Abstract
The alluvial aquifer underlying the city of Douala comprises shallow Quaternary deposits where groundwater is the main source for domestic and drinking purposes. Shallow groundwater in the area show signs of acidification with average pH range of 3.8–6.8. Long-term groundwater chemistry data (1998–2013), hydrogeochemical and R mode factor analysis were used to establish the acidification process of shallow groundwater and also determine possible origin and implications for water quality and use in the area. Twenty-six groundwater sample points, three streams and three rain sample points were studied seasonally in the 2006–2008 and the 2010–2013 study periods. The data were compared with result of a study, 7 years earlier (1998–1999). The results show evidence of acidification manifested by depletion of HCO3, a decrease in the pH and increase in SO4 and NO3 concentrations of shallow groundwater. Average groundwater pH range is 3.94–7.70 (1998–1999), 3.8–6.91 (2006–2008), and 3.7–6.8 (2010–2013). Only approximately 16 % (1998–1999), 14 % (2006–2008) and 11.11 % (2010–2013) of water samples fall within the range of pH (6.5–8.5) for potable water according to WHO (1993). The alkalinity/acid neutralizing capacity of the shallow groundwater has decreased significantly coupled with increase in the number of zero alkalinities recorded in the 2010–2013. The shallow groundwater is generally undersaturated with common carbonate minerals (calcite, dolomite), therefore providing insufficient acid buffer. Principal component analysis in combination with hydrogeochemical studies revealed that four main factors are responsible for the groundwater chemistry and acidity: (1) acid atmospheric deposition, (2) anthropogenic activities (industrial effluent discharges and acid spill, (3) chemical weathering, and (4) coastal atmospheric deposition/cation exchange. In general, the shallow groundwater is not suitable for drinking and domestic purposes with respect to the low pH and elevated nitrate concentration. In view of the implications such as increase in corrosion and increased mobilization of toxic elements (e.g. Al, Pb, Cu, Zn, Mn) as well as their possible harmful effect on health, it is recommended that the causes, rate of acidification and the mobility of trace elements be investigated with more details.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acidic groundwater and soils have been reported in many parts of the world principally in North America and Europe (Knutsson 1994; Kjoller et al. 2004; Appleyard et al. 2004). For a majority of the cases, the acidification of groundwater and soils has been instigated by acidic atmospheric deposition (Gower et al. 1995; Kjoller et al. 2004; Fest et al. 2007). Acidic gases and aerosol particles are incorporated into rainwater and cloud water and deposited by precipitation or transported by turbulent mixing and gravitational settling to soil, vegetation and exposed water and surface water bodies. The occurrence of acids in precipitation is linked to increase in the levels of anthropogenic gaseous atmospheric pollutants such as nitrogen oxide and sulphur oxide. In highly industrialized cities, the use of fossil fuels in industries and the high traffic density lead to increasing atmospheric deposition of acidic gases SO2 and NO x gases etc. which produces acid precipitation. Acid precipitation can infiltrate into the shallow groundwater system and where the acid neutralization capacity of the aquifer is low (absence of carbonates which give rise to poorly buffered ground waters) may lead to the acidification of groundwater. Soil and groundwater acidification also occurs in areas where there has been no atmospheric pollution (Krug and Frink 1983), commonly referred to as natural acidification. Natural acidification of soils and groundwater is a biogeochemical process caused by soil respiration (giving carbonic acid), dissociation of humic acids, and oxidation of sulphur and nitrogen compounds, oxidation and hydrolysis of ferrous iron in the soil (Knutsson 1994). It is evident in areas with weathering resistant rocks and soils in sandy terrains and very common in humid tropical climates with high leaching (Knutsson 1994). In coastal areas, natural acidification can also be caused by the deposition of sea salt rich precipitation, which can result in the cation exchange of marine sodium with the hydrogen and/or aluminium ions bound in the soil SFT (1993), which may cause episodic acidification of ground and surface water (Knutsson 1994). The acidification of shallow groundwater in poorly buffered, sandy soils in industrial areas with high traffic density is an emerging problem in many areas (Appleyard et al. 2004; Kjoller et al. 2004).
There are many problems related to acidification including effects on surface water, plants, vegetation and infrastructure. Acidification of groundwater can impact the geochemical equilibrium in soils and aquifers (Hansen and Postma 1995; Kjoller et al. 2004; Appleyard and Cook 2008). Acidic groundwater may promote the mobility of elements such as Al, Mn and other toxic metals and make the waters unfit for dinking (Kjoller et al. 2004; Fest et al. 2005). These problems call for concern in the sandy soils and aquifers of the unconfined aquifer of the coastal, urban town of Douala where according to Takem (2012) the average pH of shallow groundwater goes down in some places to pH 3.8, while the average pH of soils is about pH 4.6.
The coastal low-lying urban city of Douala, the economic capital of Cameroon in West Africa, hosts more than 80 % of industries in the country. The city, which rests directly on the Quaternary alluvial deposits, hosts the largest urban population in the country (3,000,000 inhabitants) with a population density of about 1000 inhabitants/km2. Such rapid growth in population, as well as the intensive urban and industrial development, has not been matched by the provision of basic infrastructures such as water supply, sanitation. The supply of potable water in the city of Douala is insufficient as the network of pipe-borne water supplied by CAM WATER cannot expand to meet the rapid population growth. According to Guevart et al. (2006), only about 32.5 % of the population of Douala has access to potable water from CAM WATER network. The total production increased to 80,000 m3 (Djuikom et al. 2010) meanwhile the daily water needs are estimated to be about 250,000 m3. The remaining population therefore depends on springs, hand-dug wells and boreholes for drinking and other domestic purposes. According to Takem et al. (2010), 80 % of the low-income populations in the informal settlements use groundwater for part or their entire drinking and other domestic water needs. However, due to scarcity of reliable data on water quality, the use of groundwater as a source of domestic water supply is usually based on availability alone.
Few studies have been carried out on the quality and chemistry of groundwater in this area (Mafany 1999; Ndjama et al. 2008; Djuikom et al. 2010; Takem et al. 2010; Takem 2012). These studies have established a close relationship between anthropogenic activities in the area and groundwater quality, especially nitrate contamination. The studies on groundwater chemistry have also shown that Na and Cl are the dominant ions with Na–Cl the main water type for shallow groundwater in the area and the groundwater chemistry is controlled by chemical weathering of feldspars, sea spray and anthropogenic inputs (Mafany 1999; Takem et al. 2010; Takem 2012). These studies have also indicated the acidity of groundwater in the area; however, there is still little known about the extent and the level of acidification and more importantly the source and the rate of acidification. Inadequate management of soil and groundwater acidification in the area can produce immediate and long-term impacts with significant health, environmental and economic consequences such as:
-
1.
Polluting water supplies with aluminium, iron arsenic and other metals and possibly causing significant health problems if untreated water is used for drinking.
-
2.
Corrosion problems leading to high maintenance cost. Furthermore, the interaction of groundwater with surface water bodies may lead to the acidification of streams and other surface water bodies and added environmental consequences.
The study therefore seeks to use long-term hydrogeochemical data in combination with multivariate statistical analysis to gain an initial understanding of the groundwater acidity, identify possible sources of acidity and implications for groundwater quality and use in the area.
Materials and methods
Description of the study area
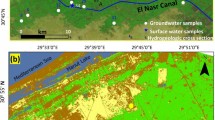
The study area is located 60 m above mean sea level in Douala, in the Littoral province of Cameroon, approximately 30 km from the Gulf of Guinea (Fig. 1). The hydrographical network (Fig. 1) is such that springs flow into streams that in turn flow into minor rivers like the Tongo Bassa and Ngoua, which feed the major rivers of Wouri and Dibamba that eventually join the Atlantic Ocean (Olivry 1986). The area has a humid equatorial climate modified by the relief of Mount Cameroon and has two main seasons, a rainy and a dry season. Peak rainfall occurs in the months of July and September, with an average annual precipitation of 4000 mm/year. Temperature ranges between 23 and 33 °C, with January and February being the hottest months of the year. The soil type varies from brown to black shallow sandy ferralitic to deep sandy clayey soils (Asaah et al. 2006).
Geological setting
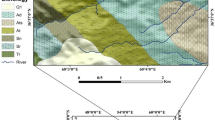
The study area is part of the Phanerozoic Cretaceous–Quaternary Douala sedimentary basin, covering an area of about 7000 km2 with a maximum width of 60 km (Dumort 1968; Regnoult 1986). It is one of the several divergent margin basins along the Southwest African Coast whose origin and structure are associated with the opening of the South Atlantic Ocean during the breakup of Gondwanaland (Tamfu and Batupe 1995). The geology of this basin has been described by several workers (Dumort 1968; Regnoult 1986; Tamfu and Batupe 1995). The stratigraphy of the Douala sedimentary basin, according to Tamfu and Batupe (1995), consists of Precambrian basement, unconformably overlain by a sedimentary sequence ranging in age from Cretaceous to Recent (Fig. 2). The city of Douala rests directly on the Mio-Pliocene to Recent alluvial sediments of this basin, which constitute the Wouri Formation of the basin. The entire study area is dominated by this formation generally consists of unconsolidated fine- to coarse-grained sand and gravel mixed with minute quantity of clay in different proportions. The alluvium is composed predominantly of quartz and kaolinite (Regnoult 1986; Takem et al. 2010), with a general thickness that ranges between 50 and 60 m (Djeuda-Tchapnga et al. 2001).
Hydrogeology
Two shallow and deep aquifer units have been identified in the Douala sedimentary basin by SNEC (1988) based on the work of Dumort (1968) and Regnoult (1986). A generalized stratigraphic sequence of the major aquifer units of the Douala sedimentary basin modified from Mafany (1999) and Regnoult (1986) is given in Fig. 2. The aquifer system in the study area can be classified into two major classes, the shallow aquifers (<50 m depth) and the deep aquifers (>50 m depth) on the basis of depth (Fig. 2). All groundwater samples considered in this study are shallow groundwater from wells with water table generally less than 15 m deep which tap the shallow aquifers (<50 m depth).
The shallow aquifer
The shallow aquifer is made up of the Mio-Pliocene sands at the base and the Quaternary alluvium at the top (Takem et al. 2010), which together form the Wouri Formation. It consists of fine- to coarse-grained sand and gravel mixed with minute amount of clay, and lies on top of the Miocene shale of the Matanda Formation, which serves as an aquiclude (Fig. 2). According to Djeuda-Tchapnga et al. (2001), the aquifer’s thickness ranges between 50 and 60 m. Several lentils of channel-filled sands, hosted in minute clay layers, occur within this main aquifer, which act as perched aquifers. The water table is generally less than 5 m below the surface (Mafany 1999) and fluctuates with the yearly dry and rainy season conditions. The aquifer is mainly recharged during the rainy season (April–September) when the area receives more than 80 % of its precipitation (about 3500 mm/year). Minor recharge also comes from flood water and waste water from drainage channels infiltrating into this aquifer. Several streams drain the area and may also recharge the aquifer depending on the season and the water levels. Many springs flow from valleys at the base of small cliffs where the topography intersects the water table in the shallow aquifer.
The deep aquifer
The deep aquifer consists of the basal sandstones of the Moundeck Formation, underlain by the Precambrian granites and overlain by shale and marl of the Logbaba Formation and the Palaeocene sands of the Nkappa Formation (Fig. 2). The Palaeocene sand aquifer, with a thickness of about 200 m, is exploited by bore wells towards the northeastern part of the study area with discharges of about 250 m3/h/well (Djeuda-Tchapnga et al. 2001). Although the exact depths of these bore wells are not known, according to Djeuda-Tchapnga et al. (2001), most of the wells owned by private individuals tap the Mio-Pliocene alluvial aquifer (shallow), whereas those owned by industries tap the Palaeocene (deep aquifer).
Sampling
The details about sampling and analytical techniques for the 1998–1999 groundwater study are given in Mafany (1999). Twenty-two groundwater water sample points, four rain and three streams were sampled in two sampling campaigns: January–February and August–September, corresponding to the dry and rainy season, respectively, from 2006 to 2008 and then from 2010 to 2013. Four shallow wells were equally sampled away from the urbanized and industrialized areas (giving a total of 26 groundwater sample points studied in each sampling campaign). These four wells provided background or control data to compare with the results of the highly urbanized and industrialized areas so that the results of these activities could be investigated. Samples were collected in pre-cleaned polythene bottles following standard procedures on a seasonal basis. The water sampling points are presented in Fig. 1. Physico-chemical parameters such as, electrical conductivity, pH were measured in the field using the electrode probe field meters WTW Cond 330i and WTW pH 330i, respectively. Samples for chemical analyses were filtered through 0.45 µm Millipore filter paper. Two sets of samples were collected at each site in 250-ml sterilized plastic bottles for chemical analysis. One set of samples was acidified (1:1 dilute nitric acid) for cation analysis. Samples were stored and transported in a cool flask (4 °C) to the laboratory where they were stored in a refrigerator pending analysis. Soil/sediment samples were collected using a soil spade that was well cleaned and rinsed with distilled water they were later on put in pre-cleaned polyethene bags.
Chemical analysis
Chemical analysis of water samples was carried out at the Indian Institute of Technology Bombay and the Geochemical Analysis Laboratory of the Hydrologic Research Center of Cameroon. HCO3 − was determined as total alkalinity by titration as outlined in APHA/AWWA/WEF (1998). Cations were analysed by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES): Na and K, and Ca, Mg and total hardness by the EDTA titrimetric method (APHA/AWWA/WEF 1998). A UV–visible light spectrophotometer was used to analyse SO4 2− by turbidimetry according to APHA/AWWA/WEF (1998). Chloride was determined by the Ion Selective Electrode Meter (ORION). The anions NO3 −, SO4 and Cl were again determined by ion chromatography with the instrument model DX-120 (Dionex). The accuracy of analyses was tested by carrying out duplicate analyses on three selected samples. The analytical precision of cations (Na+, K+, Ca2+, M2+) and anions (Cl−, HCO3 −, SO4 2− and NO3 −) was verified using ionic balance error (IBE) on the basis of ions expressed in meq/l (Appelo and Postma 1999). IBE was observed to be within a limit of ±5 % for all the samples represented in this study.
Laboratory analysis of soils (chemical composition) was carried out using the XRF analytical technique at the Indian Institute of technology Bombay while the Mineralogical composition was carried out using the XRD analytical instrument of the said institution. Soil pH was determined according to the method by Black (1965).
Statistical analysis
Microsoft excel was used for descriptive statistics and to produce correlation matrices for the hydrogeochemical data analysis and interpretation.
Factor analysis/principal component analysis
Principal component analysis is a multivariate statistical procedure designed to classify variables based on their correlations with each other. The main purpose of factor analysis procedures and PCA in particular is to reduce a large number of variables into smaller number of factors (components) that can be more easily used to explain the interrelations of the chemical factors. Factor analysis is widely used in hydrochemical research to identify major mechanisms influencing groundwater chemistry (Cloutier et al. 2008). Factor analysis has been used to determine natural and anthropogenic processes affecting groundwater chemistry (Shrestha and Kazama 2007; Dragon 2006), to determine the origin and hydrogeochemical evolution of groundwater chemistry (Cloutier et al. 2008; Oyebog et al. 2012). In the present study, the data were subjected to factor analysis/principal component analysis to provide an improved understanding of the geogenic and anthropogenic origin of the groundwater chemistry, to better interpret the acidification process of the shallow groundwater.
To carry out PCA, the statistical software SPSS 11.5 for Windows was used. Prior to the analysis, the entire data set was subjected to the KMO and Bartlett’s test of sphericity, to test the suitability of the data for factor analysis and this was successful. The principal component analysis technique was used to extract factors in this work. This technique extracts uncorrelated linear combinations of variables. The first factor that is obtained explains the biggest amount of the variance, and the factors extracted subsequently explain progressively less variance. Principal component extraction and varimax rotation on the data yielded two factors or components with Eigen values >1. Factor 1, explaining 53.366 % of the variance and factor 2, explaining 21.070 % of the variance.
Results and discussion
Groundwater chemistry
Descriptive statistical analysis of shallow groundwater chemistry data showing changes from 1998-1999 through 2006-2008 to 2010-2013
Table 1 shows the descriptive statistics (minimum, maximum and average values) of chemical constituents (pH, EC, nitrates and major ion concentrations) of groundwater samples (15 × 2 for the 1998–1999 study, 26 × 3 for the 2006–2008 study and 26 × 4 for the 2010–2013 study). Average cation concentration varies in the order Na > K > Ca > Mg, and the average anion concentration varies in the order Cl > HCO3 > SO4 > NO3 for the 1998–1999 study and full details are given in Mafany (1999). Average cation concentration for the 2006–2008 occurs in the order Na > Ca > K> Mg and anion concentration occurs in the order NO3 > Cl > SO4 > HCO3. A similar trend is observed for the 2010–2013 study. The average cation concentration occur in the order Na > Ca > K> Mg and anion concentration vary in the order NO3 > Cl > SO4 > HCO3. Taking the natural concentration of groundwater, Na and Cl are dominant ions in groundwater and the main water types are Na–Cl, Ca–Cl, Na Ca-HCO3 (Mafany 1999; Takem et al. 2010; Takem 2012). The average pH for the different study periods varies from 3.94 to 7.70 (1998–1999), 3.82–6.91 (2006–2008) and varied from 3.70 to 6.81 in the 2010–2013 groundwater study period. Figure 3 shows a decreasing trend for pH from the 1998–1999 to the 2010–2013 study. Only approximately 16 % (1998–1999), 14 % (2006–2008) and 11.11 % (2010–2013) of water samples fall within the pH range of potable water (pH 6.5–8.5) according to WHO (1993). Trends of decreasing pH in shallow groundwater from wells and springs in south and west Sweden were observed to indicate groundwater acidification in the area von Bromssen (1989). Accordingly the trend of decreasing pH of shallow groundwater in the area from 1999 to 2013 could be an indication for groundwater acidification. The trends in pH alone, however, are not sufficiently reliable for the interpretation of natural groundwater acidification; as such the temporal variations of the most important elements relating to acidification are examined in the following section.
Change in alkalinity and acidity of the shallow groundwater
Alkalinity is the water’s capacity to resist changes in pH that would make the water more acidic. This capacity is commonly known as “buffering capacity.” Alkalinity indicates the ability for groundwater to neutralize the inputs of acidity and generally consist of the bicarbonate (HCO3) and the carbonate (CO3 2−) concentration. In the area, alkalinity of groundwater is very low and is composed of only the bicarbonate concentration. Figure 4 shows that average groundwater alkalinity for the different study periods has decreased from the 1998–1999 groundwater study through the 2006–2008 to the 2010–2013 study, and the number of zero alkalinities recorded within each study period has increased in the 2010–2013 study period (Fig. 4). Alkalinity in the groundwater is low, generally less than 10 or completely absent in some samples which shows the buffering capacity is exhausted in these areas. According to von Bromseen (1989), the atmospheric load of acids exceeds the base production by weathering of non-carbonate and slowly weathering rock materials, i.e. granites, gneisses, quartzites, sandstones which leads to a decrease in the base saturation and eventually to the acidification of shallow groundwater. According to XRD analysis, the aquifer sediments contain no carbonate minerals like calcite and dolomite (Takem 2012). The mineralogical constituent of the sediment is made of almost 80 % quartz with minor amounts of clay minerals and feldspars (Takem 2012). Takem (2012) established that groundwater samples in the area are undersaturated with common carbonate minerals (calcite, dolomite and ankerite). According to Ako et al. (2012), the undersaturated state for carbonates in the groundwater samples indicates that soluble carbonate mineral phases are absent in the aquifer sediments. The absence of carbonates leads to low alkalinity and therefore poor buffering capacity. Edmunds and Kinniburgh (1986) in a regional survey of groundwater in the United Kingdom observed that low alkalinity ground waters were most susceptible to acidification.
The acid neutralizing capacity of groundwater, defined by SFT (1993) is:
and when calculated for groundwater in this area shows a general decrease during the study periods. Acid neutralizing capacity of groundwater in the area varies from 0.0 to 4.66 in 2008/2009. In the 2006/2008 study, the acid neutralizing capacity gets negative, varying from −8.01 to 0.64, and the same negative trend is observed for the 2010/2013 study, varying from −22.1 to 0.42 (Fig. 5). The negative acid neutralizing capacity equally indicates a drop in the buffering capacity of the groundwater and the process of acidification.
Figure 6 shows the average of major anion concentration for groundwater samples in the area for the different study period. The concentrations of major anions SO4, Cl and NO3 have increased from the 1998–1999 study to the 2010–2013 study. The mean nitrate concentration is less than 5 (maximum 6 mg/l) for the 1998–1999 study, with a mean of 47.4 (maximum 140.4 mg/l) in the 2006–2008 survey and mean of 55.1 (maximum 153 mg/l) for the 2010–2013 study. Average sulphate concentrations have also increased from less than 5 (maximum 17.2 mg/l) in the 1998–1999 study to 24.2 (maximum 62.4 mg/l) in the 2006–2008 study and then to 26.1 (maximum 67 mg/l) in the 2010–2013 groundwater study. Average chloride concentration has increased from about 26.8 (maximum 71 mg/l) in the 1998–1999 study to 37 (maximum 168 mg/l) in the 2006–2008 study and then to 39 (maximum 170 mg/l) for the 2010–2013 groundwater study. SO4 and NO3 are indicators of acidification from acid precipitation (Lahermo 1991). Major ions in groundwater could come from both natural and anthropogenic origin. Nitrate contamination of groundwater from anthropogenic sources in the area has been reported by Takem et al. (2010). The increase in the anions of strong acid associated to a decline in pH could possibly be an indication of acidification process as will be seen later.
Comparing the chemistry and acidity of shallow groundwater in industrial/urban and undeveloped areas in the study area
To test for the incidence of anthropogenic inputs on the shallow groundwater acidification, test samples were collected from wells away from industrial/urban activities. Tables 2 and 3 present a summary of groundwater chemistry for the 2010–2013 groundwater study with group 1 samples (test samples) collected in areas with little or no anthropogenic inputs (industries, population and traffic density) and group 2 samples collected in urban/industrial centres. The results show that the group 1 samples generally show moderate acidity pH (4.6–6.8) with EC values relatively lower than for group 2 samples which generally show a strongly acidic character (pH <4.5), with some exceptions. According to Appelo and Postma (1999), natural groundwater pH not affected by anthropogenic activities is expected to be not less than 4.5. Therefore, the group 1 samples with moderate acidity have little or no form of anthropogenic inputs of acidity. The moderate acidity shown by the group 1 sample which is accompanied by low EC suggests natural processes such as the production of carbonic acid from atmospheric carbon dioxide during rainfall, or other natural processes like the dissolution of carbon dioxide generated from soil root respiration (Knutsson 1994). The absence of anthropogenic inputs for the group 1 samples is indicated by the low levels of nitrates in the water samples. Samples from industrial/urban area which show a strongly acidic character (pH 4.5 and below) equally have high concentration of the major anions particularly nitrates reflecting the influence of anthropogenic inputs. The presence of many industries and high traffic density in the area suggests that acidic atmospheric deposition could be a source of anthropogenic input on the shallow groundwater quality and acidity. The alkalinity level in the group 1 or control wells (away from the urbanized areas) is equally relatively higher than for the group 2 wells and this could probably be due to the absence of anthropogenic sources of acidity. As such the base production from soils in the undeveloped areas is greater than the acid production which leads to increase in the buffering capacity.
Comparing the chemistry/acidity of rainwater, stream and shallow groundwater in the area
A major source of solutes to groundwater is rain falling on recharge areas (Rosenthal 1987). Table 4 shows the average chemical composition of precipitation derived from three representative rainwater sampled in three sites in the area (Table 4). The average pH of rainwater ranges from 4.79–5.51 to 4.88–5.53 for the 2006–2008 and the 2010–2013 study periods, respectively. Na and Ca are the dominant cations (7.5 and 3.3 mg/l mean concentrations, respectively, for the 2010–2013 study) followed by K and Mg the least abundant (0.7 and 2.5 mg/l mean concentration, respectively, for the 2010–2013 study). Cl and SO4 are the most abundant anions (9 and 5 mg/l mean concentration, respectively) with bicarbonates and nitrates appearing least (0.3 and 1.3 mg/l mean concentration, respectively, for the 2010–2013 study). Average major ion composition in rainwater also increases from the 2006–2008 study to the 2010–2013 study (Table 4). The dominance of sodium and chloride ions could indicate the influence of coastal sea spray atmospheric deposition and the increasing trend of the major anions SO4 and NO3 from the 2006–2008 study to the 2010–2013 study probably indicating the influence of acid rain. Mean annual rainfall is about 4000 mm/year which is very abundant and like in most tropical soils, the large volume of rainfall (4000 mm/year) in the area increases the washing of base metals from the sandy top soils with the rapid recharge to deeper horizons leaving the top soils with little buffering capacity. The continuous infiltration of acid rainwater would cause the acidification of soils and thereby leading to a deterioration of the soils ability to absorb acidic inputs as has been observed by Christophersen et al. (1990). A study of groundwater acidification in Norway (Henriksen and Kirkhusmo 1982) showed that groundwater found in sensitive areas had acidity in the range of pH 5.0–5.5 and within this pH the soil had exhausted almost its entire bicarbonate buffering capacity.
The average pH of stream water samples in the area ranges from 6.21 to 7.43 with an average of 6.88 in the 2006–2008 study, and for the 2010–2013 study, the pH of streams samples varies from 6.33 to 9.38 with an average of 7.54 (Table 4). Chloride and bicarbonate are the most abundant anions in stream water (140.3 and 83.5 mg/l mean concentrations, respectively, for the 2010–2013 study), sulphate is the least abundant (37.6 mg/l mean concentration for the 2010–2013 study). Na is the dominant cations (219.7 mg/l mean concentration for the 2010–2013 study) followed by K and Ca (11.7 and 9.6 mg/l mean concentration, respectively). Mg is the least abundant (3.1 mg/l mean concentration for the 2010–2013 study periods). Like rainfall the dominance of Na and Cl in stream water probably results from coastal atmospheric deposition. The atmospheric deposition of sulphuric and nitric acids is the dominant source of acidification of surface waters (Cai 2010). The sensitivity to acidification of surface waters is controlled by the geology of the basin (availability of weathering products in the watersheds and the hydrological factors related to transport, i.e. the rate at which the weathering products are delivered to the surface waters) (Wels et al. 1990; Campbell et al. 2004). The acidity in some surface waters could be related to the lack of weathering products to neutralize the inputs of acidic atmospheric deposition, considering the weathering resistant nature of the sandy soils and sediments, constituting the surface geology of the watershed. The alkaline pH of some stream samples probably results from a temporal availability of abundant weathering products stored in the watershed in these areas or from the effects of anthropogenic wastes discharges. The uncontrolled disposal of both industrial effluents and domestic wastes into surface water courses in the area has been reported by many workers (Guevart et al. 2006; Ndjama et al. 2008; Takem et al. 2010).
Local geology/soil and buffering capacity
Groundwater quality is usually determined by the geohydrological conditions prevailing in the area where it is formed and hence the composition of the groundwater usually also reflects the soil and bedrock in the area. The reactions from acidification vary depending on the geology and the quality of the soil, i.e. the chemical and physical composition of soil. Under conditions in which rainfall exceeds evapotranspiration (leaching), the basic soil cations (Ca, Mg, K) are gradually depleted and replaced with cations held in colloidal soil reserves, leading to soil acidity. Weathering resistant rocks and soils are generally vulnerable to acidification (Campbell et al. 2004). The local geology is generally made up of unconsolidated sands and gravels mixed with minute amounts of clay (Mafany 1999) while the soils are brown to black feralitic sandy soils (Asaah et al. 2006). Table 5 shows the chemical composition of soils and sediments samples from the area, pH ranging from 4.2 to 5.1 with average pH 4.6 (Table 5). The chemical content of the soil is dominantly made up of SiO2 and Al2O3, making up more than 70 % of the sample. Fe2O3 content is also important with some samples having above 6 %. The rest of the oxides of the major elements have less than 2 %. XRD analysis (Takem 2012) equally shows that the soils are composed of predominantly quartz with minor amounts of clay minerals and feldspars.
Origin of acidification of the shallow groundwater
The drop in the pH of rain water passing from a mean pH of 5.21 in the 2006–2008 study to a mean pH of 5.13 in the 2010–2013 study indicates that the average rainwater composition is getting acidic. Natural rainwater that is not influenced by any anthropogenic factors has a pH of approximately 5.7 (Berner and Berner 1987). Accordingly, the rainwater in this area is not under normal atmospheric conditions. The acidic nature of the rainwater is similar to the results of Sigha-Nkamdjou et al. (2003) for rainfall in the equatorial forest ecosystem in the Southern part of Cameroon and rainwater from the Mt Cameroon area (Ako et al. 2012). Unlike the acidity of rainfall in the Southern part of Cameroon and the Mt Cameroon area which is the result of biomass burning (Sigha-Nkamdjou et al. 2003; Ako et al. 2012), the acidity of rainfall in this case can be attributed to SO2 and nitrous emissions considering that the area hosts a large number of industries coupled with a high population and traffic density. Acid precipitation is primarily most often attributed to SO x and NO x emissions especially in industrial areas (Das et al. 2005; Migliavacca et al. 2005). Like rainfall, the streams and shallow groundwater in the area show trend of increasing acidity which is matched by an increasing trend for the major anions, sulphate, nitrate and chloride, which could be related to acidic atmospheric deposition. Natural sources of sulphates and chlorides in groundwater most often indicate the dissolution of evaporate, coastal atmospheric sea salt deposition and seawater intrusion. Saturation index (SI) values for chloride minerals in groundwater samples in the area (Takem 2012) show that groundwater samples are undersaturated with respect to chloride minerals like halite and sylvite. This suggests that soluble chloride minerals phases are absent in the aquifer system. The water samples are equally undersaturated with respect to sulphate minerals, gypsum and anhydrite (Takem 2012). Thus, evaporate mineral phases are absent in the aquifer despite the presence of SO4 and Cl. Another source of sulphate and chloride in the shallow groundwater could be due to seawater intrusion into the coastal aquifer. According to Vengosh and Rosenthal (1994), the Na/Cl ratio during mixing between groundwater and seawater is 0.86 and for fresh groundwater Na/Cl >1, while Na/Cl = 1 indicates dissolution of halite minerals. According to Takem (2012), the Na/Cl ratio of groundwater in the area shows that less than 20 % of groundwater samples have a Na/Cl ratio less than that of seawater (0.86). More than 70 % of groundwater samples in the area have high Na/Cl ratios >0.86 (Takem 2012) arguing against seawater intrusion as the source of sulphate and chloride. Acid rainfall could therefore be the source of these anions in groundwater. The continuous input of acid rain into sandy soils can cause the acidification of shallow groundwater. This has been observed in many regions of the world (Gower et al. 1995). Chloride and sulphate in the atmosphere may also originate from marine sea spray aerosol, atmospheric sea salt deposition (Avila and Alarcon 1999; Edmunds et al. 2003). Sea spray is a possible source of chloride and sulphate in the rainwater considering the close proximity of the area to the coast. Sea spray leads to the deposition of salts on the ground both by wet precipitation and dry fall outs, which can be washed into streams and shallow groundwater system.
To better identify the process controlling the groundwater chemistry and origin of groundwater acidification in the area, multivariate statistical analysis was employed.
Multivariate statistical analysis
Multivariate statistical analysis is a quantitative approach of groundwater classification which allows grouping of groundwater samples and the making of correlations between chemical parameters and ground water samples (Cloutier et al.2008).
Correlation matrices
Pearson’s correlation matrices were used to find relationships between two or more variables. Table 6 presents the matrix of Spearman’s correlation coefficient for physico-chemical and chemical data of the study area. Correlations with r > 0.5 are highlighted in Table 6, correlations with r > 0.7 are termed strong correlations while those from r = 0.5–0.7 are described to be moderate correlations. A positive correlation is observed between Na, K, Ca, HCO3, Cl, SO4, NO3 and EC shown by r > 0.5 in Table 6. This indicates the main elements contributing to the groundwater salinity and probably signifies both natural and anthropogenic sources of ions in the groundwater. The strong positive correlation between Ca and HCO3 (r = 0.9) is indicative of the natural processes of weathering, while anthropogenic sources of ions can be identified by the positive correlation between NO3 vs Cl and SO4, r = 0.63 and 0.53, respectively (Table 6). There is a very strong positive correlation between pH and HCO3 (r = 0.81) Table 6. This is shown in the trend of decreasing pH and alkalinity for groundwater during the different study periods (Fig. 3, 4). There is a strong positive correlation between Na and Cl (r = 0.9), K and Cl (r = 0.84) indicating the effects of sea water on the groundwater (coastal atmospheric deposition in the absence of seawater intrusion). This is equally shown by the positive correlation between Cl and SO4 (Table 6). There is a positive correlation between SO4 and NO3 which could equally indicate the effects of acid atmospheric deposition on the groundwater quality. The positive correlation between Na vs SO4 and Cl and that between K vs SO4 and Cl is an indication that these parameters have a common source which is most probably seawater coastal atmospheric deposition. The positive correlation between Na and K is an indication of the natural weathering of clay minerals and feldspars. The result of correlation matrix indicates a combination of many origins (sea spray, anthropogenic inputs and natural weathering of feldspars and clay minerals) for the chemical composition and acidification of groundwater.
Principal component analysis
Correlation analysis reveals similarities or differences in the behaviour of pairs of ions and does not conveniently identify groups of ions that behave similarly. PCA/FA is usually employed for improved hydrogeochemical interpretation (Ako et al. 2012).
Principal component analysis (PCA) was undertaken on the eleven variables listed in Table 2 for all the groundwater samples. For PCA, first principal components were calculated and this gives the Eigen values and percentage of the variance explained by each factor. The principal components were extracted in decreasing order of importance, such that the first PC accounts for much of the variation as possible and successive components have relatively less. Eigen values and Eigen vectors were calculated for the covariance matrix and the data were transformed into factors. Table 7 presents the Eigen values, percent of variance, cumulative Eigen value and cumulative percent of variance associated with each factor or component. These values are summed to express a cumulative Eigen value and percentage of variance, respectively. In this study only factors with Eigen values greater than one were retained based on the Kaiser criterion (Kaiser 1958). Two factors explain 74.43 % of the total variance in the data set. The two components which explained 74.43 % of the variance in the data set were rotated by varimax method to make interpretations. Table 7 shows the result of PCA after varimax rotation. The principal component loadings for the two components as well as their respective explained variance are presented. According to Liu et al. (2003), absolute loading values of >0.75, 0.75–0.5 and <0.5 are expressed using the terms ‘strong’, ‘moderate’ and ‘weak’ respectively.
Table 8 presents the principal component loadings for the two components as well as their respective explained variance. Component 1 explains 53.366. % of the total variance and is characterized by strong positive loadings in EC, Na, Cl, NO3, K, Ca, moderate loading for SO4, and Mg and weak loading for HCO3, Ca, pH and SiO2 (negative). The relationship among Cl, SO4 and NO3 in factor 1 is associated with the influence of human activities and is termed ‘anthropogenic’. There are many potential human related sources of chloride and sulphate in groundwater in the area, such as house hold sewage landfill leachate, poorly disposed domestic and industrial effluents, leakages from septic tanks and sewage systems and local use of fertilizers (Guevart et al. 2006; Ndjama et al. 2008; Takem et al. 2010). Apart from the above mentioned source, many industries in Douala use H2SO4, HCl and HNO3. Sulphuric acid, for example, is used in the metal processing industries, chemical manufacturing industries, manufacture of fertilizers, and manufacture of plastics, paints, perfumes and disinfectants. HCl is used in metal work shops, garages, during welding and soldering etc. and these are abundant in the area. HNO3 acid is used in many industrial processes such as in industries manufacturing nitrate fertilizers, also used in industries to manufacture explosives, food processing and diary sectors. Spilling of these acids and possible leakage from acid storage tanks do occur. The sandy nature and the high porosity and permeability of soils in the area, can lead to easy washing into groundwater system during rains. According to Ford et al. (1992), oxidation/reduction of inorganic chemicals like NH4 and NH3 from sewage and industrial processes could result in a drop in pH as seen in the equations below:
The association of Na, Cl, EC and K in factor 1 explains the effect of seawater in rainfall to the groundwater salinity. Atmospheric sea salt deposition is a possible source of chloride in groundwater considering the close proximity of the area to the coast. According to SFT (1993), the atmospheric deposition of sea salt rich precipitation could also lead to cation exchange of sea salt sodium with H+ ions bound in the soil. This could lead to the natural acidification of the shallow groundwater (SFT 1993). This phenomenon, however, needs to be verified in the future.
Component 2 explains 21.070 % of the variance and has strong positive loadings for pH, alkalinity and Ca and moderate loading for SiO2 and weak loading for SO4, Cl, NO3, Na, and K. The strong association for pH and alkalinity is an indication of acidification of the shallow groundwater. Both the pH and alkalinity of the shallow groundwater show a (Figs. 3, 4) general decreasing trend which according to Knutsson (1994) is an indication of groundwater acidification. Hultberg and Wenblad (1980) established that decreases in alkalinity over time are a good warning against future groundwater acidification. Ca, HCO3 and SiO2 also show strong positive loadings for factor 2. This factor can thus be related to the natural hydrogeochemical evolution of groundwater by chemical weathering of silicate minerals present in the aquifer sediments and soils.
Possible implication of acidity for groundwater quality and use
There are many problems related to acid groundwater including corrosion of well casings, pipes and plumbing fixtures releasing toxic metals like lead, zinc, copper and cadmium into the water (Lahermo 1991). Groundwater pH less than 4.0 produces a sour taste to the water especially when it is used as a drinking supply. One of the most important implications for soil and groundwater acidification is the mobilization of trace metals into the groundwater system. Soil acidification brings about the dissolution of trace metals in the groundwater. Acidic rainwater infiltration could dissolve heavy metals, e.g. Cd, Mn, Zn, Pb, Fe and aluminium from the soil. Aluminium is weakly soluble in neutral or slightly acidic soils, but following a decrease in pH that may result for example from acid deposition or from weak acids in humus, the solubility of aluminium increases. According to Harter (2002) below the pH of 5.5, most of the base cations (Na, Ca, Mg, K) are leached from the soil and other trace metals such as aluminium ions take over the role of acid buffer according to the following buffer reaction, \({\text{Al}}\left( {\text{OH}} \right)_{ 3} + {\text{ 3H}}^{ + } = {\text{ Al}}^{ 3+ } + {\text{ 3H}}_{ 2} {\text{O}}\). The dissolved aluminium is then susceptible to move into groundwater and surface water supplies. Takem (2012) indicated that Al in the water samples in the area exceeds the WHO permissible limits reaching more than nine (1877.89 µg/l) times the WHO guideline limits (200 µg/l) for some samples during the rainy season. Results from New York and New Hampshire equally demonstrated that comparatively high concentrations of dissolved aluminium occur in groundwater in that area due to mineral weathering by acid precipitation (Cronan and Schofield 1979).
Iron concentrations (320 µg/l) above the WHO guidelines limit for potable water have been observed in the area (Takem 2012). Iron is derived from the dissolution of iron bearing minerals such as goethite and hematite following the dissolution by acidic groundwater in the presence of organic matter. The common iron form in groundwater is the soluble ferrous iron (Fe2+) form and when it is exposed to air, the ferrous form (Fe2+) is oxidized to the ferric state (Fe3+) that is insoluble and this will precipitate as ferric hydroxide. This effect causes brown coloration of the water as is commonly the case with some water wells in the area (Mafany 1999) and could produce stains to sinks and textile. Takem (2012) has identified manganese concentrations of 80 µg/l in the area exceeding the WHO (1993) maximum guidelines limit (50 µg/l) for groundwater. Manganese is most often naturally the result of the reaction of moderately acidic groundwater on manganiferous oxides. In the presence of reducing conditions, the manganese dissolves into the mobile divalent manganous (Mn2+) ion. On exposure to air, the manganous ion is oxidized to the hydrated ion. This causes odour, colour and produces a black coloration in the water and stains plumbing equipments and washed textiles.
Conclusion
Long-term groundwater chemistry data (1998–2013), rainwater and stream quality data together with the soil chemical composition have been used in combination with multivariate statistical analysis to establish the acidification process of soils and shallow groundwater of the city of Douala. The study shows average groundwater pH range between 3.7 and 6.8, with only 11.11 % (2010–2013 study) of water samples falling within the range of pH (6.5–8.5) for potable water according to WHO (1993). The acidification process is indicated from decreases in pH, acid neutralizing capacity/alkalinity and increase in the number of zero alkalinities and increase in the concentrations of anions of strong acids SO4, NO3 and Cl. The groundwater chemistry and acidity probably result from four many origins: (1) acid atmospheric deposition, (2) coastal atmospheric deposition, (3) anthropogenic activities (industrial effluent discharges and acid spill), and (4) cation exchange of marine sodium with hydrogen and/or aluminium ions bound in the soil. The study also indicates that shallow groundwater in Douala is highly susceptible to acidification for the following reasons.
-
The soils and aquifer sediments generally consist of sands and gravels (resistant to weathering) with little or no carbonate minerals (calcite and dolomite). The rate of weathering is low giving rise to poorly buffered waters with inadequate alkalinity for acid neutralization.
-
High rainfall 4000 mm/year coupled with rapid recharge through coarse-grained sandy soils which are highly permeable to acidic rainwater and anthropogenic waste water.
-
Due to the shallow groundwater table, the contact time between water and soil/geologic media is short and hence the dissolution rate on minerals is low.
It is postulated that the continuous acid inputs into the acidic soils have led to a weakening in the buffering capacity of the soils which results to the acidification of the shallow groundwater. With regard to the implications of soil and groundwater acidification such as the increased mobilization of toxic elements (e.g. Al, Pb, Cu, Zn, Mn) as well as their possible harmful effect on health, it is recommended that the extent and the causes of groundwater acidification be investigated with more details, the rate of acidification and the mobility of trace elements should equally be investigated.
References
Ako AA, Shimada J, Hosono T, Kagabu M, Ayuk AR, Nkeng EJ, Takem GE, Takounjou ALF (2012) Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environ Geochem Health 34:615–639
APHA/AWWA/WEF (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, American Water Works Association and Water Environment Federation Publication, Washington, p 1220
Appelo CAJ, Postma D (1999) Geochemistry, groundwater and pollution. A.A. Balkema, Rotterdam, p 536
Appleyard S, Cook T (2008) Reassessing the management of groundwater use from sandy aquifers: acidification and base cation depletion exacerbated by drought and groundwater withdrawal on the Gnangara Mound, Western Australia. Hydrogeol J 17(3):579–588
Appleyard S, Wong S, Willis JB, Angeloni J, Watkins R (2004) Groundwater acidification caused by urban development in Perth, Western Australia: source distribution and implication for management. Aust J Soil Res 42:579–585
Asaah VA, Abimbola AF, Suh CE (2006) Heavy metal concentration and distribution in surface soils of the Bassa industrial zone 1 Douala Cameroon. Arab J Sci Eng 31(2A):147–158
Avila A, Alarcon M (1999) Relationship between precipitation chemistry and meteorological situations at a rural site in NE Spain. Atmos Environ 33:1663–1667
Berner EK, Berner RA (1987) The global water cycle. Prentice-Hall, Englewood Cliffs
Black CA (ed) (1965) Method of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy Inc. Publisher, Madison, p 1572
Bromssen von U (1989) Acidification trends in Swedish ground waters. Report 3576, National Swedish Environmental Protection Board, Solna
Cai M (2010) Long-term acid deposition effects on soil and water chemistry in the Noland Divide Watershed, Great Smoky Mountains National Park, USA. PhD dissertation, University of Tennessee
Campbell DH, Muths E, Turk JT, Corn PS (2004) Sensitivity to acidification of subalpine ponds and lakes in north-western Colorado. Hydrol Process 18:2817–2834
Christophersen N, Robson A, Neal C, Whitehead PG, Vigerust B, Henriksen A (1990) Evidence for long-term deterioration of stream water chemistry and soil acidification at the Birkenes catchment, southern Norway. J Hydrol 116:63–76
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313
Cronan CS, Schofield CL (1979) Aluminium leaching response to acid precipitation: effects on high elevation watersheds in the north east. Science 204:204–306
Das R, Das S, Misra V (2005) Chemical composition of rainwater and dust fall at Bhubaneswar in the east coast of India. Atmos Environ 39:5908–5916
Djeuda-Tchapnga HB, Tanawa E, Ngnikam E (2001) L’eau au Cameroun. Presses Universitaires, Yaounde, pp 115–129
Djuikom E, Temgoua E, Jugnia LB, Nola M, Banne M (2010) Pollution bactériologique des puits d’eau utilisés pas les population dans la communautés urbaine de Douala-Cameroon. Int J Biol Chem Sci 3(5):967–978
Dragon K (2006) Application of factor analysis to study contamination of a semi-confined aquifer (Wielkopolska Buried Valley aquifer, Poland). J Hydrol 331:272–279
Dumort JC (1968) Notice explicative sur la feuille de Douala-Quest, vol 69. Direction Mines et de la Geologie du Cameroun, Yaounde, p 56
Edmunds WM, Kinniburgh DJ (1986) The susceptibility of UK groundwaters to acid deposition. J Geol Soc 143:667–672
Edmunds WM, Shand P, Hart P, Ward RS (2003) The natural (baseline) quality of groundwater: a UK pilot study. Sci Total Environ 310:25–35
Fest EPMJ, Temminghoff EJM, Griffioen J, Van Riemsdijk WH (2005) Proton buffering and metal leaching in sandy soils. Environ Sci Technol 39:7901–7908
Fest EPMJ, Temminghoff EJM, Griffioen J, Van Der Grift B, Van Riemsdijk WH (2007) Groundwater chemistry of Al under Dutch sandy soils: effects of land use and depth. Appl Geochem 22:1427–1438
Ford M, Tellam JM, Hughes M (1992) Pollution-related acidification in the urban aquifer, Birmingham, UK. J Hydrol 140:297–312
Gower C, Rowel DL, Nortcliff S, Wild A (1995) Soil acidification: comparison of acid deposition from the atmosphere with inputs from the litter/soil organic layer. Geoderma 66:855–898
Guevart E, Noeske J, Solle J, Essomba J-M, Edjenguele M, Bita A, Mouangue A, Manga B (2006) Determinats du cholera a Douala. Medecin Tropical 66:283–291
Hansen BK, Postma D (1995) Acidification, buffering salt effects in the unsaturated zone of a sandy aquifer, Klosterhede, Denmark. Water Resour Res 31:2795–2809
Harter RD (2002) Acid soils of the tropics. An Echo technical note, ECHO, North Fort Myers. http://www.echonet.org
Henriksen K, Kirkhusmo LA (1982) Acidification of groundwater in Norway. Nord Hydrol 13:183–192
Hultberg H, Wenblad A (1980) Acid groundwater in south western Sweden, pp 220–221. In: Drablos D, Tollam A (eds) Ecological impact of acid precipitation. Proceedings of the international conference Sandefjord, Norway, SNSF project
Kaiser HF (1958) The varimax criteria for analytical rotation in factor analysis. Psychometrika 23(3):187–200
Kjoller C, Postma D, Larsen F (2004) Groundwater acidification and the mobilization of trace metals in a sandy aquifer. Environ Sci Technol 38:2829–2835
Knutsson G (1994) Acidification effects on groundwater-prognosis of the risks for the future. IAHS Publ 222:3–17
Krug EK, Frink CR (1983) Acid rain on acid soil: a new perspective. Sci 22:520–525
Lahermo P (1991) Aspects of acidification of groundwater in Finland. Geol Surv Finl Spec Pap 9:131–139
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313:77–89
Mafany (1999) Impact of the geology and seawater intrusion on groundwater quality in Douala. M. Sc. thesis, Buea University, p 100
Migliavacca D, Teixeira EC, Wiegand F, Machado A, Sanchez J (2005) Atmospheric precipitation and chemical composition of an urban site, Guaiba hydrographic basin, Brazil. Atmos Environ 39:1829–1844
Ndjama J, Kamgang KBV, Sigha NL, Ekodeck GE, Tita MA (2008) Water supply, sanitation and health risks in Douala, Cameroon. Afr J Environ Sci Technol 2(11):422–429
Olivry JC (1986) Fleuve et riviere du Cameroun. Collection monographies Hydrologiques. MESRE/ORSTOM, Paris
Oyebog SA, Ako AA, Nkeng GE, Suh CE (2012) Hydrogeochemical characteristics of some Cameroon bottled waters, investigated by multivariate statistical analyses. J Geochem Explor 112:118–130
Regnoult JM (1986) Synthese Geologique du Cameroun. Ministere de Mine et de L’energy, Yaounde, p 119
Rosenthal E (1987) Chemical composition of rainfall and groundwater in recharge areas of the Bet Shean-Harod multiple aquifer system, Isreal. J Hydrol 89:329–352
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin. Japan Environ Model Softw 22:464–475
Sigha-Nkamdjou L, Galy-Lacaux C, Pont V, Richard S, Sighomnou D, Lacaux JP (2003) Rainwater chemistry and wet deposition over the equatorial forested ecosystemof Zoetele (Cameroon). J Atmos Chem 46:173–198
SNEC (1988) Rapport technique sur le forages des Massoumbou. Societé Nationale des Eaux du Cameroon
Statlig Program for Forurensningstilsyn (SFT) (1993) Monitoring of long-transported polluted air and precipitation. Annual report, Oslo, SFT report no. 533/93, p 296
Takem GE (2012) Hydeogeochemistry and pollution characteristics of the unconfined urban aquifer of the city of Douala-Cameroon: implications for shallow groundwater management and use. PhD thesis, University of Buea
Takem GE, Chandrasekharam D, Ayonghe SN, Thambidurai P (2010) Pollution characteristics of alluvial groundwater from springs and bore wells in semi-urban informal settlements of Douala, Cameroon, Western Africa. Environ Earth Sci 61:297–298
Tamfu S, Batupe M (1995) Geologic setting, stratigraphy and hydrocarbon habitat of the Douala Basin Cameroon. Natl Hydrocarb J Cameroon 3:6
Vengosh A, Rosenthal E (1994) Saline groundwater in Israel: its bearing on the water crisis in the country. J Hydrol 156(389):430
Wels C, Cornet RJ, Lazerte BD (1990) Groundwater and wetland contribution to stream acidification: an isotopic analysis. Water Resour Res 26(12):2993–3003
World Health Organisation, WHO (1993) Guidelines for drinking water quality. Revision of the 1984 guidelines. Final task group meeting, Geneva, 21–25 Sept 1992
Acknowledgments
This work constitutes part of data generated during the PhD study of the corresponding author, who was supported by a fellowship from the Third World Organization for Women in Science (TWOWS). We thank IITB and the Institute of Geological and Mining Research (IRGM) for providing laboratory and infrastructure facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takem, G.E., Kuitcha, D., Ako, A.A. et al. Acidification of shallow groundwater in the unconfined sandy aquifer of the city of Douala, Cameroon, Western Africa: implications for groundwater quality and use. Environ Earth Sci 74, 6831–6846 (2015). https://doi.org/10.1007/s12665-015-4681-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4681-3