Abstract
Douala city, located in the littoral province of Cameroon, receives abundant rainfall quantities due to its geographical position in the Gulf of Guinea and bears considerable surface water and groundwater resources. Due to socioeconomic development and rapid demographic growth in the city and its consequences of unplanned urbanization and improper sanitation system, these water resources are poorly protected and managed. Streams in the Wouri watershed receive large amounts of wastewater discharge, and hundreds of boreholes have been drilled into the aquifer system without any management plan. A detailed hydrodynamic and hydrogeochemistry study in Douala town and its environs was conducted to get a better insight into the groundwater system functioning in order to provide information for the sustainable management and protection of the groundwater resource. Two field campaigns were carried out with 187 samples collected and analyzed for major ions, stable isotopes (18O, 2H), and tritium 3H. The results of the sampling have shown that the weathering of silicate minerals is the dominant geochemical process affecting groundwater chemistry in this system. However, acid rainfall in the humid climate has also caused carbonate mineral dissolution, amorphous silica deposition, and ion exchange reactions to occur in aquifers in the region. The various water types identified were categorized into four major clusters C1 to C4, based on the major ion composition and the local hydrogeological conditions. Environmental isotope data reveal that modern-to-submodern waters occur in the phreatic Quaternary/Mio-Pliocene and Oligocene/Upper Eocene aquifers, respectively. These results corroborate with the conceptual model built where modern groundwater types indicated silicate mineral weathering and calcite dissolution (C1 and C2), whereas submodern groundwater mostly showed silica deposition, ion exchange, and, to a lesser extent, carbonate mineral dissolution (C3 and C4). This improved understanding of the aquifer system functioning is essential to provide a reasonable basis for effective control measures and sustainable water management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustainable social and economic development in many developing countries is largely dependent on the availability of groundwater resources. Recent papers have particularly shown their importance to meet increasing industrial and domestic needs in metropolitan cities such as those in the Indian subcontinent, in Comoros Islands, in Kenya and Tanzania (Mukherjee et al. 2015; Comte et al. 2016). However, access to safe water in the urban zone is mostly limited by the lack of modern infrastructures such as properly constructed boreholes, the availability of water treatment plants, and the presence of an adequate water supply distribution network. The problem is more acute in low-income megacities which are typically characterized by rapid demographic growth and unplanned urbanization. This leads to water resource pressure both in terms of quantity and quality of the water supply in these cities. Thus, the assessment of groundwater has become an important issue with respect to appropriate water resource planning and management (Dinka et al. 2015).
In Douala (the economic capital of Cameroon), located in the littoral province of Cameroon with a population of 2.8 million inhabitants (2015) and a density of about 3000 inhabitants/km2, the water supply was historically piped from the Dibamba River at Japouma station (located downstream the river, in the south of the town). But due to saltwater intrusion into the river during low-stage water periods and increasing water needs from the 1980s, boreholes tapping the aquifer system beneath Douala town were drilled by the public water company (CDE) at different locations in the town. Presently, around 50 boreholes are operating, but there is still a significant water supply shortage (by about 25%), and additional water and infrastructure would be required to achieve the Millennium Development Goals (MDG) with regard to full access to the modern distribution network. Additionally, numerous private boreholes and dug-wells are exploiting the aquifer system beneath the town without any quality control and proper management of the valuable groundwater resource. As a consequence, waterborne diseases such as cholera, malaria, and typhoid fever are recurrent in Douala town.
Previous research carried out in the Douala hydrosystem was mainly focused on the quality of surface water and of shallow groundwater to determine levels of chemical and bacteriological pollutants. Tening et al. (2013) investigated NO3− and NH4+ levels of the Wouri and Dibamba rivers together with some selected streams and soils samples to evaluate the impact of the waste discharges on water quality and of the capacity of the soil to remove contaminants. Investigations by Takem et al. (2010) and Ngo Boum et al. (2015) mainly focused on the quality of the shallow groundwater where chemical and bacteriological pollutants were determined in the different parts of Douala town. They also investigated how anthropogenic factors and seasonal variations in rainfall affected water quality. Takem et al. (2015) used the groundwater chemistry data record (2008–2013) along with pH values to investigate the acidification of shallow groundwater in the system. These results indicated that the acidification of the shallow groundwater was mainly caused by acid atmospheric deposition and other anthropogenic factors.
Despite this previous work, the lack of a systematic hydrogeochemical investigation into the entire aquifer system has restricted the knowledge of the spatial variations and geochemical processes involved and of the regional aquifer system functioning based on the interpretation of these data. This is of prime importance for this valuable groundwater resource to sustainably support its management and protection.
The objectives of this present paper are to: (i) identify the geochemical processes in the Quaternary alluviums/Mio-Pliocene and Eocene/Oligocene sand formations and the potential external factors that have influenced groundwater chemistry; (ii) understand the spatial water types distribution related to the geological and hydrogeological conditions; and (iii) determine the origin of groundwater and the aquifer system functioning taking into account flow and recharge patterns in the Douala hydrosystem.
To address these objectives, chemical and environmental isotopic (18O, 2H, 3H) constituents of the different water bodies were evaluated using conventional tools including: the use of a Piper diagram; bivariate diagrams; saturation index (SI) calculations; Gibbs diagrams; mineral stability diagrams; and the use of multivariate statistical techniques.
Description of the study area
The study area belongs to the coastal aquifer system which is a part of the Douala Coastal Sedimentary Basin (DCSB). It is located on the Gulf of Guinea within the littoral province of Cameroon. It includes Douala town and its neighboring suburban localities (Bonéndalè, Yansokè, Carrefour Massoumbou, Lendi) and the Wouri estuary, covering a surface area of 923 km2 (Fig. 1). The region has an equatorial climate, characterized by a 9-month-long rainy season and 3-month-long dry season. The peak of the rainy season occurs from July to September, and the annual average precipitation is about 4000 mm. The temperature of the region ranges between 23 and 33.5 °C with January and February being the hottest months of the year. The altitude ranges from + 98 m in the NE of the town to + 3 m in the vicinity of the Wouri estuary. The study area bears a dense hydrographic network made up of streams, rivers of order 4 (Kopa et al. 2012), which are partly fed by groundwater seepage.
Geological setting
Regional geological setting
The DCSB, which comprises both an offshore and onshore portions, is located in the Gulf of Guinea. It covers an area of 7000 km2 and belongs to the divergent margin basin system occurring along the SW African coast. Their origins and structures are associated with the opening of the South Atlantic Ocean during the breakup of Gondwanaland (Tamfu and Batupe 1995). The sedimentation phase in the DCSB consists of sequences laying unconformably the Precambrian basement, and ranging from Cretaceous formations to recent alluviums in the Wouri estuary. According to Mvondo (2010), three main units compose the sedimentary basin (Fig. 2):
Geological and hydrogeological settings of the superficial aquifer (in Regnoult 1986; modified)
-
The Late Cretaceous (locally named the Logbaba Formation) has a thickness ranging from 400 to 2000 m. This unit is composed from bottom to top by sandstones and clays, sometimes with intercalations of sands or rare occurrence of limestones and fossils shales. Manga (2008) attributed the sandstone formation of the Early Cretaceous (basal Moundeck Formation) to this unit.
-
The intermediate unit corresponds to Paleocene to Middle Eocene formations locally named the Nkappa Formations. This unit is composed of marls, shales, and calcareous sandstones, with a thickness varying from 300 to 500 m in the upper part and 1000 m in the lower part.
-
The top unit is characterized by:
-
Sediments of Eocene to Oligocene age (named the Souellaba Formation) which range in thickness from 400 to 600 m and are composed of shales, sands, clayed sands, sands and gravels, sandstones, and marls
-
Sediments of Miocene age (the Matanda Formation) comprise shales with a maximum thickness of 1000 m.
-
Sediments of Pliocene to Pleistocene age (locally named the Wouri Formation) comprise various volcanic and continental sedimentary rocks of 500 m thickness. Volcanic rocks of Miocene age consist of basalts, scoria, and volcanic clasts covering the basement and the sedimentary basin in the Mount Cameroon area (Njike 2004).
-
In this paper, we focus mainly on the Quaternary/Mio-Pliocene sediments (named the Wouri Formation), and the Oligocene/Upper Eocene sediments which form regionally important aquifers. Boreholes and dug-wells mostly tap these formations. The configuration of these formations has been reassessed from stratigraphic data using petroleum deep wells and boreholes drilled for water supply. The results presented in Fig. 3 indicate that Quaternary alluviums and Mio-Pliocene formations unconformably overlay the Oligocene/Upper Eocene formations after an intense erosion phase. The thickness of the Quaternary/Mio-Pliocene increases toward the SW part of the Wouri River reaching 70 m and is up to 200 m in the western part of the river. These formations consist of consolidated fine-to-coarse-grained sands and gravels mixed with silts and clays in various proportions (Mafany 1999). The alluvial sediments ranging in thickness between 50 and 60 m (Djeuda et al. 2001) are predominantly composed of quartz and kaolinite (Regnoult 1986; Takem et al. 2009).
These formations are covered by the topsoils identified by many authors (Aubert and Segalen 1966; Segalen 1967; Olivry 1986; Zogning 1987; Din 2001; Ndomè 2010; Ndomè et al. 2014), as consisting of ferralitic, hydromorphic to immature soils. The mineralogy of the ferralitic soils was investigated by Ndomè et al. (2014) who indicated that the dominant mineral is quartz followed by kaolinite, hematite, goethite, and traces quantities of illite, gibbsite, and anatase. In addition to these mineral phases identified above, Ngon Ngon et al. (2016) investigating the paleoenvironmental characteristics of iron duricrusts of the Douala basin, especially at Missolè and Bomkoul, found smectite, halloysite, chlorite, feranhydrite, calcite, ilmenite, muscovite, K-feldspar, gypsum, pyrite, and corundum in the Paleocene–Eocene formations. Below the Mio-Pliocene formation, the Eocene/Oligocene formations mainly consist of clayey sand and sand.
Hydrogeological context
Many authors (Dumort 1968; Martin 1979; Regnoult 1986; SNEC 1988) have previously investigated the hydrogeological system in the DCSB and have identified three major aquifers according to the geological setting. These are:
-
The top aquifer consisting of sands, gravels, sandy clayey/clay of Quaternary/Mio-Pliocene age of the Wouri Formation (Kamta Fotio 2000; Djeuda et al. 2001) occurs in the southern part of the basin. The Quaternary alluvium unit has a thickness between 0 and 60 m and is composed of fluvial and marine sands located mostly in the west (Bonaberi) and in the southeast (at the vicinity of the main river Wouri). Dug-wells mostly tap this unconfined aquifer which has a depth to the water table of less than 1 m in the vicinity of the Wouri River and up to 20 m in the northern part of the study area. Water discharges from this aquifer to many springs and contributes to the base flow of many streams and the Wouri River. The Quaternary/Mio-Pliocene sands aquifer has a maximum thickness of 70 m in the SE (East of the Wouri) (Fig. 3) and 200 m in the NW (on the western side of the Wouri River) and consists of sands, sandy clayey sands, and lenses of clays. Boreholes tapping the Mio-Pliocene formations are screened at different depth of 60–180 m, due to the multilayered characteristic of the aquifer and pumping rates up to 250 m3/h are recorded.
-
The intermediate semi-confined to confined aquifer consists of sand lenses, sandy clays, clayey sands and clays of Upper Eocene to Oligocene age. This aquifer was previously considered by many authors (Kamta Fotio 2000; Djeuda et al. 2001; Takem et al. 2009, 2015; Ketchemen 2011; Kopa et al. 2012; Tening et al. 2013; Feumba 2015; Ngo Boum et al. 2015) as belonging to the Mio-Pliocene aquifer in the SE part of the basin. In this area, most boreholes screened at depths greater than 70 m tap the Upper Eocene/Oligocene formations at the SE part of the basin.
-
The deep Paleocene sand aquifer consists of sediments of the Nkappa Formation and the basal sandstones of Moundeck Formation which are separated by low permeability sediments of the Logbaba Formation. This aquifer outcrops in the northeastern part of the basin and is only exploited in this area despite the high potential for its use beneath the city as evidenced by yield higher than 250 m3/h.
Materials and methods
Field investigations
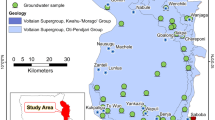
A total of 187 water samples were collected carried out during the campaigns carried out in February 2014 (dry season) and in December 2014 (end of rainy season) from a network of 4 streams (N = 21 samples), 13 springs (N = 20 samples), 38 dug-wells (N = 65 samples) and 40 boreholes (N = 81 samples) (Fig. 4). Springs and dug-wells samples represent the perched and shallow aquifer systems, respectively. The stream samples were collected during periods of base flow. Samples collected from boreholes tapping at depths between 20 and 180 m represent the Quaternary/Mio-Pliocene and the Eocene/Oligocene aquifers, respectively. In each sampling point, geographical coordinates (using a Garmin GPS Oregon 550t type receiver) and depth to the water table in dug-wells were first measured. Prior to sampling, in situ parameters such as electrical conductivity (EC), temperature, and pH were measured using a calibrated (by standard solution) multiparameter probe Consort C 931 type meter, after flushing by pumping the boreholes which were not operating.
General standard procedures for water sampling (purging boreholes) and preservation (cooled at 4 °C) were conducted. In each site, two sets of filtered samples were then collected in well rinsed 250-mL polyethylene bottles with one set acidified (1% v/v HNO3) for major cations and one unacidified set for anions analyses. Unfiltered and unacidified samples from selected sites were collected and cooled in tightly sealed 30 mL and 500 mL polyethylene bottles for stable isotopes (18O, 2H) and tritium analyses, respectively. Total alkalinity was measured by potentiometric titration with 0.16 N H2SO4 to a pH end point of 4.5 on the day of sampling. During the course of this study, no rainwater samples were collected, but existing rainfall isotope data for the period 2006–2014 of Douala station were obtained from the IAEA/GNIP website (http://www-naweb.iaea.org/napc/ih/IHS_resources_isohis.html#wiser).
Laboratory analyses
Chemical and isotopic analyses of the water samples were, respectively, performed at the Geology Department/University Cheikh Anta Diop (Dakar/Senegal) and at the Institute of Groundwater Ecology (Helmholtz Zentrum München/Germany). Major cations (Ca2+, Mg2+, Na+, and K+) were measured using an atomic absorption spectrophotometer and major anions (Cl−, SO42−, and NO3−) were analyzed using a visible–UV spectrophotometer type Jasco 7800. The accuracy was estimated from the ionic balance error which was within ± 10% for all the samples.
Stable isotopes of oxygen and hydrogen were determined using laser-based spectroscopy (Picarro, L2120-i). Both δ18O and δ2H values were determined relative to internal standards that were calibrated using the Vienna-Standard Mean Ocean Water (V-SMOW) standard. The data were normalized following Coplen (1988):
where RS represents either the 18O/16O or the 2H/1H ratio of the sample, and RV-SMOW is 18O/16O or the 2H/1H ratio of the V-SMOW.
The analytical reproducibility was ± 0.1 and ± 1.0‰ for the oxygen and deuterium measurements, respectively. Tritium analyses were performed by the electrolytic enrichment and liquid scintillation counting method (Thatcher et al. 1977), and values were reported in tritium units (TU).
The complete geochemical dataset including physical and hydrogeological characteristics is presented in tables available in Electronic Supplementary Material (ESM).
Statistical assessment
The statistical assessment of 187 groundwater samples was carried out using the hierarchical cluster analysis (HCA) method with the software package Statistica 7.1. All samples were considered in the analysis, and the statistical database was standardized to achieve the objective of a normal distribution and homogeneity. Euclidean distances were used as distances measured between the sampling sites, and Ward’s criterion is for the linkage rule. These two methods are recommended for geological studies due to the fact that they produce the most distinctive clusters (Davis 2002).
Results and interpretation
Hydraulic characteristics of the aquifers
In this study, only the depth of water table at dug-wells was measured and converted to head levels which were contoured to determine groundwater flow directions. Thus, the general groundwater flow patterns of the top Quaternary/Mio-Pliocene aquifer indicate E–W direction in the Eastern part of the Wouri estuary system (Fig. 5). Head values are as high as + 33 m in the east and decrease to + 1.8 m at wells located close to the Wouri River, suggesting that groundwater from the top aquifer drains into the river. A piezometric mound is also observed in the middle part of the system that corresponds to a topographic high point. In the western part of the system, measured heads are lower (between + 3.85 and + 8.55 m) with flow directions being toward the Wouri River. Transmissivity (T) and specific capacity (Qs) values measured during pumping tests of the top aquifer vary from 7 to 1641 m2/d and from 6.2 to 900 m3/d/m, respectively. For the Oligocene/Upper Eocene aquifer, the general NNE–SSW flow pattern is taken as only being indicative, since head values were not measured synoptically as boreholes were constructed at different times. These measurements indicate the semi-confined/confined characteristics of the Oligocene/upper Eocene aquifer and possible vertical flow exchange with the top aquifer in the southern part of the system. Transmissivity and specific capacity values obtained from pumping tests are slightly lower than those measured in the Mio-Pliocene aquifer and vary from 2.41 to 1555 m2/d and from 3.3 to 443 m3/d/m.
General hydrogeochemistry
For a better comprehension of the DCSB functioning, the chemical characteristics of streams, springs, unconfined/superficial Quaternary/Mio-Pliocene aquifer, and the Oligocene/Upper Eocene aquifer were investigated accordingly. In this project, water samples where analytical data indicated that charge balance errors were < 10% were assessed with regard to the order of abundance of major ions and were compared to the WHO (2006) water quality guidelines. These results were plotted on Piper diagrams to define their hydrochemical water types and to determine the likely geochemical processes responsible for their chemical composition.
Hydrogeochemical characteristics of surface water (streams)
Table 1 represents the descriptive statistics of the major hydrochemical parameters of surface water (streams) in the study area. Mean values of major ion concentrations are within acceptable ranges for the WHO drinking water guidelines. However, there are few cases where NO3− concentrations (58.0 mg/L) slightly exceed the WHO guideline value for this ion. The total hardness of the water, which ranges from 28 to 233.4 mg/L as CaCO3 (with a mean of 138.40 mg/L), indicates a high hardness value. The pH values range between 6.6 and 8.2 with a mean of 7.5 indicating circumneutral to slightly alkaline characteristics. The EC values range between 98.7 and 1538 µs/cm with a mean of 548.4 µs/cm, evidencing low-to-moderate mineralization. The order of abundance of major ions expressed in meq/L is Na+ > Ca2+ > K+ > Mg2+ and HCO3− > NO3− > Cl− > SO42− for cations and anions, respectively.
Water types for both sampling campaigns vary from mixed Ca/Na–HCO3/Cl, Na–Cl, Na–HCO3 to Mg–Cl. The variation is more pronounced in the cations triangle where scattered points shift from Na+ and Mg2+ poles for February samples set to mixed cations for December samples set. These trends are likely to have been derived from mixing of rainfall runoff associated with spring runoff in addition to local anthropogenic inputs (wastewaters poured in streams) as evidenced by elevated Cl− (mean 52 mg/L) and NO3− (58.02 mg/L) contents. The higher HCO3− contents (mean value of 171.3 mg/L) indicate that streams behave as an open system where there is equilibration of water with atmospheric CO2.
Hydrogeochemical characteristics of the unconfined aquifer (springs and dug-wells)
The shallow groundwater samples were collected from springs and dug-wells which had depths to the water table that ranged between 0.85 and 20 m. Spring waters represent perched groundwater in parts of the superficial aquifer system. Values of pH vary from 3.3 to 7.6 with a mean of 4.9, EC from 14.5 to 758 µs/cm with a mean of 263.8 µs/cm, and total hardness from 6.7 to 306.4 mg/L as CaCO3 with a mean of 63.0 mg/L (Table 2). The groundwater of this shallow aquifer is moderately soft with respect to the total hardness. However, few sampling points exhibit higher values of NO3− (263 mg/L), Na+ (157.8 mg/L), and Ca2+ (102.34 mg/L) contents. These latter values are from sites located near streams, where solid and liquid wastes are discharged by local residents of informal settlements zones in addition to seepage from latrine pits. The order of abundance of ions in this aquifer is Na+ > Ca2+ > K+ > Mg2+ and NO3− > HCO3− > Cl− > SO42− (in meq/L) for cations and anions, respectively.
The top phreatic aquifer samples exhibit various water types namely Na–Cl, mixed Na/Ca–Cl, Ca–HCO3 to mixed-HCO3 and to a lesser extent Na–HCO3. No clear trend appears for dug-wells samples; however, water types are mostly Na–Cl to mixed-Cl at the exception of P32 (Na–HCO3), P11–P16–P17 (Ca–HCO3), P15 (Ca–Mg–Cl) and P27 (Ca/Mg–SO4/Cl). The observed changes in water types between February and December sets of samples are likely to have been caused by mixing with recharging waters in addition to ion exchange and anthropogenic inputs. Conversely, spring waters collected from upstream areas in the basin (S1, S11, S12, and S13) display major changes in cations contents as compared to samples located downstream. In the downstream water samples, the Na+ content is higher, most likely due to longer travel time to reach downstream outflow where ion exchange proceeds to increase Na+ and decrease Ca2+ and Mg2+ in the solution. The mixed-Cl and Ca–HCO3 types found in the upstream springs, respectively, during the second and the first campaigns, may be partially derived through ion exchange and carbonate mineral dissolution reactions. It is worthwhile noting that the SO42− concentration is low in the spring samples (0.6–13.1 mg/L) and gypsum dissolution on the topsoil is unlikely to be taking place.
Hydrogeochemical characteristics of the semi-confined/confined aquifer
Groundwater samples in the semi-confined to confined Mio-Pliocene and Oligocene/Upper Eocene aquifers were collected from the boreholes tapping depths between 60 and 180 m. Table 3 shows measured and calculated parameters of these boreholes samples. The pH values range between 3.0 and 8.5 with a mean of 4.7. The EC is low ranging from 14.5 to 326 µs/cm with a mean of 82 µs/cm evidencing soft water relative to hardness as compared to WHO requirements. The relative abundance of the major ions in meq/L is Na+ > Ca2+ > Mg2+ > K+ and HCO3− > NO3− > Cl− > SO42− for cations and anions, respectively.
Boreholes tapping these two aquifers system are screened at different depths and they were accordingly classified into three categories to depict variations in water types. In this study, boreholes less than 70 m deep, boreholes more than 70 m deep, and boreholes with an unknown depth are considered. These latter constitute the majority of private wells for which lithostratigraphic logs aand technical design were not made available by owners. However, due to the low yield (water needs by owners), we can imagine that these boreholes tap mostly the Mio-Pliocene aquifer. Taking into account these considerations, the various water types obtained from samples were scrutinized with regard to screen depths. They reveal that boreholes with screen depths less than 70 m and those which have unknown screen depths, exhibit mostly Na/Ca–Cl water type with a small percentage of bicarbonate water types (Na–HCO3, Ca/Na–HCO3, Mg/Na–HCO3, and Ca–HCO3), whereas deeper boreholes (screened depth > 70 m) mostly exhibit mixed water types in both cations and anions. These samples may reflect mixed waters from the different depths, geochemical reactions, and also the effects of seepage through the borehole nest caused by poor construction practices.
Groundwater chemical variation and groundwater types
Conventional methods are used here to depict changes in ionic contents and water types which may occur in the system. Piper (Fig. 6) and bivariate diagrams were plotted according to sample types (streams, springs, dug-wells, and boreholes) and to which of the two campaigns that the sample was collected.
Due to the variability in ionic contents, water types and number of samples involved, the HCA dendrogram method was applied and four clusters (C1, C2, C3 and C4) were distinguished using a linkage distance of about 40 (Fig. 7). This phenon line was arbitrary set at higher linkage distance to minimize the number of clusters in more homogeneous groups. The dendrogram shows cluster couples C1/C2 and C3/C4 that are linked at a lower distance, indicating a similar relationship, while linkage at high distance between the two couples indicates dissimilarities. Table 4 summarizes details for each cluster relative to the main and minor types found, their hydrogeological conditions and median ionic concentrations.
The first cluster C1 (47 samples) is characterized by low mineralization (median value is 28 µs/cm), the lowest ionic contents of the samples (median values of all ion < 10 mg/L), and predominantly mixed water types (Fig. 8a). Samples in this group were mainly collected from shallow boreholes, hand-dug wells, and springs. The Stiff diagram exhibited for this group does not show any dominant ions and the lowest mineralization probably reflects recently infiltrated water and a short residence time in the aquifer. The C2 cluster which presents similarities with C1 exhibits ionic contents that are slightly higher for most ions and particularly Cl− (median value 14 mg/L), NO3− (median value 30 mg/L), and Na+ (median value 13 mg/L) as shown in the stiff diagram (Fig. 7b). This group typically consists of shallow boreholes, hand-dug wells, and springs, is Na–Cl water type, and probably reflects mineralization as waters migrate through the aquifer in addition to anthropogenic inputs. Cluster C3 represents water samples with the highest level mineralization (median EC values of 358 mg/L) and the highest ionic concentrations except for SiO2 (Fig. 8b). This group also contains a pollution signature as indicated by elevated NO3− and Cl− concentrations which have median values of 64 and 40 mg/L, respectively. This group, which is represented by water samples collected from hand-dug wells, streams, springs and shallow boreholes, is Na–Cl water type, and the pattern of its Stiff diagram differs from those of the other clusters because of the high concentrations of ions (the highest of all the groups). Water of this cluster probably reflects mineralization-caused geochemical reactions in the aquifer and anthropogenic inputs. Water in the cluster C4 is less mineralized (median EC value 215 µs/cm and of typically Na–Cl/HCO3) than C3, and is mostly encountered in deeper boreholes and a few surface water and shallow water samples. The high silica and bicarbonate ion contents together with the water type exhibited suggest that these groundwater samples are chemically more evolved and have a longer residence time from the recharge source to the confined aquifer. Hydrolysis of silicate minerals and to a lesser extent base ion exchange seem to be the dominant geochemical processes involved and responsible for the chemical changes of this group.
In order to further investigate the sources of the solutes responsible for the variations in water types and ionic contents, major ions concentrations and molar ratios are discussed relative to Cl−, SiO2, and HCO3− using conventional bivariate diagrams. Chloride is considered to behave conservatively, and its normalization to other chemical species is used to infer depletion or release of ions from/to the solution derived from geochemical reactions other than evapotranspiration and dilution induced by infiltrating water. Plots of major ions/Cl ratios relative to Cl− (Fig. 9) are high for Na/Cl (up to 5), Ca/Cl (up to 4.5), SiO2/Cl (up to 6), HCO3/Cl (up to 9), whereas values for Mg/Cl (up to 1.7) and K/Cl (up to 0.7) are low in water that has low EC values. The clusters C3 and C4 are more shifted toward increasing Cl− contents as compared to clusters C1 and C2 which are less mineralized. Conversely, there are no marked cluster trends shown in ionic ratios, except for SiO2/Cl, where C1 and C2 exhibit higher ratio values. Plots of major ions normalized against HCO3 and SiO2 contents suggest that silicate mineral weathering is the dominant process involved in C3 and C4 clusters as evidenced by their scatter patterns (Fig. 8a). Carbonate mineral dissolution (calcite, dolomite) reactions are also likely to occur since they release HCO3− to groundwater, although such a process is far less consistent (Fig. 10). The high concentrations of Na relative to Cl in the fresh groundwater may be derived from the weathering of Na-bearing silicate minerals such as albite which produce kaolinite and dissolved Na+ ions as reaction by-products (Eq. 1). This reaction would result in a Si/Na–Cl molar ratio of 2 as compared to values found in the Douala aquifer system which range from 0 to 165. Another source of Na+ may be from ion exchange reactions, but this is unlikely to be a significant process due to low pH values in the Douala groundwater system. Additionally, plots of (Ca + Mg)–(HCO3 + SO4) versus Na–Cl (not shown) do not show evidence of a negative linear regression line (− 1/1) which would be expected if this process was significant in the system.
Examination of the Ca + Mg versus HCO3 plot (Fig. 8) indicates a positive correlation with clusters below and above the 1:1 slope, suggesting processes in addition to the congruent dissolution of carbonate minerals are taking place in the aquifer. The high molar ratios of HCO3/Cl, Ca/Cl, and to a lesser extent Mg/Cl in the fresher groundwater, combined with increases in both Ca + Mg and SiO2 contents suggest that the weathering Ca–Mg silicate minerals such as K-feldspar, plagioclase, pyroxene, and amphibole (Eqs. 2, 3 and 4) are taking place, which would release both these ions in the solution under the acidic conditions that are present in the aquifer (pH less 5) (Appelo and Postma 2005). The high Ca + Mg/HCO3 ratio (more than 0.5) supports the occurrence of these processes under acidic conditions in groundwater.
Sulfate concentrations in groundwater are generally low except in a few samples of the top aquifer tapped by dug-wells and shallow boreholes. The mixed water types may be derived either from local pollution around well heads as evidenced by higher NO3− contents and perhaps gypsum dissolution in shallow sediments.
Overall, the hydrogeochemistry in the DCSB aquifer system is predominantly affected by silicate mineral weathering, carbonate mineral (dolomite, calcite) dissolution and, to a lesser extent, by ion exchange reactions. Weathering of plagioclase and, to a lesser extent, amphibole and pyroxene would be driven by the low pH and humid conditions in the Douala system as indicated by weathered products consisting of quartz, kaolinite, and accumulation of iron and aluminum oxyhydroxide minerals in the topsoil. The Gibbs diagrams plots (Fig. 11) using TDS versus Cl/Cl + HCO3 and TDS versus Na/Na + Ca indicate groundwater freshening processes from the rock dominance zone for C2, C2, C4 and the precipitation (rain) domain (low TDS) for C1 cluster.
Saturation indices and mineral stability diagrams
The degree to which groundwater is in equilibrium with minerals that are likely to be present in the aquifer matrix or have been produced as by-products of weathering was assessed by calculating saturation index (SI) values for a number of key phases.
The SI of a particular mineral can be defined as:
where IAP indicates the Ion Activity Product and K is the equilibrium constant of the mineral.
SI values near to zero are interpreted as mineral in equilibrium with solution and SI > 0 and SI < 0 provide information on whether the mineral is thermodynamically likely to precipitate and to dissolve, respectively. However, in this latter case, negative SI values may also mean the absence of a particular mineral in the aquifer matrix.
The results of the SI values computed using PHREEQC (Table 5) reveal that evaporite (anhydrite, gypsum, halite) and carbonate (aragonite, calcite, dolomite) minerals are undersaturated in all groups, suggesting that their soluble components are not limited by mineral equilibrium since these minerals are reactive. Conversely, silicate minerals such as quartz, chalcedony, and amorphous silica exhibit SI values at equilibrium to oversaturation and are prone to deposit. This latter process would limit increase in SiO2 solution to reach equilibrium to oversaturation. Thus SiO2 is likely derived from the hydrolysis of silicate minerals under acidic conditions which would release both cations (Na+, K+, Ca2+, Mg2+), H4SiO4, and HCO3− in addition to solid products. It is worthwhile noting that carbonate dissolution and ion exchange reactions contribute to changes in cation concentrations in groundwater. Investigating the sand aquifer matrix in Louga (Senegal), Faye (1991) found similar results where groundwater is saturated with respect to a number of silicate minerals at depth in the aquifer. However, in low-temperature systems, quartz does not precipitate directly from solution, but instead is deposited initially as an amorphous silica. These less crystalline phases are more prone to precipitate as amorphous silica. The weathering of the more reactive minerals in aquifer sediments would increase solute SiO2 concentrations to a level where groundwater becomes saturated with respect to amorphous silica and to possibly allow this phase to precipitate from solution.
Mineral stability diagrams are another approach to assess what chemical processes are taking place that affect the evolution of groundwater (Drever 1988). The graphical approach of the equilibrium state of groundwater is used here taking in account log a 2+Ca /a 2+H , log a +Na /a +H and log a +K /a +H against log \( a_{{{\text{H}}_{4} {\text{SiO}}_{4} }} \). The results of this assessment (Fig. 12) show that the bulk of groundwater groups of samples fall in the stability field of kaolinite except for a few that plot in the gibbsite field. It is noted that data cluster close to the quartz equilibrium constant of − 3.98, suggesting that silica is being precipitated. The predicted stability of kaolinite is consistent with the observed of the mineralogy of sediments in the Douala system. In fact, Ndomè et al. (2014) and Ngon Ngon et al. (2016), studying weathering processes and paleoenvironmental characteristics of iron duricrusts of Douala basin soils, proposed that these materials could be classified into three main groups: (i) siliceous materials characterized by high quartz content; (ii) aluminum-rich materials with predominance of Al2O3 in kaolinite, gibbsite, and illite; and (iii) iron-rich materials indicated by occurrence of goethite and hematite. In hot and humid conditions, silica is more soluble than alumina and iron, and the occurrence of gibbsite and hematite on the topsoil of Douala is an evidence of these processes.
Stability relationships among some minerals in the system K2O–Al2O3–SiO2–H2O. a Muscovite and its weathering products with respect to groundwater groups. b Albite and its weathering products with respect to groundwater groups. c Anorthite and its weathering products with respect to groundwater groups
Groundwater isotopic signature
Stable isotopes
Environmental stable isotopes (18O, 2H, 3H) are commonly used in regional groundwater studies to identify flow regimes, sources, and mechanisms of recharge, mixing process, and relative residence times of water in an aquifer (Fritz et al. 1979; Das et al. 1988; Leontiadias et al. 1988). Frequently, groundwater retains its stable isotopic signatures unless diluted or mixed with waters of different isotopic compositions (Fontes 1980; Gat 1981). Therefore, water from different sources or those that are exposed to different processes, such as evaporation and/or mixing, often acquires identifiable isotopic contents which serve as natural tracers. In addition to the stable isotopes, 3H can be used to evaluate different ages of water molecules under conditions where the concentration of this isotope is not significantly affected by reactions other than radioactive decay or mixing. Detectable 3H concentrations in groundwater indicate either that recharge has taken place after the period of atmospheric nuclear bomb tests (1952–1953) or that mixing has taken place between recent tritiated water and water recharged prior to the 1950s.
In this study, stable isotope signature of surface water (streams), phreatic aquifer (springs and dug-wells) and semi-confined/confined aquifers (boreholes) were considered in addition to 3H contents in some selected sampling points (Fig. 13).
Isotopic composition of rainwater
Records of stable isotope data in precipitation were obtained from the GNIP-IAEA website for 84 monthly bulk samples, collected between July 2006 and December 2014 at the Hydrac-Douala site (altitude 18 m). Table 6 summarizes statistical values of the isotopic contents together with climatic parameters which show that air temperature and vapor pressure are in a relatively narrow range compared to rainwater quantity (Fig. 14).
Plotted on a conventional δ18O versus δ2H graph, the isotopic data have a linear trend where the line of best fit is given by equation: δ2H = 7.3 δ18O + 11.4 (R2 = 0.92). This trend appears to be close to the GMWL defined by Graig (1961) and to those given by Ketchemen (2011) and Nlend (2015); which are, respectively, δ2H = 8.17 δ18O + 12.74 (June 2006 to December 2009) and δ2H = 8 δ18O + 11.4 (June 2006 to December 2012). However, the slightly lower slope of 7.3 and intercept of 11.4 as compared to the GMWL (Graig 1961) are likely to have been derived from the effects of local climatic conditions such as the origin of vapor mass, re-evaporation during rainfall and the seasonality of precipitation (Clark and Frirtz 1997). The lower slope of the line indicates the occurrence of secondary evaporation which is more pronounced in the lighter rains.
Isotopic composition of surface water
The δ18O and δ2H isotope composition of streams samples ranges from − 4.0 to − 2.0‰ and from − 15 to − 9‰ with respective averages of − 2.9 and − 11‰. The depleted δ18O values are measured upstream, while downstream, enriched δ18O signature is observed. The increase in evaporation through the flowpath from upstream to downstream, especially during the dry season, could be the main factor for this enrichment. In Fig. 11, the isotopic data trend deviates from the meteoric line indicating that samples had undergone some degree of evaporation as a result of non-equilibrium kinetic process fractionation. However, the average isotope values are close to the rainwater values.
Stable isotopic composition of groundwater
Unconfined aquifer
Samples collected from springs and dug-wells were considered to represent the top phreatic Quaternary sand aquifer of the Douala system. Stable isotope values measured in groundwater from this aquifer range from − 2.2 to − 3.5‰ for δ18O and from − 7 to − 16‰ for δ2H with respective means of − 2.8 and − 11‰. The spring signatures exhibit a narrow range (− 2.6 to − 3.1‰ for δ18O and − 10 to − 12‰ for δ2H). Plotted on a conventional δ18O versus δ2H diagram, the scattered points slightly deviate from the GMWL indicating fractionation has taken place that is likely to have been caused by a combination of local processes such as surface water contribution, direct infiltration, and some degree of fractionation both on the ground surface and within the unsaturated zone (Adomako et al. 2011). It is worthwhile noting that mean signature values of the unconfined aquifer are close to mean values derived from isotopic records of monthly rainfall from 2006 to 2014 as well as values in surface water.
Semi-confined/confined aquifer
Samples collected from boreholes represent different depths from 21 to 88 m. Measured isotopic data range from − 2.6 to − 4.5‰ for δ18O and from − 10 to − 18‰ for δ2H with respective means of − 3.9 and − 15‰. Plotted against the GMWL, data scatter above and parallel to the reference line and display a bulk depleted trend except for two boreholes. Their signatures are more depleted than the mean local rainfall isotopic values and the monthly bulk rainfall isotopic data recorded. This indicates that evaporation can be neglected and the confined groundwater was either recharged at higher elevations, i.e., under an equatorial climate with rainfall > above 4000 m (Ketchemen 2011), or old infiltrated water occurring probably during a cooler and humid period in the past which has mixed with leakage from the phreatic aquifer.
Tritium
Concentrations of 3H in groundwater system depend primarily on the initial atmospheric input at the time of recharge and the radiogenic decay that had occurred since infiltration. 3H is an invaluable tracer for water flow and can give an indication of the relative age of waters on a timescale of 60 years before present and also mixing process between different water bodies. In this study, the tritium content of rainfall samples was not measured, but values in present-day rainwater approach background atmospheric level of 1–2 TU which is the reflection of the depleted 3H concentrations in the northern hemisphere.
The measured tritium values in surface water and the phreatic aquifer ranged from 0.8 to 1.9 TU (mean 1.76 TU) and from 0.9 to 2.3 TU (mean 1.3 TU), respectively. These values indicate that recharge to the phreatic aquifer is of recent age and is taking place under current climatic conditions. Groundwater samples from the unconfined aquifer that contains tritium were generally located near the recharge zones in the NE of the study area. Conversely, samples collected from boreholes tapping the semi-confined/confined aquifer exhibited lower tritium contents from below the detection limit (< 0.7 TU) to 1.3 TU. These values indicate the groundwater had received recharged prior to the atmospheric bomb tests (i.e., before 1953) but there has been locally mixing with more recent water, probably through leakage through the annular spaces around the casing of poorly-constructed boreholes tapping this aquifer. The lighter stable isotope contents as compared to the phreatic aquifer and the bulk present-day rainfall content together with lower 3H content are consistent with recharge during a humid phase and perhaps also a lower temperature than in the current climate. Unfortunately, no 14C data exist in groundwater to determine the age of groundwater in this aquifer.
Discussion
The tentative conceptual model developed below takes into account the groundwater flow dynamics in the different aquifers, the different geochemical processes identified in this context of a humid climate and acidic rainfall as well as the isotopic signatures of the waters sampled in this study (Fig. 12). On the basis of this information, it is considered that the evolution of groundwater quality in the area takes place in four steps. These are as follows.
Step 1
The infiltration of acidic rainwater in the Douala area quickly leads to the total congruent dissolution of carbonate minerals and silicate minerals. Under these conditions, the weathering of unstable silicate minerals in soil (olivine, Ca-feldspar, K-feldspar, albite, pyroxene) is likely to release Ca, Mg, and Na/K (Goldich 1938) into solution that would lead to groundwater with a chemical composition dominated by (Ca–HCO3). During the weathering processes, cations and balancing bicarbonate ions are released into solution, and new alumino-silicate minerals such as kaolinite and smectites are formed (Garrels 1984). Under the humid tropical conditions, minerals such as kaolinite, goethite, hematite, and quartz are the dominant minerals that form in lateritic soils and weathering mantles, while gibbsite and boehmite occur in bauxites and ferrallitic soils (Trolard and Tardy 1989; Ndomè et al. 2014; Ngon Ngon et al. 2016). This pedogenetic process (presumably albitization) as identified by Tardy (1971) and Garrels (1984) in a similar geographical context is mostly associated with calcite dissolution occurring in the top well-drained soil zones by modern infiltrated rainfall (3H, δ18O, δ2H) (flow pattern 1). Evaporite minerals (gypsum, halite, anhydrite) may also dissolve in this humid context, but the lower SO42− contents and negative SI values are consistent with the absence of these mineral phases. The open nature system of phreatic aquifer and local pollution may lead to other water types such as (Ca–HCO3/Cl and even Na–Cl).
Step 2
This stage occurs during vertical flow from surface sediments through the unsaturated zone down to the unconfined and semi-confined aquifer under modern climate conditions. The chemical processes outlined in Step 1 continue in shallow groundwater near the water table where the dissolution calcite and the weathering of silicate minerals are more pronounced. Higher dissolved SiO2 together with unsaturated conditions with respect to amorphous silica and chalcedony seems to be controlled by the weathering of silicate minerals under acidic conditions which would add HCO3− contents in the solution. Studying pedologic profiles in the similar context Tardy (1971) and Ndomè et al. (2014) found that bisiallitization (leading to neoformation of clay mineral such as montmonrillonite and to a full release of cations) occurs as a main pedogenetic process characterized by occurrence of kaolinite as weathering product and to a lesser extent hematite. Besides these processes, ion exchange reactions may occur leading to a mixed water type.
Step 3
This step is marked by water type transition from (Ca–Mg–HCO3) to (Na–HCO3) during horizontal flow in the confined Oligocene/Upper Eocene aquifer (flow pattern 3). Silicate weathering processes associated with base-exchange reactions are likely to be responsible for this evolution. The decrease in SiO2 concentrations and positive SI values for silica minerals suggest that the precipitation of silica is taking place. Groundwater samples from this group are mostly collected from deeper boreholes where isotopic data is submodern in age. Other water types found in this group are located mainly in the recharge zone where Ca–Mg–HCO3 evolves to mixed cations-HCO3 then to Na–HCO3.
Step 4
The transition from Na–HCO3 to Na–Cl, in the confined Oligocene/Upper Eocene aquifer tapped by many boreholes features this step. Ion exchange reactions seem to be the dominant process during horizontal flow (flow pattern 3) as evidenced by occurrence of Na–Cl, mixed cations-Cl water types. The high NO3− content and Na–Cl water type of this group are associated with spring samples collected from the unconfined aquifer.
Summary and conclusions
This study presents a comprehensive regional hydrogeochemical investigation aimed at identifying the main geochemical processes responsible for controlling the evolution of groundwater quality in aquifers in the region. Conventional hydrogeochemical methods have been used to classify groundwater into compositional types, namely Na–Cl, Na–HCO3, Ca–HCO3 and mixed type and their hydrogeological conditions of occurrence. Ionic molar ratios relative to Cl−, HCO3−, and SiO2 in bivariate diagrams suggest that the incongruent weathering of silicate minerals is likely to be the dominant process releases Na+, Ca2+, HCO3−, and SiO2 into solution. The dissolution of carbonate minerals and ion exchange reactions concomitantly occur but these are likely to be less important under the hydrogeological and climate conditions present in the study area than silicate weathering. This is suggested by the Gibbs diagram plots which indicate a groundwater freshening processes from rock-dominant zone. Computed SI values of possible mineral phases together with mineral stability diagrams suggest that the weathering of silicate minerals in soils and aquifer sediments should produce kaolinite and the precipitation of amorphous silica. The stable isotope signature (18O, 2H) of the surface water and shallow groundwater exhibits some degree of evaporation as a result of non-equilibrium kinetic fractionation and/or mixing process, as opposed to deep groundwater which values are more depleted and aligned parallel to the reference GMWL. Groundwater from the semi-confined/confined aquifer contains lower 3H contents below detection limit, which indicates that they do not currently receive direct rainfall recharge, but may still receive slow leakage from an overlying aquitard.
On the basis of the information collected during this investigation, a conceptual model was developed comprised of four steps that consider the hydrogeological conditions in the Douala aquifer system. The principal geochemical processes affecting pore-water quality in the unsaturated zone and in shallow groundwater (Steps 1 and 2) are considered to be the weathering of silicate minerals by the infiltration of acidic rainfall with minor dissolution of carbonate minerals and ion exchange reactions. These steps allowed release of solute cations and SiO2 as well as balancing bicarbonate and kaolinite formed as new product. The dominant processes in Step 3 and step 4, representing flow in the semi-confined and the confined aquifer, are considered to be the weathering of silicates associated with base ionic exchanges and amorphous silica precipitation which would lead to Na–HCO3 and Na–Cl water types occurrence. The results of this study will enable a better comprehension thus a better management of this valuable groundwater resources where there are competing demands to guarantee sustainability.
References
Adomako D, Osae S, Akiti TT, Faye S, Maloszewski P (2011) Geochemical and isotopic studies of groundwater conditions in the Densu River of Ghana. Environ Earth Sci 62:1071–1084
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Taylor & Francis Group, Amsterdam, p 647
Aubert G, Segalen P (1966) Projets de classification des sols ferrallitiques. Cahiers Office de la Recherche Scientifique et technique Outre-Mer, série Pédologie IV
Clark I, Frirtz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, Boca Raton, p 328
Comte JC, Cassidy R, Obando J, Robins N, Ibrahim K, Melchioly S, Mjemah I, Shuri H, Bourhane A, Mohamed I, Noe C, Mwega B, Makokha M, Join JL, Banton O, Davies J (2016) Challenges in groundwater resource management in coastal aquifers of East Africa: investigations and lessons learnt in the Comoros Islands, Kenya and Tanzania. J Hydrol Reg Stud 5:179–199
Coplen TB (1988) Normalization of oxygen and hydrogen isotope data. Chem Geol 72:293–297
Das BK, Kakar YP, Moser H, Sticher W (1988) Deuterium and oxygen-18 studies in groundwater of the Dehli area, India. J Hydrol 98:133–146
Davis JC (2002) Statistics and data analysis in geology, 3rd edn. Wiley, New York
Din N (2001) Mangrove du Cameroun, statut écologique et perspectives de gestion durable. Thèse de Doctorat d’Etat es Sciences. Option Ecologie Végétale, Faculté des Sciences, Université de Yaoundé I, p 252
Dinka MO, Loiskandl W, Ndambuki JM (2015) Hydrochemical characterization of various surface water and groundwater resources available in Matahara areas, Fantalle Woreda of Oromiya region. J Hydrol Reg Stud 3:444–456
Djeuda THB, Tanawa E, Ngnikam E (2001) L’eau au Cameroun. Tome I: Approvisionnement en eau potable. Collection Connaissances des Presses Universitaires de Yaoundé, p 359
Drever JI (1988) The geochemistry of natural waters. Surface and groundwater environments. Prentice Hall, New Jersey, p 436
Dumort JD (1968) Notice explicative sur la feuille Douala-Ouest carte géologique de reconnaissance (1/500 000). Publication Direction des Mines et de la Géologie du Cameroun, p 69
Faye S (1991) Geochemical processes occurring in the unsaturated zone of a quaternary sandstone aquifer (Louga-Senegal), Master of Sciences thesis, University of Leeds, UK, p 103
Feumba R (2015) Hydrogéologie et évaluation de la vulnérabilité des nappes dans le bassin versant de Bessekè (Douala-Cameroun). Thèse de Doctorat PhD, Département de Sciences de la Terre, Faculté des Sciences. Université de Yaoundé I, p 226
Fontes JC (1980) Environmental isotopes in groundwater hydrology. In: Fritz P, Fontes JC (eds) Handbook of environmental isotope geochemistry. Elsevier, Amsterdam, pp 75–140
Fritz P, Hennings CS, Suzuki O, Salari E (1979) Isotope hydrology in northern Chile. In: Isotope hydrology (1978). IAEA, Vienna, pp 525–544
Garrels RM (1984) Montmorillonite/Illite stability diagrams. Clays Clay Miner 32(3):161–166
Gat JR (1981) Groundwater In: Gat JR, Gonfiantini R (eds) Stable isotopes in precipitation. The GNIP database, Release 3, Oct 1999. http://www.iaea.org/programs/ri/gnip/gnip-main.htm
Goldich SS (1938) A study in rock weathering. J Geol 46:17–56
Graig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Kamta Fotio GA (2000) Contribution à l’étude hydrogéologique du bassin de Douala: Essai de corrélation des niveaux captés dans l’aquifère quaternaire. Mémoire de maîtrise Université de Douala, p 36
Ketchemen TB (2011) Déterminants hydrogéologiques de la complexité du système aquifère du bassin sédimentaire de Douala (Cameroun). Thèse doctorat d’Etat, Faculté des Sciences et Techniques, Univ Cheick Anta Diop de Dakar, p 211
Kopa NKA, Likeng HDJ, Nono A (2012) Hydrodynamique et qualité des eaux souterraines dans le bassin sédimentaire de Douala (Cameroun): Cas des aquifères sur formations quaternaires et tertiaires. Int J Biol Chem Sci 6(4):1874–1894
Leontiadias IL, Payne BR, Christodoulou T (1988) Isotope hydrology of the Aghios Nikolaosarea of Crete, Greece. J Hydrol 98:121–132
Mafany GT (1999) Impact of the geology and seawater intrusion on groundwater quality in Douala. M.Sc. Thesis. Buea University Cameroon, p 100
Manga CS (2008) Stratigraphy, structures and prospectivity of the southern onshore Douala basin Cameroon-Central Africa. Cameroon and neighbouring basins in the Gulf of Guinea. Petroleum geology, tectonics, geophysics, paleontology and hydrogeology. Afri Geosci Rev Spec Publ 1–2:14–37
Martin G (1979) Alimentation en eau de la ville de Douala à partir des eaux souterraines. Etudes et travaux. Rapport BRGM, 79 AGE 021, p 145 + annexes
Mukherjee A, Saha D, Harvey CF, Taylor RG, Ahmed KM, Bhanja SN (2015) Groundwater systems of the Indian subcontinent. J Hydrol Reg Stud 4:1–14
Mvondo OF (2010) Surrection cénozoïque de l’Ouest de l’Afrique à partir de deux exemples: le plateau sud namibien la marge nord camerounaise. Thèse de Doctorat PhD, Université de Rennes 1, p 309
Ndomè EPE (2010) Minéralogie, géochimie et applications géotechniques des produits d’altération sur roches sédimentaires de Douala. Thèse de Doctorat PhD, Département des Sciences de la Terre, Faculté des Sciences, Université de Yaoundé I, p 210
Ndomè EPE, Onana VL, Boubacar L, Kamgang KBV, Ekodeck GE (2014) Relationships between major and trace elements during weathering processes in a sedimentary context: Implication for the nature of sources rocks in Douala, Littoral Cameroon. Chemie der Erde-Geochemistry, p 17
Ngo Boum NS, Ketchemen TB, Ndje Y, Emvoutou H, Ebonji CR, Huneau F (2015) Origin of mineralization of groungwater in the Bassa watershed (Douala-Cameroon). J Hydrogeol Hydrol Eng 4:1–9
Ngon Ngon G, Etamè J, Ntamak-Nida MJ, Mbesse CO, Mbai JS, Bayig EC, Gerard M (2016) Geochemical and paleoenvironmental characteristics of Missolè I iron duricrusts of the Douala sub-basin (Western Cameroon). CR Geosci 348:127–137
Njike NPR (2004) Palyno-stratigraphie et reconstitution des paléo-environnements du Crétacé de l’Est du bassin sédimentaire de Douala (Cameroun). Thèse de Doctorat d’Etat, Faculté des Sciences, Université de Yaoundé I, p 259
Nlend YB (2015) Signature isotopique des précipitations à Douala—Cameroun (Station GNIP Douala—HYDRAC, No 6491001). Département de Géologie. Faculté des Sciences. Université de Douala, p 46
Olivry JC (1986) Fleuves et rivières du Cameroun. Paris: Collection “Monographies hydrologiques ORSTOM”, p 781
Regnoult M (1986) Synthèse géologique du Cameroun. Ministère des Mines et de l’Energie du Cameroun. Yaoundé, p 119
Segalen P (1967) Altération des minéraux primaires, synthèse des minéraux secondaires au cours de la ferrallitisation. Les sols ferrallitiques. Cah ORSTOM Séri Péd 4(4):5–13
SNEC (1988) Rapport technique sur les forages de Massoumbou, Société Nationale des Eaux du Cameroun
Takem EG, Ayonghe SN, Thambidurai P (2009) Pollution characteristics of alluvial groundwater from springs and bore wells in semi-urban settlements of Douala, Cameroon, western Africa. Environ Earth 61:287–298
Takem EG, Chandrasekharam D, Ayonghe NS, Thambidurai P (2010) Pollution characteristics of alluvial groundwater from springs and bore wells in semi urban settlements of Douala, Caleroon, Westhern Africa. Environ Earth Sci 61:287–298
Takem GE, Kuitcha D, Ako AA, Mafany GT, Takounjou FA, Ndjama J, Ntchancho R, Ateba BH, Chandrasekharam D (2015) Acidification of shallow groundwater in the unconfined sandy aquifer of the city of Douala, Cameroon, Western Africa: implication four groundwater quality and use. Environ Earth Sci 74:6831–6846
Tamfu S, Batupe M (1995) Geologic setting, stratigraphy and hydrocarbon habitat of the Douala basin (Cameroon). Natl Hydrocarb J Cameroon 3:6
Tardy Y (1971) Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs. Chem Geol 7:253–271
Tening AS, Chuyong GB, Azinwie AG, Ambo FB, Likowo LL, Ketchemen TB (2013) Nitrate and ammonium levels of some water bodies and their interaction with some selected properties of soils in Douala metropolis, Cameroon. Acad J AJEST 7:648–656
Thatcher LL, Janzer VJ, Edwards RW (1977) Methods for determination of radioactive substances in water and fluvial sediments. Techniques of water resources investigations of the US Geological Survey US Government Printing Office, Washington, pp 79–81
Trolard F, Tardy Y (1989) A model of Fe3+-kaolinite, Al3+-hematite, Al3+-hematite equilibria in laterites. Clays Miner 24(1):21
WHO (2006) Guidelines for drinking-water quality, third edition, volume 1—recommendations. World Health Organization, Geneva
Zogning A (1987) Les formations superficielles latéritiques dans la région de Douala: morphologie générale et sensibilité aux activités humaines. In : Séminaire régional sur les latérites : sols, matériaux, minerais : sessions 1 et 3. Paris: ORSTOM, pp 289–303
Acknowledgements
The first author is very grateful to the financial support from the French cooperation (SCAC) and UNESCO-ANESIVFW. She also thanks the UCAD hydrochemistry laboratory and the Institute of Groundwater Ecology (Helmholtz Zentrum München/Germany) for carrying out the analyses. She would like to thank Prof. Pape Malick NGOM, University Cheikh Anta Diop, Dakar, for reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Emvoutou, H.C., Ketchemen Tandia, B., Ngo Boum Nkot, S. et al. Geologic factors controlling groundwater chemistry in the coastal aquifer system of Douala/Cameroon: implication for groundwater system functioning. Environ Earth Sci 77, 219 (2018). https://doi.org/10.1007/s12665-018-7400-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7400-z