Abstract
Delta State of the Niger Delta, Nigeria, is an oil exploration and production region that is characterized by huge revenue generation but with its attendant waste generation and oil spillage that impact the environment. The variability in the hydrochemical characteristics, hydrochemical controlling processes and quality in space has been investigated. The pH of the groundwater samples ranged from slightly acidic to slightly alkaline nature. Biological oxygen demand and chemical oxygen demand of the coastal area are higher than those of the inland area indicating more domestic and industrial contamination. Total dissolved solid values across the region indicated fresh and brackish water for the coastal area and fresh water only for the inland area. The orders of abundance of the cations and anions for the coastal and inland areas are Ca>Mg>Na>K/Cl>HCO3>SO4>NO3 and Na>Ca>K>Mg/HCO3>Cl>NO3>SO4 respectively. A Piper diagram identified four hydrochemical facies, namely CaHCO3, NaHCO3, NaCl and CaMgClSO4. Ionic cross plots and correlation matrix revealed that the groundwater chemistry of the inland area is predominantly influenced by silicate weathering and ion exchange processes while those of the coastal area are influenced by silicate weathering, ion exchange processes and seawater tidal flushing. The groundwater from the coastal area is more polluted by heavy metals than those from the inland area. The observed variability may be attributed to effects of industrial wastes and exploration activities. In terms of water quality for domestic and irrigation, the groundwater of the coastal and the inland areas are not generally potable and suitable as drinking and irrigation water sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Access to secure, safe and sustainable source of fresh water is a fundamental requirement for the survival, well-being and socio-economic development of all humanity. About 97% of the water on earth is in the oceans and is too salty to be used for drinking, agriculture and manufacturing. Only about 3% of the Earth’s water is fresh. Yet, the majority of this is not easily accessible and constantly being threatened by both natural and anthropogenic environmental pollutions. The Niger Delta, by nature, is susceptible to both natural and artificial environmental impacts. The proximity of its coastline to the Atlantic Ocean makes it highly prone to saline water incursion. The existing oil fields and producing oil well heads in the area make it highly susceptible to anthropogenic environmental impact.
The Niger Delta is the hub of oil exploration and production in Nigeria. The amount of oil produced and transported between points of production, processing and distribution or export terminals has greatly increased as the demand for and dependence on oil increased. Although this increase in oil production level contributes to the national economic growth, it also presents increased potential for environmental pollution and degradation. It has been observed that thousands of barrels of oil have been spilled into the environment through oil pipelines and storage facilities failure (Awobajo 1981; Ajakaiye 2008; Aroh et al. 2010).
Apart from exploration and production of crude oil in Nigeria which can greatly impact groundwater, the rate of urbanization characterized by high population concentration, increasing industrial and agricultural activities coupled with environmental pollution/degradation and indiscriminate disposal of all kinds of wastes are perceived to pose serious pollution threats with all its concomitant health hazards on groundwater quality especially in urban areas (Kehinde 1998; Adelana et al. 2005; Ocheri 2006; Adelana et al. 2008; Eni et al. 2011).
The shallow character and high permeability of the coastal plain-sand aquifer of Niger Delta make the groundwater system highly vulnerable to contamination (Amadi and Olasehinde 2009). According to Amadi (2007), the coastal plain-sand aquifer in Niger Delta is characterized by shallow water table, high porosity and hydraulic conductivity, which makes the aquifer highly prolific quantitatively but highly vulnerable qualitatively. The strategic position of the Niger Delta in the socio-economic activities of Nigeria makes it imperative to have a good knowledge of the groundwater pollution status in the area. A good understanding of the groundwater pollution scenario of the area will help in economic planning, management and development of groundwater resources as the main source of freshwater supply. This is what the present study tends to achieve.
Study area: description, geology and hydrogeology
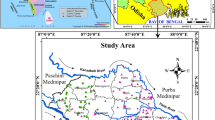
The study area, Delta State (Fig. 1), is an oil- and agricultural-producing state in Nigeria, situated in the South-South geo-political zone of Nigeria. The State has a total land area of 16,842 km2 (6503 mi2), the southern flank is the Bight of Benin on the Atlantic Ocean which covers about 160 km of the state’s coastline. Delta State is generally low-lying without remarkable hills. The state has wide coastal belt inter-lace with rivulets and streams, which form part of the Niger Delta. The state is located within the Niger Delta with the coordinate description of 5° 00′ E to 5° 30′ E and 5° 00′ N to 6° 30′ N.
Delta State is situated in the tropics and therefore experiences a fluctuating climate, ranging from the humid tropical in the south, to the subhumid in the northeast. The temperature condition in the study area (Table 1) is generally high with an overall mean daily value of 30.0 °C, mean maximum of 32.5 °C and mean minimum of 20.0 °C respectively. Rain falls throughout the year in form of precipitation. Over 80% of the rainfall is received in the month of April–October (Table 1). This period usually, records high-intensity rainfall, flooding and storms. However, during the months of October and early November, rainfall especially thunderstorm may be induced by conventional activities.
Relative humidity in the study area is high with mean daily value of 82.6%. Maximum relative humidity of 100% was recorded in some days while minimum relative humidity was 50%. Apart from January and February (peak dry months), other months experienced relative humidity above 72.5%. Relative humidity values were slightly higher during the rainy season months than the dry season. The vegetation varies from the mangrove swamp along the coast to the evergreen forest in the middle and the savannah in the northeast.
Drainage generally is limited by its flat topography resulting in swamps being developed in the lower areas and waterlogging of adjourning lands during the rainy reasons. In the central and southern part of the state, watersheds showed complex morphologic features. These watersheds were drastically different from those in the northern. In the southern areas, parts of the surface drainage system include water-filled depressions, waste pits, burrow pits and ponds that contribute to the drainage and surface runoff only at high stages. However, such water held in depressions above the water table recharges the groundwater. Major hydrologic effects include creating more local, shallow groundwater flow systems and increasing recharge to the groundwater systems among others. In the northern part of the state watershed, groundwater flow is predominantly from recharge areas in the uplands between streams toward discharge area along the streams (Table 1). The diverse topography and scattered depressions (burrow pits, ponds and waste pits) at the southern areas create patterns of groundwater flow that are more localized than those at the northern areas. Recharge is from infiltration of precipitation through the soil and percolation from water-filled depressions above the water table. Groundwater commonly flows to areas of discharge along streams, wetlands or lakes that are at lower altitudes but not necessarily within the watersheds.
Delta State falls within the Niger Delta Basin (Short and Stauble 1967). The basin is an extensive continental margin basin situated in the Gulf of Guinea built out into the Central South Atlantic Ocean at the mouths of the Niger-Benue and Cross River systems during the Eocene (Hosper 1971). It is an arcuate delta that is wave-dominated and tidally influenced sand bodies whose thickness may be influenced by growth faulting (Avbovbo and Ayoola 1981).
Materials and methods
Field data collection, field measurements and laboratory analyses
A total of 150 groundwater samples, 110 samples from the coastal area and 40 samples from the inland area were collected from existing monitoring hand-dug wells and boreholes across the study area. Groundwater samples were collected following the procedure described by Omonona et al. (2013). All physical parameters measurements and chemical analyses followed standard guidelines (APHA 2005). Samples were collected in prewashed polystyrene bottles after pumping the wells for about 30 min and filtered through 0.45-μm-membrane filter paper to avoid contaminations. The samples for cations and heavy metal analysis were preserved by acidifying them with concentrated HNO3 to a pH < 2 and stored at 4 °C.
Physical parameters which are transient in nature were measured in situ. These parameters included pH, temperature, colour, turbidity, total dissolved solid (TDS) and electrical conductivity. Temperature, pH, EC and TDS were measured using a HANNA multi-meter hand-held equipment (HI 9835) while colour and turbidity were determined with Hach DR/2000 spectrophotometer. Sodium (Na+), calcium (Ca2+), magnesium (Mg2+), potassium (K+), iron (Fe2+), lead (Pb), nickel (Ni) and cadmium (Cd) were analysed using an atomic absorption spectrophotometer (Varian AAS, 680FS). Bicarbonate (HCO−3), Chloride (Cl−), nitrate (NO−3) and sulphate (SO42−) were analysed using the colorimetric method with UV spectrophotometer (WPAS110). All the laboratory analyses were carried out at the Petroleum Analysis Laboratory (PAL) of the Petroleum Training Institute both laboratories located at Effurun, Delta State, Nigeria.
Statistical data analyses
Statistical data analysis, descriptive analysis (DA) and correlation analysis were carried out on the field tests and laboratory tests results. The data were first standardized before they were statistically analysed to correct the effects of varied range of measurements of the various parameters and differences in the units of measurements. All the statistical analyses were carried out using Statgraphics centurion while all graphical plots were created using Aquachem 2011 1.14 version.
Heavy metal enrichment index
HEI is an index that gives the overall quality of groundwater with respect to multiple heavy metal contaminations and it is used for rapid interpretation of the degree of pollution (Krzysztof et al. 2003; Prasanna et al. 2012). HEI was computed as follows:
where Si is the standard permissible concentration, Mi is the concentration value of the monitored heavy metal and n is the number of the examined heavy metals.
Irrigation water quality indices
A total of seven indices were employed to evaluate the present status of the groundwater of the area for irrigation purposes. Total hardness (TH), sodium percentage (%), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), magnesium hazard (MH), permeability index (PI) and Kelley’s ratio (KR) were calculated using their respective equations (Eqs. 2–8). All the parameters used in the equations were expressed in the milli-equivalent unit of measurement except for parameters in Eq. 1 that were expressed in mg/l.
TH was estimated using the relation (Todd 1980; Ragunath 1987):
Na% was computed as Todd (1980):
The SAR was estimated using the following equation (Richards 1954; Hem 1991):
RSC was calculated using the expression (Gupta 1983):
MH was computed as (Ragunath 1987):
Doneen (1964) expressed PI as:
Finally, Kelly’s ratio (KI) was computed using the relation (Kelley 1940):
Geostatistical approach
A geostatistical approach which entails variogram and kriging has been extensively used in groundwater and hydrological studies with attendant success. An extensive description of the method has been presented in Liang et al. 2016 and Jang et al. 2016. Geostatistics provides a variogram of data with a statistical framework which can be used to quantify the spatial variability of random variables between two locations (Liang et al. 2018 and Liang et al. 2017). The semivariogram, γ(h), is defined as follows:
where h denotes the lag, Z(xi) is the value of the regional variable of interest at location xi, and Z(xi + h) is the value of the regional variable of interest at location xi + h, N(h) is the number of pairs of sampling points separated by h. In practices, the probability of the distance between the sampling pairs being exact is low, and thus, h is represented by a distance interval.
Kriging is regarded as an optimal spatial interpolation method in which the values of the random field at an un-sampled location x0 are estimated on the basis of the given measured values in a linear form
where Z∗(x0) is the value to be estimated at (x0); (Zxi) denotes the given measured values at xi; N is the total number of given measured values used for estimation; and λi0 is Kriging weight for Z(xi) Zxi to estimate Z∗(x0).
All geostatistical analyses were done in the Arc GIS environment.
Results and discussion
Spatial patterns of the physicochemical characteristics
The univariate statistics of the values for all the physicochemical parameters in the coastal and inland groundwater samples of Delta State and the corresponding maximum allowable limits as stipulated by World Health Organization (WHO) 2011 are presented in Table 1. The pH of the groundwater samples ranged from4.21 to 7.41 with an average value of 6.13 for the coastal area and 4.48 to 6.82 with an average of 5.61 for the inland area, indicating slightly acidic to slightly alkaline nature with 53.64% and 90% of the groundwater samples from coastal and inland areas respectively outside the World Health Organization (WHO) (2011) stipulated limits of 6.5 to 8.5. It was observed that majority (90%) of the water samples from the inland area has pH value below 6.5, thus indicating that they are mostly slightly acidic in nature. The observed acidic nature may be attributed to the effect of humic acid released from decaying vegetation of the soil zone that percolates down to the saturated zone below (Edet 2018). Total dissolved solid (TDS) ranged from 6.20 to 6540 mg/l and between 7.5 and 490 mg/l for the coastal and inland areas respectively. Electrical conductivity (EC) measured in μS/cm varied from0.60 to 1279 and 16 to 964 for the coastal and inland areas respectively. All the groundwater samples of the inland area are below the guideline values for TDS (500 mg/l) and EC (1500 μS/cm) while 18.18% and 0.00% of groundwater from coastal area have TDS and EC respectively above the guideline values. Extremely high TDS values ranging from 2540 to 6540 mg/l found in groundwater in the southern coastal region may be attributed to the effect of tidal influence of seawater (Edet and Worden 2009). Based on TDS (Hem 1982), all the groundwater from the inland area belong to freshwater class (TDS, <1000 mg/l) while 83.64% and 16.36% of groundwater from the coastal area belong to freshwater class and brackish water class (TDS, 1000–10,000 mg/l) respectively.
The biological oxygen demand (BOD) and chemical oxygen demand (COD) are parameters that are used for determining waste concentration and applied primarily to pollutant mixture such as domestic sewage and agricultural and industrial wastes. The BOD and COD of groundwater from the coastal area varied from 0.1 to 50 mg/l and 1.48 to 857 mg/l respectively while BOD and COD of groundwater from the inland area varied from 1.02 to 2.03 mg/l and 1.2 to 3.02 mg/l respectively. The recommended BOD and COD values for drinking water are 6 and 10 mg/L, respectively (WHO 2004).It was observed that 51.82% and 14.55% of groundwater from the coastal area have BOD and COD values respectively greater than the drinking water limits. Relatively high BOD and COD values are found in the western and southern areas of the coastal region where most of the petrochemical industries are located and are densely populated too. The inland area has extremely low BOD and COD values and these may be attributed to the low population density and scarcity of industries in that area.
The prevalence of the cations for groundwater from the coastal area are Ca>Mg>Na>K while for the samples from the inland area are Na>Ca>K>Mg. The concentration of calcium ranged from 0.001 to 65.60 mg/l for the coastal and from 0.001 to 48.30 mg/l for the inland area. All the groundwater analysed for the entire study area are below the maximum allowable limit of 200 mg/l set by World Health Organization (WHO) (2011). The concentration of magnesium ranged from 0.08 to 74.30 mg/l and from 0.08 to 10 mg/l for groundwater from the coastal area and inland area respectively. These results revealed that all the inland samples have values within the World Health Organization (WHO) (2011) stipulated guideline value of 50 mg/l while 4 samples (3.64%) from the coastal area are above the guideline value. Sodium concentration ranged from 0.25 to 51 mg/l for groundwater from the coastal area and varied from 0.05 to 85.21 mg/l for samples from the inland area. All the groundwater samples analysed have concentration lower than 200 mg/l set as maximum allowable concentration for Na in drinking water. The concentration of potassium for the coastal area groundwater samples ranged from 0.01 to 42 mg/l and from 0.04 to 36 mg/l for groundwater from inland area. 30.91% and 10% of groundwater from the coastal and inland areas respectively have concentrations above the maximum allowable concentration of 12 mg/l set by World Health Organization (WHO) (2011).
The prevalence of the anions for groundwater from the coastal area are Cl>HCO3>SO4>NO3 while for the samples from the inland area are HCO3>Cl>NO3>SO4.The concentration of sulphate for groundwater from the coastal area varied from 0.01 to 82 mg/l and from 0.01 to 105.90 mg/l for groundwater from the inland area. All the groundwater samples analysed for the entire study area are within the acceptable limit for drinking water purpose. Chloride concentration varied from 0.01 to 666 mg/l for the coastal area and 0.05 to 18.1 mg/l for the inland area. All the groundwater samples from the inland area have concentration within the stipulated limit of 250 mg/l while two of the samples from the coastal area have concentrations above the guideline value. The concentration of bicarbonate for groundwater from the coastal area ranged from 0.01 to 230 mg/l and from 0.01 to 152.5 mg/l for sample from the inland area. The concentration of HCO3 in groundwater across the entire study area is within the World Health Organization (WHO) (2011) stipulated guideline value of 600 mg/l. The concentration of nitrate varied between 0.01 and 189 mg/l for groundwater from the coastal area and 0.01 and 44 mg/l for groundwater from the inland area. A relatively high NO3 (> 50 mg/l) concentration was measured around the western segment of the coastal area which is densely populated. 26.36% of groundwater samples from the coastal area is not within the World Health Organization (WHO) (2011) guideline value while 12.5% of groundwater from the inland area is not within the guideline value. Excess NO3 (NO3 concentration > 40 mg/l) in groundwater causes blue eye disease in infants and children.
Water classification and facies
Four hydrochemical facies types were delineated based on the percentage values of the major ions for the entire study area (Fig. 2). The various facies included CaHCO3, NaHCO3, NaCl and CaMgClSO4. These facies are not typical of any environmental area. The CaHCO3 signifies the dominance of alkaline earths over alkali (Ca+Mg>Na+K) and weak acidic anions over strong acidic anions (HCO3>Cl+SO4). The facies are thought to be result of groundwater recharge via precipitation (Omonona et al., 2014; Owoyemi 2017). The NaHCO3 signifies the dominance of alkalis over alkaline earth (Na+K>Ca+Mg) and weak acidic anions over strong acidic anions (HCO3>Cl+SO4). The origin of this facies may be traced to base ion exchange processes, where Ca and M of the initial CaMgHCO3facies formed by direct recharge from rainfall is replaced by Na and K ions. NaCl facies denotes the dominance of alkalis over alkaline earth (Na+K>Ca+Mg) and strong acids over weak acid (Cl+SO4>HCO3). This facie can be attributed to the effect of tidal influence of seawater for samples that belong to the coastal area and brine deposits in the inland area. The CaMgClSO4 facies signifies the dominance of alkaline earths over alkali (Ca+Mg>Na+K) and strong acids over weak acid (Cl+SO4>HCO3). The facie is formed from simple dissolution and mixing processes and belongs to mixed hydrochemical facie where no one cation-anion pair exceeds 50% (Ravikumar et al. 2015).
Sources of ions
To decipher the major mechanisms controlling the geochemistry of the study area, a plot based on ratio of (Na+K)/(Na+K+Ca) as a function of TDS (Gibbs 1970) was constructed (Fig. 3). The plot revealed that most of the groundwater is influenced by geology (water-rock interactions). Some groundwater samples, mainly groundwater from the coastal area, however, plotted in the seawater section and these samples are thought to be influenced by the tidal influence of the seawater (seawater intrusion) (Olobaniyi and Owoyemi 2006). It was observed from the plot that most of the samples from the inland area are characterized by low TDS and some of which plotted around the freshening section. No sample plotted around the rainfall section.
A plot of Ca + Mg against HCO3 (Fig. 4) revealed that most of the inland area groundwater samples are low in Ca, Mg and HCO3 concentrations. The plot also revealed that several of coastal area and inland area groundwater samples are plotted along the theoretical line ([Ca2+] = 0.5[\( {HCO}_3^{-} \)]) of calcite and anorthite dissolution (Montcoudiol et al. 2014). Ca-rich mineral dissolution can therefore be suggested for the process that leads to enrichment of Ca, Mg and HCO3in groundwater of the area. The dissolution of calcite and Ca-rich silicates (anorthite) is presented by Eqs. 11 and 12.
However, a plot of Ca versus Mg (Fig. 5) revealed that most of the groundwater samples from the two areas plotted above the [Ca2+] = 2[Mg2+] line, indicating the dominance of silicate weathering over carbonate dissolution (Rajmohan and Elango 2004). Excess Ca+Mg over HCO3 observed mainly in the coastal area groundwater samples suggest other sources and this must be balanced up by SO4 and/ or Cl. The excess of HCO3 over Ca+Mg requires that part of the HCO3 should be balanced by Na and/or K. The plot of Ca+Mg versus total cations (Fig. 6) showed that the points fell below the 1:1 line, reflecting an increasing contribution of Na and K with increasing dissolved solids (Edet 2018).
In Fig. 7, the groundwater samples from the coastal and inland area show enrichment in Ca and Na concentrations which could be explained by silicate weathering. Some of the inland area samples show high Na concentrations associated with a lower Ca concentration than the other samples. These samples appear to be influenced by ion exchange processes. Appelo and Postma (2005) noted that ion exchange may be the geochemical process responsible for the deficit of calcium versus sodium in groundwater. A bivariate plot of Cl versus Na (Fig. 8) revealed the possible sources of Na in groundwater of the area. The plot showed that most samples from the inland area are influenced by silicate weathering and some by ion exchange. The sources of Na in groundwater of the area may be attributed to silicate weathering and ion exchange. Also, groundwater from the coastal area is influenced by weathering of silicates minerals and seawater tidal flushing which accounts for excess of Cl over Na.
Physicochemical parameter relationship
Correlation matrix has been effectively and extensively employed in understanding relationships among constituent parameters in groundwater (Omonona et al. 2013; Onwuka and Omonona 2017; Tiwari et al. 2017; Amano et al. 2018). The correlation coefficient (CC) always ranges from − 1 to + 1 and CC > 0.5 is regarded as statically related or correlated. In addition to the CC values, the P value measures the statistical significance of the test. P value < 0.05 (95% confidence level) is always accepted as significant for any test. The results of the computed Pearson correlation test for the two areas for the present study are presented in Table 2.
A number of significant correlations were observed in the physicochemical concentrations in the study area groundwater. Positive correlations were found between TDS and COD, Na and EC, K and EC, K and Na, HCO3andEC, HCO3 and K, SO4 and EC, SO4 and K, SO4 and HCO3, NO3 and EC, NO3 and SO4 for groundwater from the coastal area (Table 2). These correlations revealed that K, HCO3, SO4and NO3 constitute the main ions that contribute to the electrical conductivity property of the groundwater. HCO3, K, Na, SO4 have similar source from weathering of silicate minerals and the high correlation coefficient (r2 = 0.5369, P = 0.00) between SO4 and NO3suggest that SO4 has other sources (domestic wastes and agricultural activities) other than weathering of silicate minerals. For the inland areas, the following pairs are identified TDS-EC, Na-EC, K-EC, HCO3-EC, SO4-EC, TDS-Na, TDS-K, TDS-HCO3, SO4-TDS, K-Na, Na-HCO3, Na-SO4, K-HCO3, K-SO4, Ca-Mg and SO4-HCO3. These ions might be thought to be released from weathering of silicate minerals.
Heavy metal spatial characteristics
The summary statistics of heavy metals in groundwater from the study area is presented in Table 3. Heavy metals are mobilized in aqueous solutions at different rates, some metals like Zn, Cu, Ni and Cd are more mobile than others like Cr and Pb (Baran and Tamawski 2015; Ramose Silva et al. 2017). The pH of water plays a major role in the transport of metals in water. Low pH is favourable for metal concentration in water. Caboi developed a diagram that classifies water into different classes from a plot of the sum of dissolved metals as a function of pH (Caboi et al. 1999; Ficklin et al. 1992). Based on the Caboi diagram, the groundwater from the two areas may be classified into four categories, namely “acid high-metal”, “acid low-metal”, “near neutral high-metal” and “near neutral low-metal” (Fig. 9). The concentrations of Cu and Cd were below the World Health Organization (WHO) (2011) guideline values of 2 mg/l and 0.003 mg/l respectively. Cr, Pb and Ni concentrations were found to be above the World Health Organization (WHO) (2011) guideline values of 0.05 mg/l, 0.01 mg/l, and 0.07 mg/l respectively. 13.64% and 0% of groundwater from the coastal area and inland area respectively have Cr concentrations above the stipulated guideline value, 31.82% and 7.50% of Pb concentrations from the coastal area and inland area respectively are above the World Health Organization (WHO) (2011) guideline value and 14.55% and 0% of Ni concentrations from the coastal area and inland area respectively are above the World Health Organization (WHO) (2011) guideline value. The concentrations of Fe (32.73 % and 20% from coastal area and inland area respectively) and Mn (3.64% and 2.50% from coastal area and inland area respectively) in groundwater were found to be above the World Health Organization (WHO) (2011) guideline value. The concentrations of Zn in groundwater from the coastal area and inland area are within the World Health Organization (WHO) (2011) guideline value.
Heavy metal enrichment and pollution
The evaluation of the degree of multiple heavy metal pollution was carried out using heavy metal enrichment index (HEI). The following heavy metals, Cr, Cd and Ni were not included in the computation because their concentration values were largely below detection limits in most of the stations especially for groundwater from the inland area. The summary statistics of the results of the computed HEI are presented in Table 3. The HEI values for the coastal area range from 0.04 to 16.96 with a mean of 1.18 and those for the inland area range from 0.28 to 4.13 with a mean of 0.69. The higher HEI values in the coastal area than the inland area may be attributed to the effects of the concentration of all oil and gas exploration and exploitation activities in the coastal area, the prevalence of the petrochemical industries and their allied firms in the coastal area and lastly, the coastal area is densely populated (urbanized) with little or inadequate waste disposal facilities. The HEI values also showed that groundwater samples from 31.82% of the stations examined from the coastal area and from 15% of the samples tested from the inland area exceeded the threshold value 1 (HEI > 1), thus indicating pollution by heavy metals and are therefore regarded as unfit for domestic use. Areas where very high HEI concentration were observed such as Ughelli, Isoko, Kwale (Inland areas) and Burutu, Bomadi, Warri (coastal areas) as presented in Fig. 10 are areas with relatively high oil well concentration in the oil-rich Niger Delta and may have impacted these regions.
Water quality for drinking purposes
The suitability of groundwater from the study area was assessed by comparing the monitored values of the physicochemical parameters and heavy metals with the standard values stipulated by World Health Organization (WHO) (2011) for drinking and domestic purposes. The results of the physicochemical parameters (Table 1) and the results of the heavy metals (Table 3) revealed that the concentrations of TDS, Mg, K, Cl, NO3, Fe, Cd, Cr, Ni, Pb and Zn are not within the stipulated guideline values for drinking and domestic purposes for groundwater from the coastal area while the concentrations of K, NO3, Fe and Pb are not within the guideline values for groundwater from the inland area.
Water quality for irrigation purposes
Various irrigation water quality indices and USSL diagram were used to assess the suitability of groundwater from the study area for irrigation and agricultural purposes.
Table 4 presents the summary of irrigation assessment parameters. Water hardness plays a role in determining the suitability of water for irrigation purposes. Extremely hard water causes scaling in irrigation pipes and equipment thereby limiting its use for irrigation purposes (Okogbue et al. 2012). Water hardness is measured as a function of total hardness (as CaCO3) measured in mg/l. TH for the coastal area ranged from 0.09 to 31.87 with a mean of 3.74 while the inland area ranged from 0.03 to 9.32 with a mean of 1.32. According to Sawyer et al., 2003, all the groundwater (100%) from the two areas belong to soft water class, thus making them suitable water sources for irrigation purpose. EC measures the amount of ions available in water to conduct electric current and measures the salinity hazard of irrigation water. EC ranged from 0.60 to 1279 μS/cm with a mean of 287.13 μS/cm for the coastal area and from 16 to 964 μS/cm with a mean of 106.85 μS/cm for the inland area. 60%, 30.91% and 9.9% of groundwater from the coastal area belong to excellent, good and permissible categories respectively while 87.5%, 10% and 2.5% of groundwater from the inland area belong to the excellent, good and permissible categories respectively.
SAR irrigation water quality parameter evaluates sodium hazard in relation to Ca and Mg concentrations (Table 5). High sodium ion in water makes soil structure becomes compact thereby reducing soil permeability and soil aeration (Ayuba et al. 2013). Increase in the concentration of Ca and Mg in water on the other hand increase soil permeability. SAR ranged from 0.03 to 8.86 with a mean 0.98 for groundwater from the coastal area and from 0.01 to 21.40 with a mean of 2.50 for groundwater from the inland area. 100% of the groundwater samples from the coastal area fall to the excellent category and 90%, 7.5% and 2.5% of groundwater samples from the inland area fall to the excellent, good and doubtful categories respectively. The United States Salinity Laboratory classification diagram (USSL Salinity Laboratory 1954) of irrigation water is a plot of sodium hazard as a function of salinity hazard. Based on the USLL classification, the groundwater of the study area was classified into 6 categories, C1S1,C2S1, C3S1,C1S2, C2S2 and C3S3 (Fig. 11). Groundwater samples belonging to C1S1, C2S1 and C1S2 are suitable for irrigation purposes while those of the C3S1, C2S2 and C3S3 categories are not suitable for irrigation purposes. A high concentration of Na in relation to other ions leads to alkaline soils and saline soils when it combines with CO3 or HCO3 and Cl respectively. Both alkaline soils and saline soils are not favourable for irrigation purposes (Roy et al. 2018). Na% for groundwater from the coastal area ranged from 5.09 to 96.17 with a mean of 44.56 and from 14.51 to 99.17 with a mean of 58.14 for groundwater from the inland area. For the coastal area, 19.09%, 29.09%, 20.00%, 20.91% and 10.91% of the groundwater samples belong to excellent, good, permissible, doubtful and unsafe classes respectively while for the inland area, 7.5% belongs to excellent class, 22.5% to good class, 30% to permissible class, 7.5% to doubtful class and 32.5% to unsafe class.
RSC defines the effects of excess bicarbonates in irrigation water. When irrigation water has high bicarbonate concentration it gives rise to precipitation of calcium bicarbonate and magnesium bicarbonate which tend to increase water hardness. RSC ranged from − 8.65 to 3.02 with a mean of − 0.59 for the coastal area and varied from − 3.06 to 2.28 with a mean of − 0.13 for the inland area. These values indicate good to unsuitable water for irrigation for coastal area and good to doubtful water for irrigation for the inland area. MH defines the relationships between Mg and Ca in irrigation water. High Mg concentration leads to increase in soil alkalinity and which in turn reduces crop yield (Ravikumar et al. 2011). Estimated MH values ranged from 1.75 to 99.96 with a mean of 45.21 for groundwater from the coastal area and varied between 4.03 and 99.97 with a mean of 54.61 for groundwater from the inland area. MH above 50 is unsuitable for irrigation water, 60% and 52.5% of the coastal area and inland area respectively are suitable water for irrigation while 40% of the coastal area and 47.5% of the inland area are unsuitable water for irrigation uses.
KR measures the ratio of Na to the sum of Ca and Mg in irrigation water. KR > 1 indicates an excess of Na in relation to the other ions. KR varied between 0.094 and 44,348 with a mean of 758 for the coastal area and from 0.28 to 74095 with a mean of 8086 for the inland area. 31.82%, 20% and 48.18% of the groundwater from the coastal area belong to suitable, marginally suitable and unsuitable classes respectively while 35%, 20% and 45% of the groundwater from the inland area belong to the suitable, marginally suitable and unsuitable respectively. PI developed by Doneen (1964) is also used in evaluating irrigation water quality. PI is influenced by Na, Ca, Mg and HCO3 concentrations. The PI values for the coastal area ranged from 10.93 to 410.79 with a mean of 93.68 and that for the inland area varied from 14.39 to 4010.84 with a mean of 240.70. 60% of the groundwater from the coastal area and 75% from the inland area are suitable for irrigation, 27% and 22.5% of the coastal area and the inland area respectively belong to moderate class, and 13% of the coastal area and 2.5% of the inland area are not suitable for irrigation uses.
Determining water quality safe zones based on the stipulated standards for drinking, irrigation and aquaculture using a geostatistical approach
Table 6 presents the summary of observation wells in which different hydrochemical parameters exceeded the water quality standards for different purposes: drinking, irrigation and aquaculture.
For each water utilization purpose, two distinct zones, safe zone and unsafe zone, were defined. The unsafe zone for different utilization uses is defined as when the estimated hydrochemical parameters values do exceed the stipulated guidelines of the specified water quality standards in any given locality.
The results of the spatial distributions of the safe zones and unsafe zones for drinking water quality, irrigation water quality and aquaculture water quality are presented in Figs. 12, 13 and 14 respectively. The safe zones for drinking water are located in parts of the northern section and southeastern of the inland area and most part of the central spread of the coastal area of the Delta State (Fig. 12). The unsafe zones for water quality for drinking purpose of the Delta State may be attributed to contamination emanating from anthropogenic activities and poor sanitation practices. The safe zones for irrigation water quality are located mainly in the northern and central portions of the coastal area and around the northwestern and western sections of the inland area of the Delta State (Fig. 13). The unsafe zones for aquaculture water are predominantly confined within the inland area and the southern portion of the coastal area (Fig. 14). The safe zones occupied mainly the coastal area and very small portion of the inland area.
The inland area of the Delta State is occupied with more unsafe zones for irrigation water and aquaculture water than the coastal area (Figs. 13 and 14). The poor state of water quality for irrigation and aquaculture purposes can be attributed to agricultural and industrial wastes inputs and poor general sanitation practices in the area.
It was observed that the southernmost part of the Delta State is predominantly characterized by unsafe zone for all the three (drinking water, irrigation water and aquaculture water) water utilization purposes. This observation can be attributed to the impact of seawater intrusion phenomenon.
Generally, groundwater exploration and exploitation should be concentrated in the safe zones while already existing developed groundwater sources in the unsafe zones should be carefully managed, encouraging usage of groundwater from the unsafe zones only after adequate treatment measures have been taken.
Conclusions
A systematic study of the variability in space of groundwater hydrochemical characteristics, hydrochemical controlling processes and quality is presented in this study. The distribution characteristics of the physicochemical parameters (pH, TDS, EC, BOD and COD) are higher in groundwater from the coastal area than groundwater from the inland area. All the groundwater from the inland area belongs to freshwater while those from the coastal are freshwater and brackish in nature. The observed discrepancies in the values of the physicochemical parameters especially EC, TDS, BOD and COD may be attributed to the effects of seawater tidal flushing, seawater intrusion from groundwater over-abstraction and industrial effects that are prevalent in the coastal area. The prevalence and order of abundance of the cation/anion in groundwater from the coastal area and inland area are Ca>Mg>Na>K/Cl>HCO3>SO4>NO3 and Na>Ca>K>Mg/HCO3>Cl>NO3>SO4 respectively. Different ionic pairs were revealed from the correlation analysis. Common ionic pairs identified in groundwater from both coastal and inland areas which are thought to be released from the same sources and or through the same geochemical processes are Na-K, Mg-Ca, K-HCO3, K-SO4 and HCO3-SO4.
The hydrochemical data for the entire study area revealed four distinct hydrochemical facies, namely CaHCO3, NaHCO3, NaCl and CaMgClSO4. All the different hydrochemical facies are distributed across the two distinct environments (coastal area and inland area). The presence of all the facies type in the area showed the variability in the hydrochemical controlling processes that are influencing the chemistry of the groundwater of the area. It also points the fact the groundwater of the area are not all recent in age that is of recharge era but that the initial young water characterized with CaHCO3 facies has been transformed to NaHCO3 through ion exchange process and then to NaCl facies via reversed ion exchange process made possible only in a saline environment and finally to CaMgClSO4 facies formed as a result of mixing processes.
Heavy metal characteristics revealed that Cd, Cr and Ni are below detection limit of 0.01 mg/l in all the groundwater from the inland area while they are above the detection limit in some monitored wells of the coastal area, and are even above stipulated guideline values for drinking and domestic purposes in some of the monitored wells. 31.82% and 7.50% of Pb concentrations from the coastal area and inland area respectively are above the World Health Organization (WHO) (2011). Also, the concentrations of Fe in groundwater from the coastal area (32.73%) are greater than the guideline value while those of the inland area (20%) are above the guideline value. Mn concentrations (3.64% and 2.50% from coastal area and inland area respectively) in groundwater were found to be above the World Health Organization (WHO) (2011) guideline value. The concentrations of Zn in groundwater from both the coastal area and inland area are within the World Health Organization (WHO) (2011) guideline value. Groundwater from the coastal area has more heavy metal enrichment than those from the inland area. The HEI values also showed that groundwater samples from 31.82% of the stations examined from the coastal area and from 15% of the samples tested from the inland area exceeded the threshold value 1 (HEI > 1), thus indicating pollution by heavy metals and are therefore regarded as unfit for domestic use.
In terms of groundwater quality for drinking and domestic purposes, the concentrations of TDS, Mg, K, Cl, NO3, Fe, Cd, Cr, Ni, Pb and Zn are not within the stipulated guideline values for drinking and domestic purposes for groundwater from the coastal area while the concentrations of K, NO3, Fe and Pb are not within the guideline values for groundwater from the inland area. The suitability of groundwater for irrigation was based on different irrigation water quality indices (EC, SAR, CSR, PI, KR) and USLL diagram. The irrigation water quality indices indicated that the groundwater from the study area ranged from excellent rating to unsuitable rating with more groundwater samples classified under permissible to unsuitable ratings for inland area and more samples classified under excellent to permissible for the coastal area. USSL diagram classified the groundwater of the entire study area into 6 categories, C1S1, C2S1, C3S1, C1S2, C2S2 and C3S3. Groundwater samples belonging to C1S1, C2S1 and C1S2 are suitable for irrigation purposes while those of the C3S1, C2S2 and C3S3 categories are not suitable for irrigation purposes. Spatial distribution patterns for safe and unsafe zones were defined based on the stipulated standards of different purposes using a kriging geostatistical approach.
References
Adelana, S. M. A., Bale, R. B., Olasehinde, P. I., Wu, M. (2005). The impact of anthropogenic activities over groundwater quality of coastal aquifer in Southwestern Nigeria. Proceedings on Aquifer vulnerability and Risks. 2nd International Workshop and 4th Congress on the Protection and Management of Groundwater. Raggia di Colornoparma.
Adelana, S. M. A., Abiye, T. A., Nkhuwa, D. C. W., Tindinugaya, C., Oga, M. S. (2008). Urban groundwater management and protection in Sub-Saharan Africa in: Adelana, S.M.A and MacDonald, A.M Applied groundwater studies in Africa, International Association of Hydrogeologists, selected papers, 222-259.
Ajakaiye, B. A. (2008). Combating oil spill in Nigeria: primary role and responsibility of the national oil spill detection and response agency (NOSDRA) Stakeholders’ Consultative Workshop. August 4 – 6, 2008, Calabar, Nigeria.
Amadi, A. N. (2007). Aquifer characterization and groundwater vulnerability around Owerri, Southeast Nigeria. Unpublished M. Tech. Thesis, Federal University of Technology, Minna.
Amadi, A. N., & Olasehinde, P. I. (2009). Assessment of groundwater potential of parts of Owerri, Southeastern Nigeria. Journal of Science, Education and Technology, 1(2), 177–184.
Amano, H., Nakagawa, K., & Berndtsson, R. (2018). Surface water chemistry and nitrate pollution in Shimabara, Nagasaki, Japan. Environmental Earth Sciences. https://doi.org/10.1007/s12665-018-7529-9.
APHA. (2005). Standard methods for the examination of water and wastewater (20th ed.). Washington: APHA, AWWA and WEF.
Appelo, C.A.J., Postma, D. (2005). Geochemistry, groundwater and pollution, 2nd Edition Balkema, Leiden, The Netherlands, pp. 668.Beaudry, C., (Master dissertation) 2013. Hydrogéochimie de l’aquifèrerocheuxrégionalen Montérégie-est, Québec. INRS, 193.
Aroh, K. N., Ubong, U. I., Eze, C. L., Harry, I. M., Umo-Otong, J. C., & Gobo, A. E. (2010). Oil spill incidents and pipeline vandalization in Nigeria: Impact on public health and negation to attainment of millennium development goal: the Ishiagu example. Disaster Prevention and management: An International Journal, 19(1), 70–87.
Avbovbo, A. A., & Ayoola, O. (1981). Petroleum Prospects of southern Nigeria’s Anambra Basin. Oil Gas Journal, 79, 334–347.
Awobajo, S. A. (1981). An analysis of oil spill incidents in Nigeria – 1970-1980. In Proceedings of the international Seminar on the Petroleum Industry and the Nigeria Environment, (Lagos: NNPC).
Ayuba, R., Omonona, O. V., & Onwuka, O. S. (2013). Assessment of groundwater quality of Lokoja Basement Area, North-Central Nigeria. Journal Geological Society of India, 82, 413–420.
Baran, A., & Tamawski, M. (2015). Assessment of heavy metals mobility and toxicity in contaminated sediments by sequential extraction and a battery of assays. Ecotoxicology, 24(6), 1279–1293.
Caboi, R., Cidu, R., Fanfani, L., Lattanzi, P., & Zuddas, P. (1999). Environmental mineralogy and geochemistry of the abandoned Pb–Zn Montevecchio-Ingurtosu mining district, Sardinia. Italy. Chron. Rech. Min, 534, 21–28.
Doneen L. D. (1964). Notes on water quality in agriculture, Published as a water science and engineering, Paper 4001, Department of Water Sciences and Engineering, University of California.
Eaton F.M (1950). Significance of carbonates in irrigated water. Soil Sciences, 69,127–128
Edet, A. (2018). Seasonal and spatio-temporal patterns, evolution and quality of groundwater in Cross River State, Nigeria: implications for groundwater management. Sustainable Water Resources Management, 5, 667–687. https://doi.org/10.1007/s40899-018-0236-6.
Edet, A., & Worden, R. (2009). Monitoring of Physical parameters and evaluation of the chemical composition of river and groundwater in Calabar (Southeastern Nigeria). Environmental Monitoring and Assessment, 157, 243–258.
Eni, D. V., Obiefuna, J., Oko, C., & Ekwok, I. (2011). Impact of urbanization on sub-surface water quality in Calabar municipality, Nigeria. International Journal of Humanities and Social Sciences, 1(10), 167–172.
Ficklin, D. J. W. H., Plumee, G. S., Smith, K. S., & McHugh, J. B. (1992). Geochemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest AS (eds) Water–rock interaction. Balkema Rotterdam, 7, 381–384.
Gibbs, R. J. (1970). Mechanism controlling world water chemistry. Science, 170, 1088–1090.
Gupta, S. K. (1983). Variations of water table in Yamuna drainage basin of Haryana-implications and management strategies. Paper presented at the Seminar on Strategies for Irrigation Water Management, Patna.
Hem, J. D. (1982). Conductance: a collective measure of dissolved ions. In: Water analysis, vol. 1, Inorganic species, Part 1 (ed. by R. A. Minear and L. H. Keith), 137-161. Academic Press, New York.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural waters. 3rd Edition Book, 2254. Scientific Publishers, Jodhpur, p 263
Hosper, I., (1971). The geology of the Niger delta area: In Delany, F.M., (1971). The Geology of Eastern Atlantic continental margin, Great Britain Institute of Geological Science Report, 70:121-142.
Jang, C. S., Chen, C. F., Liang, C. P., & Chen, J. S. (2016). Combining groundwater quality analysis and a numerical flow simulation for spatially establishing utilization strategies for groundwater and surface water in the Pingtung Plain. Journal of Hydrology, 533, 541–556.
Kehinde, M. O. (1998). The impact of industrial growth on groundwater quality and availability. In: Osuntokun, A. (eds) Current Issues in Nigerian Environment. Ibadan Danidan Press. p145
Kelley, W. P. (1940). Permissible composition and concentration of irrigated waters. Proc ASCF, 66, 607.
Kelly W.P. (1963). Use of saline irrigation water. Soil Science 94, 355–391.
Krzysztof, L., Wiechula, D., & Korns, I. (2003). Metal contamination of farming soils affected by industry. Environment International, 30, 159–165.
Liang, C. P., Jang, C. S., & Chen, J. S. (2016). Zonal management of multi-purposes groundwater utilization based on water quality and impact on the aquifer. Environmental Monitoring and Assessment, 188, 431. https://doi.org/10.1007/s10661-016-5414-0.
Liang, C. P., Chien, Y. C., Jang, C. S., Chen, C. F., & Chen, J. S. (2017). Spatial analysis of human health risk due to arsenic exposure through drinking groundwater in Taiwan’s Pingtung Plain. International Journal of Environmental Research and Public Health, 14, 81. https://doi.org/10.3390/ijerph14010081.
Liang, C. P., Chen, J. S., Chien, Y. C., Jang, C. S., & Chen, C. F. (2018). Spatial analysis of the risk to human Health from exposure to arsenic contaminated groundwater: a kriging approach. Science of the Total Environment, 627, 1048–1057.
Montcoudiol, N., Molson, J., & Lemieux, J. M. (2014). Groundwater geochemistry of the Outaouais Region (Québec, Canada): a regional-scale study. Hydrogeology Journal, 23, 377–396. https://doi.org/10.1007/s10040-014-1190-5.
Ocheri, M. I. (2006). Analysis of water consumption pattern in Makurdi metropolis. Journal of Geography and Development, 1(1), 71–83.
Okogbue, C. O., Omonona, O. V., & Aghamelu, O. P. (2012). Qualitative assessment of groundwater from Egbe–Mopa basement complex area, north-central Nigeria. Environmental Earth Sciences, 67, 1069–1083.
Olobaniyi, S. B., & Owoyemi, F. B. (2006). Characterization by factor analysis of the chemical facies of groundwater in the deltaic plain sands aquifer of Warri, western Niger Delta, Nigeria. African Journal of Science and Technology, 7(1), 72–80.
Omonona, O. V., Onwuka, O. S., & Okogbue, C. O. (2013). Characterization of groundwater quality in three settlement areas of Enugu metropolis, southeastern Nigeria, using multivariate analysis. Environmental Monitoring Assessment., 186, 651–664.
Omonona O. V., Onwuka O. S., Okogbue C. O. (2014). Characterization of groundwater quality in three settlement areas of Enugu metropolis, southeastern Nigeria. Environmental Monitoring and Assessment 186, 651–664. https://doi.org/10.1007/s10661-013-3405-y
Onwuka, O. S., & Omonona, O. V. (2017). Hydrogeochemical characteristics of coastal aquifers from Port Harcourt, southern Nigeria. Environmental Earth Sciences. https://doi.org/10.1007/s12665-017-6933-x.
Owoyemi, F. B. (2017). Geophysical and geochemical investigation of the groundwater quality of Delta State, Nigeria. Unpublished Ph.D. Thesis, University of Benin, 149–204.
Prasanna, M. V., Praveena, S. M., Chidambaram, S., Nagarajan, R., & Elayaraja, A. (2012). Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environment and Earth Science, 67, 1987–2001.
Ragunath, H. M. (1987). Groundwater (p. 563). New Delhi: Wiley Eastern Ltd.
Rajmohan, N., & Elango, L. (2004). Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environmental Geology, 46, 47–51.
Ramose Silva, C. A., Da Fonseca, E. M., Grotto, B. W., De Souza, F. E. S., & Baptista Neto, J. A. (2017). Potentially mobile of heavy metals on the surface sediments in tropical hyper-saline and positive estuaries. Annals of the Brazilian Academy of Sciences, 89(4), 2597–2607.
Ravikumar, P., Venkatesharaju, K., Prakash, K. L., & Somashekar, R. K. (2011). Geochemistry of groundwater prospects evaluation, AnekalTuluk, Bangalore Urban District, Karnataka, India. Environmental Monitoring Assessment, 179, 93–112.
Ravikumar, P., Somashekar, R. K., & Prakash, K. L. (2015). A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Earth Science, 80, 31073–31077.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. Agricultural hand book 60 (p. 160). Washington D.C.: U.S. Dept. of Agriculture.
Roy, A., Keesari, T., Mohokar, H., Sinha, U. K., & Bitra, S. (2018). Assessment of groundwater quality in hard rock aquifer of central Telangana state for drinking and agriculture purposes. Applied Water Science. https://doi.org/10.1007/s13201-018-0761-3.
Sawyer CN, Macarthy PL, Parkin GF (2003). Chemistry for environmental engineering and science McGraw-Hill, New York 5 Ed., 752
Short, K. C., & Stauble, A. J. (1967). Outline geology of the Niger Delta, Nigeria. American Association of Petroleum Geologist, 5, 761–779.
Tiwari, A. K., De Maio, M., & Amanzio, G. (2017). Evaluation of Metal Contamination in the Groundwater of the Aosta Valley Region, Italy. International Journal of Environmental Research, 11, 291–300. https://doi.org/10.1007/s41742-017-0027-1.
Todd, D. K. (1980). Groundwater Hydrology (p. 552). New York: Wiley International Edition. Wiley.
USSL Salinity Laboratory (1954). Diagnosis and Improvement of Saline and Alkaline Soils. US Department of Agriculture Handbook, Washington DC 60:160 p
Wilcox L.V. (1995). Classification and use of irrigation waters. US Department of Agriculture. Cire.969, Washington D.C.USA.: p.19
World Health Organization (WHO). (2004). Guidelines for drinking water quality, 1st ed. Health criteria and other supporting information (p. 540). Geneva: WHO.
World Health Organization (WHO). (2011). Guidelines for drinking water quality (4th ed.p. 564). Geneva: WHO.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Owoyemi, F.B., Oteze, G.E. & Omonona, O.V. Spatial patterns, geochemical evolution and quality of groundwater in Delta State, Niger Delta, Nigeria: implication for groundwater management. Environ Monit Assess 191, 617 (2019). https://doi.org/10.1007/s10661-019-7788-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7788-2