4246
Zhang Q, Xu P, Qian H (2020a) Groundwater quality assessment using improved water quality index (WQI) and human health risk (HHR) evaluation in a semi-arid region of Northwest China. Expos Health 12:487–500. https://doi.org/10.1007/s12403-020-00345-w
Zhang Q, Xu P, Qian H, Yang F (2020b) Hydrogeochemistry and fluoride contamination in Jiaokou Irrigation District, Central China: assessment based on multivariate statistical approach and human health risk. Sci Total Environ 741:140460. https://doi.org/10.1016/j.scitotenv.2020.140460
Zhang Q, Liu H, Zhu P, Xu W, Su X (2021a) Evaluation of the influence of river bank infiltration on groundwater in an inland alluvial fan using spectral analysis and environmental tracers. Hydrogeol J 29:1117–1128. https://doi.org/10.1007/s10040-020-02290-z
Zhang Q, Qian H, Xu P, Li W, Feng W, Liu R (2021b) Effect of hydrogeological conditions on groundwater nitrate pollution and human health risk assessment of nitrate in Jiaokou Irrigation District. J Clean Prod 298:126783. https://doi.org/10.1016/j.jclepro.2021.126783
Zhang Q, Xu P, Chen J, Qian H, Qu W, Liu R (2021c) Evaluation of groundwater quality using an integrated approach of set pair analysis and variable fuzzy improved model with binary semantic analysis: a case study in Jiaokou Irrigation District, east of Guanzhong Basin, China. Sci Total Environ 767:145247. https://doi.org/10.1016/j.scitotenv.2021.145247
Zhang Y, Dai Y, Wang Y, Huang X, Xiao Y, Pei Q (2021d) Hydrochemistry, quality and potential health risk appraisal of nitrate enriched groundwater in the Nanchong area, southwestern China. Sci Total Environ 784:147186. https://doi.org/10.1016/j.scitotenv.2021.147186
Zhu H, Zhou J, Song T, Feng H, Liu Z, Liu H, Ren X (2020) Influences of natural and anthropogenic processes on the groundwater quality in the Dagujia River Basin in Yantai, China. J Water Supply Res Technol AQUA 69:184–196. https://doi.org/10.2166/aqua.2019.113
Acknowledgements
This research was funded by the Natural Science Foundation of China (42007183), the Fundamental Research Funds for the Central Universities (2682021ZTPY063; 2682020CX10), the Science and Technology Plan Project of Qinghai Province (2021-ZJ-T08), the Bureau of Qinghai Environmental Geological Prospecting (2021-KJ-001-02; 2021-KJ-011), the Student Research Training Program of Southwest Jiaotong University (210815), and the Research Project on Teaching Reform of Southwest Jiaotong University (20201023-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a Topical Collection in Environmental Earth Sciences on Groundwater quality and contamination and the application of GIS, guest edited by Narsimha Adimalla and Hui Qian.
Rights and permissions
About this article
Cite this article
Xiao, Y., Liu, K., Yan, H. et al. Hydrogeochemical constraints on groundwater resource sustainable development in the arid Golmud alluvial fan plain on Tibetan plateau. Environ Earth Sci 80, 750 (2021). https://doi.org/10.1007/s12665-021-10076-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-10076-z