Abstract
In order to study the heavy metal accumulation and distribution in the roots, stems, and leaves of Spartina alterniflora, we collected S. alterniflora samples and the associated sediments along three transects at the Andong tidal flat, Hangzhou Bay. Co, Ni, Cd, Pb, Cu, and Zn were mainly accumulated in the aerial parts (stems and leaves) of the plants, and their distributions depended on their mobility and their roles during the metabolism processes of S. alterniflora. The concentrations of Cu, Zn, Cd, Hg, and Pb were significantly enhanced with the increasing of heavy metal concentrations in the sediments, while those of Co and Ni remained relatively constant. Bioaccumulation factors results showed that the serious heavy metal contamination in the sediments from the transect A may overwhelm the accumulation capability of the plants. In addition, the physicochemical properties of the sediments and the pore water therein also play a role in the heavy metal concentrations and accumulations in the plants, because they can influence the behaviors and bioavailabilities of heavy metals during nutrition and bioaccumulation processes of the plants. The sediments with vegetation did not show significantly decreased heavy metal concentration with respect to the unvegetated sediments, although the plants did absorb heavy metals from the sediments. Principal component analysis and correlation analyses indicated that Co–Ni, Cu–Cd–Hg behaved coherently during accumulation, which may be ascribed to their similar accumulation mechanisms. This work provided essential information on the heavy metal accumulation by plants in a tidal flat, which will be useful for the environmental control through phytoremediation at estuaries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are harmful and widespread in the environment, especially in the estuary zone such as tidal flats and salt marshes. They may originate from both natural and anthropogenic processes (Markert and Friese 1999). These pollutants are characterized by high toxicity, persistence, and bioaccumulative behaviors. Previous studies suggested that heavy metals may pose a significant threat to human health and living organisms and therefore produce a serious damage for natural ecosystem (Bryan and Langston 1992; Williams et al. 1994; Wong et al. 2002; Diagomanolin et al. 2004; DeForest et al. 2007). Consequently, it is important to study the heavy metal pollution within the studied coastal environment (Marcovecchio 2000; Hempel et al. 2008).
Numerous studies reported the occurrence and distribution of heavy metals in the sediments and plants from tidal flats at estuary zones (Marcovecchio 2000; Ferrer et al. 2006). The periodical tide can carry large quantities of pollutants which can be accumulated in the sediments of tidal flats. In addition, the tidal flat plays an important role in the biogeochemical cycling of pollutants through their active and positive circulation mechanisms (Weis and Weis 2002). Vegetation can absorb the nutrients and metals from the sediments when their concentrations are relatively high (Reboreda and Cacador 2007a; Cacador et al. 2009; Almeida et al. 2004; Weis et al. 2003; Windham et al. 2003). The bioaccumulation process is depended on the mobility and bioavailability of metals as well as the physicochemical characteristics of the sediments such as pH, salinity, redox potential, organic matter content, grain size (Alloway et al. 1990). These physicochemical properties could change the microenvironment and therefore affect the bioavailability of metals in the tidal flats (Windham and Lathrop 1999; Windham et al. 2003). The uptake and accumulation of heavy metals by plants follow two different paths: (1) by the root system and (2) by the foliar surface (Sawidis et al. 2001). Path (1) is usually the dominated process. Therefore, tidal flat is often considered to be a sink for heavy metals (Cacador et al. 1996, 2000; Weis et al. 2004; Reboreda and Cacador 2007b), where the heavy metals play key roles for the local ecological systems.
Hangzhou bay is a typical macro-tidal estuary along the east coast of China, and the Andong tidal flat is one turning point of the hydrodynamic environment within Hangzhou Bay. It is widely accepted that the Andong tidal flat is the transition zone from middle tidal to spring tidal, open sea to semi-enclosed sea, and also the influence boundary between the Qiantang River and Yangtze River (Su and Wang 1989). In recent years, Hangzhou Bay suffered high urbanization and industrial activities. Large quantities of pollutants were discharged into Hangzhou Bay (Marcovecchio and Ferrer 2005; McGann 2008). Therefore, the heavy metal contamination in Hangzhou Bay attracted great attentions (Zhang et al. 2001; Deng et al. 2004). For instance, Liu et al. (2012) investigated the distribution of major and trace elements in the surface sediments of Hangzhou Bay and suggested that the anthropogenic impact was enriched near the Qiantang River mouth.
The Andong tidal flat in Hangzhou Bay is largely covered by Spartina alterniflora. In China, S. alterniflora was intentionally introduced from North America in 1979 to control the erosion of flats, improve the soil quality and protect the dikes. Nowadays, S. alterniflora has been a predominant macrophytes in the estuaries of China (Huang et al. 2008; Jiang et al. 2009; Zuo et al. 2009). S. alterniflora can accumulate metals from sediments via roots and transport some of them to its aerial structures. The metals can be stored in the belowground parts as well (Weis and Weis 2004). In addition, S. alterniflora can transport oxygen through aerenchyma to the rhizosphere, and therefore the absorbed metals are enriched in the roots (Burke et al. 2000). This phenomenon is similar to the cases of other marsh plants that the absorbed metals tend to accumulate in the belowground tissues (Burke et al. 2000; Reboreda and Cacador 2007a; Hempel et al. 2008; Duarte et al. 2010). Current studies on the S. alterniflora at coastal zones of China mainly focused on its ecological and physiological characteristics (Gallagher et al. 1980; Fang et al. 2004; Davis et al. 2004), its restoration for tidal flats (Gallagher et al. 1976; Gallagher and Plumley 1979; Valery et al. 2004; Mendelssohn and Kuhn 2003), its effect on the biogeochemical processes of intertidal ecosystem in coastal region (Zhou et al. 2008), and its influence on the uptake and distribution of N, P, and metals in the Yangtze River estuary (Quan et al. 2007).
To date, however, the research on the accumulation and distribution of heavy metals in the organs of S. alterniflora within Hangzhou Bay is scarce. The main objectives of this study are to: (1) assess the accumulation and distribution of heavy metals (Co, Ni, Cd, Hg, Pb, Cu, and Zn) in the organs of S. alterniflora and the associated sediments from the Andong tidal flat; (2) comprehend the relationship between heavy metal concentrations in the plants and sediments and explore the role of S. alterniflora on the translocation of heavy metals in the ecosystem of tidal flat; (3) preliminarily identify the accumulation mechanisms of heavy metals by the S. alterniflora.
Geological settings
Hangzhou Bay, which lies in the northeast of Zhejiang province and covers an area of about 8500 km2, embodies one of the largest tidal gulfs in the world (Xie et al. 2010). Hangzhou Bay can be divided into an inner bay and an outer bay according to the hydrological and sediment characteristics of the bay (Fig. 1) (Chen et al. 1990). The sediments of the triangle shaped Hangzhou Bay mainly come from: (1) the particles carried by the Qiantang River and Yangtze River and forced by the river flows and tidal currents (Milliman et al. 1985; Su and Wang 1989); (2) sediments formed by the erosion of the seabed of the East China Sea (Dai et al. 2014). The sediments from the Yangtze River and Qiantang River played an important role in the distribution of heavy metals in the sediments and the anthropogenic influences were concentrated near the Qiantang River mouth (Li and Xie 1993; Liu et al. 2012; Pan and Wang 2012).
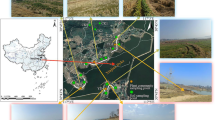
a Detailed sampling sites and b the geographic location of the studied area in Andong tidal flat, Hangzhou Bay, China. The blue lines indicate the main tidal creeks along the sampling transects. The yellow line shows the passageway of local fishermen to catch aquatic products. The red tags indicate the plant samples and the associated sediments samples, while the green tags are the unvegetated sediment samples. The red spheres in (b) indicate the factories near the Andong tidal flat, including chemical plants, steel plants, plastics factories
The Andong tidal flat, which is located at the southern part of Hangzhou Bay, situates near the boundary between the inner bay and outer bay. The Andong tidal flat is composed by tidal flats, tidal slopes, and tidal creeks, covering an area of about 300 km2. The tidal flat is dominated by silt and clayed silt (Li and Xie 1993). The clayed silts are mainly distributed in the nearshore areas, and the silt sediments are widespread in the tidal flat and tidal creek areas. The sedimentation rate of the Andong tidal flat was 2.0–4.5 cm a−1 in recent centuries (Li 1993; Guo et al. 2004), and the average annual deposition volume of sediments was 6 × 107 t a−1 in recent decades (Li and Xie 1993). The Andong tidal flat received considerable anthropogenic pressures from engineering constructions, local aquaculture, and industrial activities from adjacent towns and cities (red spheres in Fig. 1b).
The Andong tidal flat is mainly covered by halophyte vegetation with a width of about 5 km from shore to offshore and an area of 100–150 km2. S. alterniflora is the most commonly and widely distributed speciation. Our previous study investigated the heavy metal concentrations in the sediments from the Andong tidal flat and found that a transect was severely polluted by heavy metals (Pang et al. 2015). Therefore, it is essential to investigate the accumulation and distribution of heavy metals in the S. alterniflora from the Andong tidal flat, which may provide important information for the ecological, environmental, and geochemical studies on estuaries.
Materials and methods
Sampling
We collected 17 plant samples and 29 associated sediment samples from the Andong tidal flat during low tide. All of our samples were collected by hands that wore pre-cleaned gloves. We conducted three transects in this tidal flat, and the sample locations were recorded by a handhold GPS (Fig. 1). The samples from the transect A (A1–A8) were collected on August 14, 2014; the samples from the transect B (B1–B10) and transect C (C1–C11) were collected on September 14, 2014. Both sampling days were cloudy with light raining. The transect A is situated along a major passenger way and a creek in this area and therefore may be significantly affected by human activities (Pang et al. 2015). The transects B and C are relatively natural and should be excluded from significant human pollution. The sample locations include both vegetated and unvegetated areas. Therefore, these transects are suitable to investigate the accumulation of heavy metals by the plants and the resilience of plants to the heavy metal contaminations. In each site, duplicate S. alterniflora samples were collected and the adjacent sediment samples were also collected for referencing. Furthermore, offshore sediments (A5–A8, B8–B10, C7–C11) where plants were absent were also collected by the same method for comparison. The collected samples were stored in polyethylene bags and preserved in an icebox (T = 10–15 °C). The samples were taken back to laboratory for subsequent analyses.
Pretreatment of the samples
Digestion of the sediment samples
The sediment samples were dried in an oven at 50 ± 5 °C overnight to a constant weight and were ground in an agate grinder until homogeneous particles were obtained. Then, the samples were digested by concentrated HNO3 and H2O2 (Fernandez-Cadena et al. 2014) using the following procedures: 1.00 g sediment sample was weighted and placed into flasks with distillation device. 10.0 mL (1:1) HNO3 was added to each flask. Each mixture was gently shaken using a magnetic stirrer and then placed in an aluminum-heating block. The digestion of the sediment samples was performed as the following sequence of operations: heat the sample to 200 °C, stop heating to non-boiling, and reflux the mixture for 10–15 min. After cooling, 5 mL concentrated HNO3 was added to the residual mixture and the mixture was heated at 200 °C for 30 min again. The above steps were repeated until there was no brown fume. Then, the solution was evaporated to about 5 mL in the state of non-boiling. After finishing the above steps, 2 mL distilled water and 3 mL H2O2 were added into the cooled sample, and the mixture was heated again till there was no bubble. The mixture was shaken gently and poured into polyethylene tubes. Then, it was diluted to 40 mL and centrifuged prior to elemental analyses.
Pretreatment of the plant samples
All of the plant samples were washed thoroughly by tap water to remove the attached impurities such as mud and sand. Then, each sample was divided into leaves, stems, and roots for our subsequent study. Each part was cut into small pieces by plastic scissors in order to avoid metal interference. Then, these pieces were washed thoroughly by distilled water (Phillips et al. 2015; Bonanno and Lo Giudice 2010). The samples were dried in a temperature-controlled oven at 50 ± 5 °C for 48 h and then were calcined in a muffle furnace (Nabertherm, L3/11/B180) at 500 °C for 90 min. The ashed plant samples were ground by a mortar for the following digestion procedures (Cacador et al. 2000; Padinha et al. 2000).
A portion of ashed plant samples (0.20 g) was weighted and placed into flasks with distillation device. Then, 10 mL mixture of HNO3–HClO4 (4:1 v/v) and a magnet were added into each flask. The mixture was gently shaken using a magnet and then placed in an aluminum-heating block. The operation procedures were as follows: heat the sample to approximately 100 °C, stop heating to non-boiling until nitrous fumes formed, then boil the mixture to approximately 200 °C. Repeat the above steps till there was no brown fume, and then evaporate the solution to about 5 mL in the state of non-boiling. After finishing the above steps, cool the sample, transfer it into a centrifugal tube, and dilute it to 50 mL with deionized water (Quan et al. 2007).
Elemental determination
The digested solutions from the sediment and plant samples were centrifuged at 3000 rpm for 5 min prior to the analyses of elemental concentrations. Metal concentrations (Co, Ni, Cd, Hg, Pb, Cu, and Zn) were determined by Inductively Coupled Plasma–Mass Spectrometry (ICP–MS, Thermo Fisher Scientific, XSeries II). The standard stock solution was diluted by 1% HNO3 step by step. Some 10 μg L−1 mixed standard solutions were measured to determine the sensitivity of the analyses. Reagent blanks, duplicate samples, and multi-elements standard solutions (Chinese national standard, GBS-04 series) were used for quality controls. The errors of the analyses as relative standard deviation were less than 10%. The detection limit (unit μg L−1) of ICP-MS for each metal was: Co 0.0035, Ni 0.036, Cd 0.0051, Hg 0.00024, Pb 0.016, Cu 0.075, Zn 0.00024. The calculated detection limits (unit: μg kg−1) for each metal were: in the sediment samples, Co 0.14, Ni 1.44, Cd 0.204, Hg 0.0096, Pb 0.64, Cu 3, Zn 0.0096; and in the plant samples, Co 0.875, Ni 9, Cd 1.275, Hg 0.06, Pb 4, Cu 18.75, Zn 0.06. The recoveries for all metals in both sediment and plant samples ranged from 90 to 110%. The operating parameters of ICP-MS were: 1200 WRF power and scanning mode of peak jumping, sampling time of 20 s with a dwell time of 1000 μs, sampling depth of 120 mm, and sample extraction yield of 1.0 mL min−1. The total injection time for each sample was 60 s, and the cleaning time was maintained at 60 s. The flowing rates of cooling air, auxiliary gas, and atomization gas were 14.0, 0.75, and 0.92 L min−1, respectively.
The physicochemical characteristics of the sediments
The temperatures (T, °C) of the sediments were measured in situ using a TES-1310 thermometer with a thermocouple (TES, Taiwan). The total organic carbon (TOC, %) and total nitrogen (TN, %) of the sediment samples were measured by a 2400 Series II CHNS/O Analyzer (PerkinElmer, US). The grain size of the sediments was measured by a Microtrac S3500 Laser Particle Size Analyzer (Microtrac, US). The measuring range and accuracy of the instrument were 0.02–2800 μm and 0.6%, respectively.
The pore water was extracted by a RHIZON 1921SA soil solution sampler with a diameter of 2.5 mm. The pH values of the sediments and pore water were measured by a TZS-pH-I pH meter (Tuopu, China). The pH calibration was performed using buffer solutions of pH 4, pH 7, and pH 10. The redox potential (Eh value) was measured by a ORP-401 m (Shanghai, China) with a platinum electrode.
Statistical analysis
Statistical analyses on the data were performed by a SPSS Windows release 18.0. Pearson’s correlation coefficients were used to verify the relationships among variables. Principal component analysis (PCA) was used to distinguish the associations among these elements (Armid et al. 2014). Bioaccumulation factors (BAF) were calculated to assess the accumulation of heavy metals by the S. alterniflora. The BAF was obtained by dividing the trace element concentrations in the plant samples by that in the sediments. The calculation equation is listed as follows:
where X refers to the concentration of the assessed heavy metal. BAF value of >1 indicates the significant accumulation of heavy metals by S. alterniflora from the sediments (Idaszkin et al. 2014).
Results and discussion
Heavy metal concentrations in the plant samples
Heavy metal concentrations in the different organs of S. alterniflora from the transects A, B, and C are given in Figs. 2, 3, and 4, respectively. The raw data can be found in Table S1. All of the samples exhibited higher concentrations of Cu and Zn with respect to other heavy metals. Both Cu and Zn were enriched in the stems and leaves. The accumulations of Cu and Zn in the aerial parts may be attributed to their roles in the plants. Cu and Zn play vital roles in the nutrition and enzymatic activities of plants (Bonanno and Lo Giudice 2010). Cu can be present in many oxidizing enzymes involving the redox processes of plants. In addition, Cu can also act as the components of chloroplast and participate in the electron transfer process during the photosynthesis (Lee et al. 2012). Zn is the metal activator of enzymes. It distributes in the chloroplast and promotes the formation of carbohydrates (Grill et al. 1989). In addition, Larsen (1983) and Lehtonen (1989) proposed that Zn is essentially important for the biosynthesis of the plant growth hormone indolyl-3-acetic acid, which is primarily active in the stems.
Co was mainly accumulated in the leaves with concentrations of less than 20 mg kg−1 in all three transects. Co is an essential nutrition element for plants and plays an important role for the plant physiology, such as the responses of stress and loads of process controls (Bush 1995). In addition, Co can fix the chloroplast membrane protein complexes, which are also concentrated in the leaves (Hajar et al. 2014). Ni was also mainly concentrated in the leaves of S. alterniflora with concentrations of 2–32 mg kg−1. Ni is a toxic element for plants, and it can be significantly enriched in the leaves at the end of the growing period (Sainger et al. 2011). Therefore, it is reasonable to suggest that the accumulation of Ni in the plant leaves in this study may be ascribed to fact that we sampled mature plants in August and September.
Cd concentrations ranged from 0.03 to 4.73 mg kg−1 in the different organs of S. alterniflora. The distribution of Cd was relatively homogenous in all parts of the plants from the transect A and was mainly accumulated in the leaves in the transects B and C. Cd is a highly toxic and nonessential element, and it can hinder the growth and metabolism process of plants (Scholze et al. 1988; Divan Junior et al. 2009). Cd is rather mobile in the sediments, and it is readily available for plants (Madejon et al. 2004). It is suggested that Cd tends to be enriched in the aerial parts rather than the belowground parts of S. alterniflora (Hempel et al. 2008). Cd could also go into the root cells by competitive relationship with nutrients and then be transferred into the stems and leaves by leaf vacuoles (Almeida et al. 2004; Reboreda and Cacador 2007b; Vymazal et al. 2007). In addition, some studies suggested that ultrastructural modifications of aerial organs of plants in case of high Cd concentrations could lead to Cd accumulation in the stems and leaves (Pietrini et al. 2003).
Hg concentrations varied from below detection limit (<0.002 mg kg−1) to 0.27 mg kg−1. Hg concentrations were relatively homogenous in all parts of S. alterniflora with slightly enrichment in the roots of the samples from the transect A, and in the roots and leaves from the transects B and C. Hg is a toxic metal especially when its availability increased (Scholze et al. 1988). Recent reports suggested that the plants in aquatic environments preferably accumulate Hg in the roots (Fay and Gustin 2007; Zhang and Wang 2013), while early literatures reported that Hg was enriched in the leaves because the absorbed Hg can be quickly transported upwards (Shaw 1986; Lindberg et al. 1979). In addition, Hg enrichment in the leaves could also due to the Hg absorption from atmosphere by stomas (Ericksen et al. 2003).
Pb is considered as more toxic than other metals (Kabata-Pendias and Mukherjee 2007). Pb concentrations varied in three orders of magnitude among different samples. In the transects A and C, Pb was concentrated in the aerial parts, especially in the leaves. In the transect B; however, Pb was mainly enriched in the roots. Previous studies on the trace element accumulation in Phragmites australis showed that anthropogenic emission was a primary origin for Pb (Bonanno and Lo Giudice 2010). In addition, Pb accumulation in the leaves may be additionally affected by the exposure to the waste gas exhausted from automobiles (Schierup and Larsen 1981; Djingova et al. 2003; Bonanno and Lo Giudice 2010). Adjacent to our studied area, there are some factories (Fig. 1) producing mechanical and electrical equipment, auto parts, and nonferrous metals. These factories may act as sources for the heavy metals. In addition, Pb is an immobile element for its strong binding to organic matters and other components in plants (Aksoy et al. 2005; Mazej and Germ 2009).
The sediments from the transect A exhibited the highest heavy metal concentrations in the plants, while the transects B and C showed lower values, suggesting that the plants from the transect A were severely polluted by the assessed heavy metals (Pang et al. 2015). As shown in Fig. 5, Cu concentration in the transect A was elevated in all organs of the plant samples, Zn and Ni showed slightly increased concentrations in the stems and leaves, and Cd was especially accumulated in the stems. It is suggested that heavy metals could be adsorbed and accumulated by the plants, and some heavy metals may be eventually transported to the aerial parts of the plants according to their functions during metabolic processes. Hg concentrations in the transect A exhibited the highest values in the roots. It may be attributed to the severe contamination of Hg in the sediments, which overwhelmed the transportation ability of S. alterniflora (D’Souza et al. 2013; He et al. 2014; Wu et al. 2014). The Co concentrations in the plant samples from the transects B and C were slightly higher than those of transect A, while Co concentrations in the transect A sediments were much higher than those in the transects B and C (Table S2). This contradiction may be attributed to the fact that Co in the plants is limited by the metabolism of plants. Therefore, Co concentrations changed slightly with the background values (Bush 1995; Hajar et al. 2014).
Accumulation of heavy metals in the plants
In order to investigate the accumulation of heavy metals in the plants, we calculated the bioaccumulation factors (BAFs) of heavy metals by S. alterniflora (Idaszkin et al. 2014). As shown in Table 1, Co and Ni exhibited BAF values of less than 1 in most of the transects and organs, suggesting that the accumulations of Co and Ni by the plants were quite limited. Cd, Hg, Cu, and Zn showed BAF values between 1 and 5 in the transects B and C, indicating that these elements were significantly accumulated and were easily adsorbed by the plants (Bonanno and Lo Giudice 2010; Divan Junior et al. 2009; Zhang and Wang 2013). Most of the heavy metals presented BAF values of leaves > stems > roots, because these elements were essentially transported upward according to their functions during the metabolism of the plants. However, another possibility is that the S. alterniflora will be submerged by water during high tides. The leaves may additionally absorb metals from the water. As a consequence, the leaves presented enhanced BAF values with respect to the roots. An exception was Pb which exhibited BAF values of roots > stems > leaves, maybe because the Pb contamination in the transect B sediments has exceeded the transportation capability of the plants (Lisamarie et al. 2001; Windham et al. 2003). Note that all of the BAF values in the transect A were below 1 and were much lower than those in the transects B and C. It is ascribed to the fact that the transect A was a seriously polluted area, and the heavy metal contamination overwhelmed the accumulation capabilities of the plants (D’Souza et al. 2013; Wu et al. 2014). As a result, the BAF values were much lower although the heavy metal concentrations in the plants were significantly higher than those in the transects B and C.
The heavy metal distribution and accumulation in the plants may also be affected by the physicochemical characteristics of the sediments such as T, TOC, TN, pH, and grain size. As shown in Table S3, all of the sediment samples (and the pore water therein) from all transects exhibit relatively comparable physicochemical properties, although the samples from the transect A showed slightly higher T, lower TOC and TN, lower grain size, and lower pH values. Table S4 shows the correlations between the physicochemical properties and the heavy metal concentrations of the sediment samples. It is suggested that the temperature of the sediments was positively correlated (0.05 level) with Co, Cd, Hg, and Pb. The TOC, TN, pH, and grain size of the sediments were negatively correlated with their heavy metal concentrations, although the correlations were not significant. The pH and Eh of the pore water in the sediments, however, significantly (0.01 level) negatively correlated with almost all of the heavy metals. It is ascribed to the fact that the pH and Eh values control the balance between the sediment and the pore water, where lower pH values and a reducing environment facilitate the accumulation of heavy metals in the sediment.
These physicochemical properties can further affect the heavy metal accumulation in the plant samples. As shown in Table S5, the physicochemical properties of the sediments and the pore water therein did not show significant correlations with some of the heavy metals (Ni, Pb, and Zn) in the plants. Co positively correlated with TOC, TN, pH, and grain size, because Co is a nutrient for plants and it will be absorbed together with other nutrients. Cd, Hg, and Cu negatively correlated with the TOC and TN of the sediments and the pH and Eh values of the pore water. It is suggested that all of these heavy metals are biological toxic elements and they are unfavorable during the nutrition of the plants. In addition, the accumulations of these heavy metals were significantly affected by the pH and redox conditions of the pore water, which can change the speciation of these metals and consequently affect their bioavailabilities by the plants.
On the other hand, the bioaccumulation of heavy metals by the plants can in turn affect the heavy metal concentrations of the associated sediments. Although the plants can also absorb heavy metals from water besides the sediments, the heavy metal concentrations of the pore water within sediments are associated with the sediments. The heavy metals in the sediments and the pore water are in equilibrium and the metal concentrations of the sediments can represent the overall heavy metals that the plant can absorb (Doyle et al. 2003; Katsev et al. 2006; Santos-Echeandia et al. 2010) giving the fact that the physicochemical characteristics of the sediments are comparable. As shown in Fig. 6, however, the vegetation of the plants did not positively decrease the heavy metal concentrations of the associated sediments. The unvegetated sediments actually exhibited lower heavy metal concentrations than those with plants, although the plants do accumulate heavy metals from the sediments. This phenomenon was ascribed to two reasons: (1) The heavy metals removed by the plants were trivial for the total amounts of heavy metals in the sediments. Therefore, the heavy metal concentrations in the sediments were only slightly affected by the accumulation by plants. (2) The vegetated sediments are near shore and experienced stronger anthropogenic pollution and showed higher heavy metal concentrations with respect to the unvegetated sediments which located offshore.
Multi-statistical analyses
Pearson’s correlation coefficients and PCA analyses are widely used to distinguish the correlations among elements and the sources of these elements. As shown in Table S6, almost all of the heavy metals in the sediments significantly positively correlated among each other. It is indicated that they were either derived from similar sources, or experienced analogous biogeochemical or accumulation processes (Ghandour et al. 2014; Maanan et al. 2015; Armid et al. 2014). In the S. alterniflora samples, however, only Co–Ni and Cu–Cd–Hg exhibited significant positive correlations (Table 2). The different correlations of heavy metals in the plants and sediments may be attributed to the differences in the bioavailability of trace metals for the plants. It is determined by many factors including the affinity of heavy metals, the speciation of heavy metals, the functions of heavy metals in the plants, the physicochemical properties of sediments or waters (Baldantoni et al. 2004; Kumar et al. 2006; Mishra et al. 2008; Zhang and Wang 2013). The Cd–Hg–Cu correlations and Co–Ni correlation in the plants suggested their analogous accumulation mechanisms in the plants, which is consistent with previous arguments on the accumulation processes of these metals in the plants (Almeida et al. 2004; Reboreda and Cacador 2007b).
The PCA analyses showed similar results to the correlation analyses. As shown in Table 3, all of the heavy metals in the S. alterniflora can be grouped into two principal components, which occupied appropriately 79.30% of the total variance. The first component (PC1) generated 49.68% of total variance and exhibited high weights (>0.7) for Cd, Hg, and Cu, and moderate weights for Ni and Zn. The second component (PC2) accounted for 29.63% of the total variance, showing high weights for Co and Ni and moderate weight for Pb. Ni exhibited high loadings of >0.6 in both components. Trace metals in the sediments could be divided into two components (Table S7), accounting 86.87% of the total variance. The first component (PC1) produced 69.61% of the total variance and presented high weights (>0.7) for Co, Ni, Cd, Hg, Pb, and Zn, and moderate weight (0.62) for Cu. The second component (PC2) occupied about 17.28% of the total variance and exhibited high weight (0.76) for Cu (Bonanno and Lo Giudice 2010).
Figure 7 shows the loading plots of heavy metals in S. alterniflora and in the associated sediments, respectively. The results showed that the heavy metals in the plants could be grouped into two parts using 0.6 as a boundary value: The first group includes Co, Pb, and Ni, and the second group is comprised of Cd, Hg, Zn, and Cu. The heavy metals in the sediment samples could also be divided into two groups, the single Cu group, and the second group comprised of Zn, Cd, Hg, Ni, Co, and Pb. These results suggested that (Co, Ni, Pb) and (Zn, Cu, Hg, Cd) behaved relatively coherently during the accumulation processes although they can be divided into different groups in the sediments. Furthermore, the two-factor cluster analysis (Fig. 7c) suggested that the plant and the sediment samples are not correlated, indicating that the bioaccumulation of the heavy metals was largely affected by the bioavailabilities of the heavy metals by the plants, which is consistent with former statements.
Conclusion
In summary, we collected marsh plant (S. alterniflora) samples and the associated sediments from the Andong tidal flat, Hangzhou Bay, and investigated the heavy metals accumulation in different organs of S. alterniflora. Most of the heavy metals including Co, Ni, Cd, Pb, Cu, and Zn were accumulated in the aerial parts (stems and leaves) of the plants. The heavy metal distributions were determined by their mobility in the plants and their roles during metabolic processes. With increasing heavy metal contaminations in the transect A, the accumulation of most of the heavy metals (such as Cu, Zn, Cd, Hg, and Pb) in the plants was significantly enhanced, while that of Co and Ni remained relatively constant. Most of the heavy metals except Pb exhibited BAF values of >1 and presented BAF values in the order of leaves > stems > roots in the transects B and C. In the transect A, however, most of the BAF values were less than 1, suggesting that the heavy metal contamination in the transect A may has exceeded the accumulation capabilities of the plants. PCA and correlation analyses indicated that Co–Ni and Cu–Cd–Hg behaved coherently during accumulation, while the correlations among other elements were disturbed due to their different accumulation mechanisms. The bioaccumulation of the heavy metals may be additionally affected by the physicochemical properties of the sediments and the pore water therein, which can influence the speciation and the behaviors of the heavy metals during nutrition and accumulation processes. Although the plants can adsorb and accumulate heavy metals from the sediments, the heavy metal contamination of the sediments cannot be relieved since the sediments with dense vegetation showed analogous heavy metal concentrations to those of unvegetated sediments. This study provides essential information on the heavy metals accumulation by plants in estuaries, and we will investigate the accumulation mechanisms in depth in our subsequent study.
References
Aksoy A, Duman F, Sezen G (2005) Heavy metal accumulation and distribution in narrow-leaved cattail (Typha angustifolia) and common reed (Phragmites australis). J Freshw Ecol 20(4):783–785. doi:10.1080/02705060.2005.9664806
Alloway BJ, Jackson AP, Morgan H (1990) The accumulation of cadmium by vegetables grown in soils contaminated from a variety of sources. Sci Total Environ 91:223–236. doi:10.1016/0048-9697(90)90300-J
Almeida CMR, Mucha AP, Vasconcelos MT (2004) Role of different salt marsh plants on metal retention in an urban estuary (Lima estuary, NW Portugal). Estuar Coast Shelf Sci 91(2):243–249. doi:10.1016/j.ecss.2010.10.037
Armid A, Shinjo R, Zaeni A, Sani A, Ruslan R (2014) The distribution of heavy metals including Pb, Cd and Cr in Kendari Bay surficial sediments. Mar Pollut Bull 84(1–2):373–378. doi:10.1016/j.marpolbul.2014.05.021
Baldantoni D, Alfani A, DiTommasi P, Bartoli G, De Santo AV (2004) Assessment of macro and micro element accumulation capability of two aquatic plants. Environ Pollut 130(2):149–156. doi:10.1016/j.envpol.2003.12.015
Bonanno G, Lo Giudice R (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10(3):639–645. doi:10.1016/j.ecolind.2009.11.002
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy-metals in sediments with special reference to United-Kingdom estuaries—a review. Environ Pollut 76(2):89–131. doi:10.1016/0269-7491(92)90099-V
Burke DJ, Weis JS, Weis P (2000) Release of metals by the leaves of the salt marsh grasses Spartina alterniflora and Phragmites australis. Estuar Coast Shelf Sci 51(2):153–159. doi:10.1006/ecss.2000.0673
Bush DS (1995) Calcium regulation in plant-cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Bioil 46:95–122
Cacador I, Vale C, Catarino F (1996) Accumulation of Zn, Pb, Cu, Cr and Ni in sediments between roots of the Tagus estuary salt marshes, Portugal. Estuar Coast Shelf Sci 42(3):393–403. doi:10.1006/ecss.1996.0026
Cacador I, Vale C, Catarino F (2000) Seasonal variation of Zn, Pb, Cu and Cd concentrations in the root-sediment system of Spartina maritima and Halimione portulacoides from Tagus estuary salt marshes. Mar Environ Res 49(3):279–290. doi:10.1016/S0141-1136(99)00077-X
Cacador I, Caetano M, Duarte B, Vale C (2009) Stock and losses of trace metals from salt marsh plants. Mar Environ Res 67(2):75–82. doi:10.1016/j.marenvres.2008.11.004
Chen J, Liu C, Zhang C, Walker HJ (1990) Geomorphological development and sedimentation in Qiantang Estuary and Hangzhou Bay. J Coast Res 6(3):559–572
Dai Z, Liu JT, Xie HL, Shi WY (2014) Sedimentation in the Outer Hangzhou Bay, China: the influence of Changjiang sediment load. J Coast Res 30(6):1218–1225. doi:10.2112/JCOASTRES-D-12-00164.1
Davis HG, Taylor CM, Civille JC, Strong DR (2004) An Allee effect at the front of a plant invasion: Spartina in a Pacific estuary. J Ecol 92(2):321–327
DeForest DK, Brix KV, Adams WJ (2007) Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat Toxicol 84:236–246. doi:10.1016/j.aquatox.2007.02.022
Deng H, Ye ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut 132(1):29–40. doi:10.1016/j.envpol.2004.03.030
Diagomanolin V, Farhang M, Ghazi-Khansari M, Jafarzadeh N (2004) Heavy metals (Ni, Cr, Cu) in the Karoon Waterway River, Iran. Toxicol Lett 151(1):63–68. doi:10.1016/j.toxlet.2004.02.018
Divan Junior AM, de Oliveira PL, Perry CT, Atz VL, Azzarini-Rostirola LN, Raya-Rodrigueze MT (2009) Using wild plant species as indicators for the accumulation of emissions from a thermal power plant, Candiota, South Brazil. Ecol Indic 9(6):1156–1162. doi:10.1016/j.ecolind.2009.01.004
Djingova R, Kovacheva P, Wagner G, Markert B (2003) Distribution of platinum group elements and other traffic related elements among different plants along some highways in Germany. Sci Total Environ 308(PII S0048-9697(02)00677-01-3):235–246. doi:10.1016/S0048-9697(02)00677-0
Doyle C, Pablo F, Lim R, Hyne RV (2003) Assessment of metal toxicity in sediment pore water from Lake Macquarie, Australia. Arch Environ Contam Toxicol 44:0343–0350. doi:10.1007/s00244-002-2003-8
D’Souza Varun M, Pratasb J, Paula MS (2013) Spatial distribution of heavy metals in soil and flora associated with the glass industry in north central India: implications for phytoremediation. Soil Sediment Contam 22(1):1–20. doi:10.1080/15320383.2012.697936
Duarte B, Caetano M, Almeida PR, Vale C, Cacador I (2010) Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal). Environ Pollut 158(5):1661–1668. doi:10.1016/j.envpol.2009.12.004
Ericksen JA, Gustin MS, Schorran DE, Johnson DW, Lindberg SE, Coleman JS (2003) Accumulation of atmospheric mercury in forest foliage. Atmos Environ 37(12):1613–1622. doi:10.1016/S1352-2310(03)00008-6
Fang XB, Subudhi PK, Venuto BC, Harrison SA (2004) Mode of pollination, pollen germination, and seed set in smooth cordgrass (Spartina alterniflora, Poaceae). Int J Plant Sci 165(3):395–401. doi:10.1086/382810
Fay L, Gustin M (2007) Assessing the influence of different atmospheric and soil mercury concentrations on foliar mercury concentrations in a controlled environment. Water Air Soil Pollut 181(1–4):373–384. doi:10.1007/s11270-006-9308-6
Fernandez-Cadena JC, Andrade S, Silva-Coello CL, De la lglesia R (2014) Heavy metal concentration in mangrove surface sediments from the north-west coast of South America. Mar Pollut Bull 82(1–2):221–226. doi:10.1016/j.marpolbul.2014.03.016
Ferrer L, Andrade S, Asteasuain R, Marcovecchio J (2006) Acute toxicities of four metals on the early life stages of the crab Chasmagnathus granulata from Bahia Blanca estuary, Argentina. Ecotoxicol Environ Saf 65(2):209–217. doi:10.1016/j.ecoenv.2005.06.010
Gallagher JL, Plumley FG (1979) Underground biomass profiles and productivity in Atlantic coastal marshes. Am J Bot 66(2):156–161
Gallagher JL, Pfeiffer WJ, Pomeroy LR (1976) Leaching and microbial utilization of dissolved organic-carbon from leaves of Spartina alterniflora. Estuar Coast Shelf Sci 4(4):467–471. doi:10.1016/0302-3524(76)90021-9
Gallagher JL, Reimold RJ, Linthurst RA, Pfeiffer WJ (1980) Aerial production, mortality, and mineral accumulation-export dynamics in Spartina alterniflora and Juncus roemerianus plant stands in a Georgia salt-marsh. Ecology 61(2):303–312
Ghandour IM, Basaham S, Al-Washmi A, Masuda H (2014) Natural and anthropogenic controls on sediment composition of an arid coastal environment: SharmObhur, Red Sea, Saudi Arabia. Environ Monit Assess 186(3):1465–1484. doi:10.1007/s10661-013-3467-x
Grill E, Loffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86(18):6838–6842
Guo YX, Fan DD, Li CX, Yuan LH (2004) Grain-size characteristics and their applications to the intertidal subfacies division: a case study from Andong tidal flat in the Hangzhou Bay. Mar Geol Lett 20(5):9–14
Hajar EWI, Sulaiman AZB, Sakinah AMM (2014) Assessment of heavy metals tolerance in leaves, stems and flowers of Stevia rebaudiana plant. In: The international conference on sustainable future for human security sustain, vol 20, pp 386–393
He B, Li MW, Qiu GY (2014) Threat of heavy metal contamination in eight mangrove plants from the Futian mangrove forest, China. Environ Geochem Health 36(3):467–476. doi:10.1007/s10653-013-9574-3
Hempel M, Botte SE, Negrin VL, Chiarello MN, Marcovecchio JE (2008) The role of the smooth cordgrass Spartina alterniflora and associated sediments in the heavy metal biogeochemical cycle within Bahia Blanca estuary salt marshes. J Soils Sediment 8(5):289–297. doi:10.1007/s11368-008-0027-z
Huang HM, Zhang LQ, Guan YJ, Wang DH (2008) A cellular automata model for population expansion of Spartina alterniflora at Jiuduansha Shoals, Shanghai, China. Estuar Coast Shelf S 77(1):47–55. doi:10.1016/j.ecss.2007.09.003
Idaszkin YL, Bouza PJ, Marinho CH, Gil MN (2014) Trace metal concentrations in Spartina densiflora and associated soil from a Patagonian salt marsh. Mar Pollut Bull 89(1–2):444–450. doi:10.1016/j.marpolbul.2014.10.001
Jiang LF, Luo YQ, Chen JK, Li B (2009) Ecophysiological characteristics of invasive Spartina alterniflora and native species in salt marshes of Yangtze River estuary, China. Estuar Coast Shelf Sci 81(1):74–82. doi:10.1016/j.ecss.2008.09.018
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer, Berlin
Katsev S, Tsandev I, L’heureux I, Rancourt DG (2006) Factors controlling long-term phosphorus efflux from lake sediments: exploratory reactive-transport modeling. Chem Geol 234:127–147. doi:10.1016/j.chemgeo.2006.05.001
Kumar JIN, Soni H, Kumar RN (2006) Biomonitoring of selected freshwater macrophytes to assess lake trace element contamination: a case study of NalSarovar Bird Sanctuary, Gujarat, India. J Limnol 65(1):9–16
Larsen VJ (1983) The significance of atmospheric deposition of heavy-metals in 4 Danish Lakes. Sci Total Environ 30(SEP):111–127. doi:10.1016/0048-9697(83)90006-2
Lee SA, Yoon EK, Heo JO, Lee MH, Hwang I, Cheong H, Lee WS, Hwang YS, Lim J (2012) Analysis of Arabidopsis glucose insensitive growth Mutants Reveals the Involvement of the plastidial copper transporter PAA1 in glucose-induced intracellular signaling. Plant Physiol 159(3):1001–1012
Lehtonen J (1989) Effects of acidification on the metal levels in aquatic macrophytes in Espoo, S Finland. Ann Bot Fenn 26(1):39–50
Li Y (1993) 201Pb as a tracer for the tidal flat sedimentation in the southern Hangzhou Bay. Donghai Mar Sci 11(1):34–43
Li Y, Xie QC (1993) Dynamic development of the Andong tidal flat in Hangzhou Bay, China. Donghai Mar Sci 11(02):25–33
Lindberg SE, Robert Harriss, Turner RR, Shriner DS, Huff DD (1979) Mechanisms and rates of atmospheric deposition of trace-elements and sulfate to forest canopy. Abstr Pap Am Chem Soc. doi:10.2172/5997611
Lisamarie W, Judith SW, Peddrick W (2001) Lead uptake, distribution, and effects in two dominant salt marsh macrophytes, Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Mar Pollut Bull 42(10):811–816. doi:10.1016/S0025-326X(00)00224-1
Liu SF, Liu YG, Yang G, Qiao SQ, Li CX, Zhu ZW, Shi XF (2012) Distribution of major and trace elements in surface sediments of Hangzhou Bay in China. Acta Oceanol Sin 31(4):89–100. doi:10.1007/s13131-012-0223-y
Maanan M, Zourarah B, Sahabib M, Maananc M, Le Royd P, Mehdib K, Salhib F (2015) Environmental risk assessment of the Moroccan Atlantic continental shelf: the role of the industrial and urban area. Sci Total Environ 511:407–415. doi:10.1016/j.scitotenv.2014.12.098
Madejon P, Maranon T, Murillo JM, Robinson B (2004) White poplar (Populus alba) as a biomonitor of trace elements in contaminated riparian forests. Environ Pollut 132(1):145–155. doi:10.1016/j.envpol.2004.03.015
Marcovecchio JE (2000) Land-based sources and activities affecting the marine environment at the Upper Southwestern Atlantic Ocean: an overview. UNEP Regional Seas Reports and Studies No. 170:67
Marcovecchio J, Ferrer L (2005) Distribution and geochemical partitioning of heavy metals in sediments of the Bahia Blanca Estuary, Argentina. J Coastal Res 21(4):826–834. doi:10.2112/014-NIS.1
Markert B, Friese K (1999) Seventh international congress of ecology: symposium on “trace metals in the environment”. UWSF-Z Umweltchem Ökotox 11(3):163–166
Mazej Z, Germ M (2009) Trace element accumulation and distribution in four aquatic macrophytes. Chemosphere 74(5):642–647. doi:10.1016/j.chemosphere.2008.10.019
McGann M (2008) High-resolution foraminiferal, isotopic, and trace element records from Holocene estuarine deposits of San Francisco Bay, California. J Coastal Res 24(5):1092–1109. doi:10.2112/08A-0003.1
Mendelssohn IA, Kuhn NL (2003) Sediment subsidy: effects on soil-plant responses in a rapidly submerging coastal salt marsh. Ecol Eng 21(2–3):115–128. doi:10.1016/j.ecoleng.2003.09.006
Milliman JD, Shen HT, Yang ZS, Robert HM (1985) Transport and deposition of river sediment in the Changjiang estuary and Adjacent continental-shelf. Cont Shelf Res 4(1–2):37–45. doi:10.1016/0278-4343(85)90020-2
Mishra VK, Upadhyaya AR, Upadhyaya AR, Pandey SK, Tripathi BD (2008) Heavy metal pollution induced due to coal mining effluent on surrounding aquatic ecosystem and its management through naturally occurring aquatic macrophytes. Bioresour Technol 99(5):930–936. doi:10.1016/j.biortech.2007.03.010
Padinha C, Santos R, Brown MT (2000) Evaluating environmental contamination in Ria Formosa (Portugal) using stress indexes of Spartina maritima. Mar Environ Resour 49:67–78. doi:10.1016/S0141-1136(99)00049-5
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421:3–16. doi:10.1016/j.scitotenv.2011.03.013
Pang HJ, Chen XG, Lou ZH, Jin AM, Yan KK, Jiang Y, Yang XH (2015) Contamination, distribution, and sources of heavy metals in the surface sediments of Andong tidal flat, Hangzhou Bay, China. Cont Shelf Res 100:72–84. doi:10.1016/j.csr.2015.10.002
Phillips DP, Human LRD, Adams JB (2015) Wetland plants as indicators of heavy metal contamination. Mar Pollut Bull 92:227–232. doi:10.1016/j.marpolbul.2014.12.038
Pietrini F, Iannelli MA, Pasqualini S, Massacci A (2003) Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol 133(2):829–837
Quan WM, Han JD, Shen XY, Ping PL, Li CJ, Shi LY, Chen YQ (2007) Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River estuary, China. Mar Environ Res 64(1):21–37. doi:10.1016/j.marenvres.2006.12.005
Reboreda R, Cacador I (2007a) (a)) Halophyte vegetation influences in salt marsh retention capacity for heavy metals. Environ Pollut 146(1):147–154. doi:10.1016/j.envpol.2006.05.035
Reboreda R, Cacador I (2007b) (b)) Copper, zinc and lead speciation in salt marsh sediments colonised by Halimione portulacoides and Spartina maritima. Chemosphere 69(10):1655–1661. doi:10.1016/j.chemosphere.2007.05.034
Sainger PA, Dhankhar R, Sainger M, Kaushik A, Singh RP (2011) Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotoxicol Environ Saf 74(8):2284–2291. doi:10.1016/j.ecoenv.2011.07.028
Santos-Echeandia J, Vale C, Caetano M (2010) Effect of tidal flooding on metal distribution in pore waters of marsh sediments and its transport to water column (Tagus estuary, Portugal). Mar Environ Res 70:358–367. doi:10.1016/j.marenvres.2010.07.003
Sawidis T, Chettri MK, Papaioannou A, Zachariadis G, Stratis J (2001) A study of metal distribution from lignite fuels using trees as biological monitors. Ecotoxicol Environ Saf 48(1):27–35. doi:10.1006/eesa.2000.2001
Schierup HH, Larsen VJ (1981) Macrophyte cycling of zinc, copper, lead and cadmium in the littoral-zone of a polluted and a non-polluted lake. I. Availability, uptake and translocation of heavy-metals in Phragmites-Australis (CAV) trin. Aquat Bot 11(3):197–210. doi:10.1016/0304-3770(81)90061-9
Scholze RJ, Smith ED, Bandy JT, Wu YC, Basilico JV (1988) Biotechnology for degradation of toxic chemicals in hazardous wastes. Atmos Environ 23(4):899–900
Shaw BP (1986) Mercury absorption by some plants and the biology significance in some areas of India. Arch Environ Contam Toxicol 15:439–446
Su JL, Wang KS (1989) Changjiang River plume and suspended sediment transport in Hangzhou Bay. Cont Shelf Res 9(1):93–111. doi:10.1016/0278-4343(89)90085-X
Valery L, Bouchard V, Lefeuvre JC (2004) Impact of the invasive native species Elymus athericus on carbon pools in a salt marsh. Wetlands 24(2):268–276
Vymazal J, Svehla J, Kröpfelovád L, Chrastnýc V (2007) Trace metals in Phragmites australis and Phalaris arundinacea growing in constructed and natural wetlands. Sci Total Environ 380(1-3SI):154–162. doi:10.1016/j.scitotenv.2007.01.057
Weis JS, Weis P (2002) Contamination of saltmarsh sediments and biota by CCA treated wood walkways. Mar Pollut Bull 44(6):504–510. doi:10.1016/S0025-326X(01)00294-6
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30(5):685–700. doi:10.1016/j.envint.2003.11.002
Weis JS, Windham L, Weis P (2003) Patterns of metal accumulation in leaves of the tidal marsh plants Spartina alterniflora Loisel and Phragmites australis Cav. Trin Ex Steud over the growing season. Wetlands 23(2):459–465
Weis JS, Glover T, Weis P (2004) Interactions of metals affect their distribution in tissues of Phragmites australis. Environ Pollut 131(3):409–415. doi:10.1016/j.envpol.2004.03.006
Williams TP, Bubb JM, Lester JN (1994) Metal accumulation within salt-marsh environments—a review. Mar Pollut Bull 28(5):277–290. doi:10.1016/0025-326X(94)90152-X
Windham L, Lathrop RG (1999) Effects of Phragmites australis (common reed) invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. Estuaries 22(4):927–935
Windham L, Weis JS, Weis P (2003) Uptake and distribution of metals in two dominant salt marsh macrophytes, Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Estuar Coast Shelf Sci 56(1):63–72. doi:10.1016/S0272-7714(02)00121-X
Wong SC, Li XD, Zhang G, Qi SH, Min YS (2002) Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut 119(1):33–44. doi:10.1016/S0269-7491(01)00325-6
Wu QH, Tam NFY, Leung JYS, Zhou XZ, Fu J, Yao B, Huang XX, Xia LH (2014) Ecological risk and pollution history of heavy metals in Nansha mangrove, South China. Ecotoxicol Environ Saf 104:143–151. doi:10.1016/j.ecoenv.2014.02.017
Xie D, Gao S, Pan CH (2010) Process-based modeling of morphodynamics of a tidal inlet system. Acta Oceanol Sin 29(6):51–61. doi:10.1007/s13131-010-0076-1
Zhang HY, Wang ZD (2013) Source identification and transfer route of heavy metal pollution in environment-plant-human system (a REVIEW). Agric Eng 3(3):55–58. doi:10.3969/j.issn.2095-1795.2013.03.022
Zhang W, Yu L, Hutchinson SM, Xu S, Chen Z, Gao X (2001) China’s Yangtze Estuary: I. Geomorphic influence on heavy metal accumulation in intertidal sediments. Geomorphology 41(2–3):195–205. doi:10.1016/S0169-555X(01)00116-7
Zhou HX, Liu JE, Zhou J, Qin P (2008) Effect of an alien species Spartina alterniflora Loisel on biogeochemical processes of intertidal ecosystem in the Jiangsu coastal region, China. Pedosphere 18(1):77–85. doi:10.1016/S1002-0160(07)60105-2
Zuo P, Liu CA, Zhao SH, Wang CH, Liang YB (2009) Distribution of Spartina plantations along the China’s coast. Acta Oceanol Sin 31:101–111
Acknowledgements
This work is financially supported by the “Research of heavy metal geochemical characteristics in the sediments of Hangzhou Bay,” Self-Program of Zhejiang University.
Author information
Authors and Affiliations
Contributions
Z-HL, X-GC, and A-MJ conceived the ideas; X-GC, H-JP, K-KY, X-HY contributed to the sampling work; H-JP, S-SL, YJ, and FL analyzed the samples and investigated the data; H-JP, X-GC, and Z-HL wrote the manuscript, FL helped to revise the language of the manuscript, X-HY helped to draw the maps and diagrams; Z-HL is responsible for the whole article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is part of a Topical Collection in Environmental Earth Sciences on “Environment and Health in China II”, guest edited by Tian-Xiang Yue, Cui Chen, Bing Xu and Olaf Kolditz.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pang, HJ., Lyu, SS., Chen, XG. et al. Heavy metal distribution and accumulation in the Spartina alterniflora from the Andong tidal flat, Hangzhou Bay, China. Environ Earth Sci 76, 627 (2017). https://doi.org/10.1007/s12665-017-6948-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6948-3