Abstract
This study reported the first comprehensive research on identification of metal concentrations (Fe, Mg, Mn, Pb, Cd, Cr) in order to provide baseline data for future studies, identify possible sources, determine degree of pollution, and identify potential ecological risks of metals in surface sediments from Iran’s Choghakhor Wetland. The order of metal concentration was as follows: Fe > Mg > Mn > Pb > Cd > Cr, with mean concentrations of 6140.35, 1647.32, 289.03, 1.10, and 0.45 µg/g of dry weight, respectively. These results reveal that Choghakhor Wetland is not heavily polluted compared to other regions. The results of enrichment factor (EF) and geoaccumulation index (I geo) showed that Fe, Pb, Mg, Cr, and Mn presented low levels of contamination and probably originated from natural sources. On the other hand, the results of EF and I geo indices suggested that Cd concentrations in sediments of Choghakhor Wetland originated from anthropogenic sources. Based on the results of three sets of sediment quality guidelines, only Cd concentration in sediments of Choghakhor Wetland is a threat for aquatic organisms of Choghakhor Wetland. The results of multivariate analysis such as principal component analysis and cluster analysis showed that Fe–Mn, Cr–Mg, and Pb groups originated from natural sources, while Cd concentrations in sediments of Choghakhor Wetland originated from both natural and anthropogenic sources (mainly chemical fertilizers). To our knowledge, this is the first study about metal concentrations in sediments of Choghakhor Wetland, and because of low levels of these metals, these concentrations can be considered background levels for future investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination by metals has considerable importance because of its extreme toxicity to humans and biological systems (Benson et al. 2007). The contamination of aquatic ecosystems by metals is one of the most challenging pollution issues due to the toxicity, abundance, persistence, and, finally, bioaccumulation of metals (Fu et al. 2014). Sediments are the final destination of metals, because of adsorption, desorption, precipitation, diffusion, chemical reactions, biological activity, and a combination of these phenomena. Sediments are an important sink for metals, but when some physical disturbance occurs, or there is diagnosis, and/or there are changes in pH or redox potential, they can become a source of metal pollution, released into the overlying water column (Chatterjee et al. 2009). Moreover, it is widely known that metals are indestructible and can be retained in soils for a long time (Gao et al. 2013), as they cannot be chemically or biologically degraded. Sediments have been used as an important tool to assess the health status of aquatic ecosystems and provide a history of anthropogenic pollutant input (Zahra et al. 2014). Spatial surveys of metal concentrations in sediments are useful to assess pollution in the aquatic environment and to provide basic information for the judgment of environmental health risks (Hu et al. 2013). Without the baseline dataset, it is not possible to judge environmental health risks and apply a suitable environmental remediation program.
Like other creatures, aquatic organisms are vulnerable to pollution at both toxic and subtoxic concentrations. This weakens them and can result in decline or death of populations, by making them more vulnerable to disease, impaired fertility, and/or early death. Furthermore, certain aquatic creatures through their unique biological processes can amass trace metals far greater than those typically found in the organisms. Consequently, the risk extends beyond their lives and into upstream risk to the humans and animals that depend upon these protein resources (Kibera 2016).
To our knowledge, no study has reported on metallic pollution in the sediments of Choghakhor Wetland, located in Chaharmahal and Bakhtiari Province, southwestern Iran. To address this critical knowledge shortcoming, this study aimed to investigate the state of the metal contamination in surface sediments of this aquatic ecosystem using several indices, such as enrichment factor (EF), geoaccumulation index (I geo), and sediment quality guidelines (SQGs), and a multivariate approach. In recent years, several indices have been used to assess metal contamination and its ecological risk (Hou et al. 2013). For better management of aquatic ecosystems, their contamination assessment should be easily communicated to local managers and decision makers. Environmental quality indicators and indices are powerful tools for processing, analyzing, and conveying raw environmental information to decision makers, managers, technicians, or the public (Caeiro et al. 2005). In addition, to reveal possible sources of metals and determine relationships among metals, multivariate statistical approaches can be useful. In recent decades, Choghakhor Wetland has been affected by human activities such as dam construction, agricultural activities, livestock grazing, boating, fishing, and wastewater discharge from nearby villages. In this study, we report the first comprehensive research on identification of metal concentrations providing baseline data for future studies, identifying possible sources, determining degree of pollution, and categorizing potential ecological risks of metals in surface sediments from Choghakhor Wetland.
Materials and methods
Study area
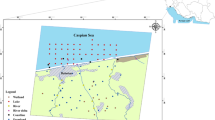
Choghakhor Wetland is internationally known, located in Chaharmahal and Bakhtiari Province, located at latitude 31°55′N, longitude 50°54′E, encompassing 1687 ha (Fig. 1). This wetland is near Tang-e-Sayad and Sabz-koh Biosphere Reserves in southwestern Iran. The wetland was designated a Ramsar site in 2011. Choghakhor Wetland is considered one of the most important sites in Iran for the endemic Zagros pupfish (Aphanius vladykovi). It is important for flood control and ground water replenishment and is generally considered a reservoir for biodiversity (RSIS 2010). As mentioned above, in recent decades Choghakhor Wetland has been affected by human activities such as construction of a dam, agricultural activities, livestock grazing, boating, fishing, and discharging wastewater from encompassing villages.
Sample collection
Fifty-two surface sediment samples were collected from the top 6 cm of sediment with a polypropylene coring device measuring 3 m long with 5 cm internal diameter. Locations of sampling points were recorded using a Global Positioning System. Samples were placed in sterile plastic bags and then stored in a refrigerator prior to analysis. Because of the high density of aquatic plants in the central area of the wetland, it is virtually impossible to collect sediment samples from the center of the wetland. Samples were air-dried and sieved through a 2-mm sieve in order to remove stone and coarse debris and then passed through a 0.125-mm sieve.
Analytical methods
For all samples, 1 g of dried and homogenized sediment was carefully weighed and digested with HNO3–HClO4–HF mixture in Teflon tubes at a temperature of 160 °C for 6 h (Gao et al. 2013). The digestion solution was filtered and diluted with deionized water to 25 ml in a volumetric flask. All samples were kept at 4 °C prior to analysis (Massolo et al. 2012). Metal concentrations in solutions were analyzed using an atomic absorption spectrometer (PerkinElmer model A Analyst 400, MA, USA). All samples were analyzed in three replicates. Chinese geochemical standard reference sample GSD 9 (stream sediments) and Standard Reference Materials (SRMs) (SRM 2711 and SRM 2709, National Institute of Standards and Technology) have been used to estimate accuracy. The average recoveries of the standard reference materials were 125, 98.4, 97, and 93.9% for Cd, Pb, Mn, and Cr, respectively.
Assessment of metal contamination
Enrichment factor (EF)
EF is commonly used to determine and quantify the anthropogenic contribution of metals over natural inputs (Li et al. 2013a; Sungur and Özcan 2014). To reduce the influence of the particle grain-sized effects on metal contamination, concentration data should be normalized by a conservative element (Wang et al. 2015). Mn was used as a reference element to calculate anthropogenic metal enrichments (Zahra et al. 2014). In addition, other metals such as Li, Cs, Sc, and Fe have been used as reference elements (Ruhong et al. 2010; Zahra et al. 2014). The enrichment factor can be calculated as follows:
In this equation, the numerator refers to the ratio of the concentration of a sample’s examined element (C EE) to the reference element (Mn). The denominator shows the ratio of the concentration of a reference material’s examined element (C EE) to the reference element (Mn) (Zahra et al. 2014). World average shale is one of the materials commonly used to provide reference elements. The regional reference elements for these metals are not available in the study area. Thus, the reference elements of heavy metals in the average shale obtained from Turekian and Wedepohl (1961) are used in this study. The EF value was interpreted as suggested by Wang et al. (2015), where EF < 2, minimal enrichment; EF = 2–5, moderate enrichment; EF = 5–20, significant enrichment; EF = 20–40, very high enrichment; and EF > 40, extreme enrichment.
Geoaccumulation index (I geo)
Geoaccumulation index is another index commonly used to calculate the metal contamination levels (Müller 1979). The equation used for the calculation of I geo is expressed as follows:
where C n is the examined element in the sediment for heavy metal n, B n is the examined reference for the metal n, and the factor 1.5 is used because of possible variations of the background data due to lithological variations (Ghrefat et al. 2011). In the present study, global average shale data from Turekian and Wedepohl (1961) were used as examined reference to calculate I geo. This index defines seven classes with respect to sediment quality: unpolluted (I geo ≤ 0); unpolluted to moderately polluted (0 < I geo ≤ 1); moderately polluted (1 < I geo ≤ 2); moderately to strongly polluted (2 < I geo ≤ 3); strongly polluted (3 < I geo ≤ 4); strongly to extremely polluted (4 < I geo ≤ 5); and extremely polluted (I geo > 5) (Müller 1981; Li et al. 2013a).
Sediment quality guidelines (SQGs)
To evaluate ecological risk of heavy metals in sediments, three sets of sediment quality guidelines were performed as follows. (1) The effect range low (ERL) and effect range median (ERM) were carried out. (2) Next, the threshold effect level (TEL) and probable effect level (PEL) values were implemented. ERL and ERM are defined as the levels below which adverse biological effects are unexpected. In contrast, ERM and PEL represent chemical concentrations above which adverse biological effects are expected to happen (Xiao et al. 2011; Li et al. 2013b). (3) Finally, the lowest effect level (LEL) and severe effect level (SEL), defined below, were determined. As expected, at LEL, there was no effect on the majority of sediment-dwelling organisms. Above that level, however, namely at SEL, serious adverse effects on the majority of sediment-dwelling organisms are expected (MOE 1993).
Multivariate statistical methods
Multivariate techniques, such as principal component analysis (PCA) and cluster analysis (CA), are commonly performed to identify possible source of heavy metals from natural or anthropogenic inputs (Bai et al. 2011; Hu et al. 2013; Li et al. 2013b; Fu et al. 2014; Wang et al. 2014, 2015). Statistical analyses were performed using SPSS 17.0 and MINITAB 17.0 software packages. Before performing PCA, Kaiser–Meyer–Olkin (KMO) and Bartlett’s tests were applied to assess the suitability of the data for testing (Fu et al. 2014). In addition, normality of data was checked before running tests. All data were reported as µg/g of dry weight (dw).
Results and discussion
Metal concentrations in sediments
Table 1 shows descriptive statistics of Cr, Pb, Cd, Mn, Fe, and Mg in surface sediments of Choghakhor Wetland. The order of metal concentrations was as follows: Fe > Mg > Mn > Pb > Cd > Cr with mean concentrations of 6140.35, 1647.32, 289.03, 1.10, and 0.45 µg/g dw, respectively. Fe, Mg, and Mn are among the most abundant metals in the Earth’s crust (Yaroshevsky 2006), and the order of these metals in this study is in adherence to their amounts in the Earth’s crust. The sequence of metal concentrations in this study is similar to previous studies, which showed high levels of Fe, Mg, and Mn compared to other examined metals(An and Kampbell 2003; Arain et al. 2008; Ennouri et al. 2010). In addition, Fe and Mn had the highest spatial variability, with a coefficient of variation of 0.68 and 0.39, respectively. The comparison of metal concentrations with other regions is presented in Table 2.
Metal concentrations in sediments of Choghakhor Wetland were lower than those reported from Shadegan Wetland of Iran (Alhashemi et al. 2012). In addition, the mean concentration of Cd in sediments of Choghakhor Wetland was higher than the mean concentration of Cd from Iran’s Anzali Wetland, but the mean concentration of Pb from Choghakhor Wetland was significantly lower than the mean reported from Anzali Wetland (Zamani-Ahmadmahmoodi et al. 2013). Overall, as shown in Table 2, metal concentrations in sediments of Choghakhor Wetland were lower than those reported from other bodies of water, except in some cases the mean concentration of Cd, Fe, and Mn in sediments of Choghakhor Wetland was higher than reported elsewhere. These results reveal that Choghakhor Wetland is not heavily polluted compared to other regions.
Contamination assessment of metals, using EF and I geo
Enrichment degree of metals was calculated using enrichment factor. The EF values for investigated metals were as following order: 4.94–16.98 (mean = 10.13) for Cd; 0.03–2.62 (mean = 0.43) for Fe; 0.09–5.16 (mean = 0.42) for Pb; 0.14–1.02 (mean = 0.37) for Mg; and 0.01–0.04 (mean = 0.02) for Cr, yielding a sequence of Cd > Fe > Pb > Mg > Cr. The results show that the EF values of Fe, Pb, Mg, and Cr were less than 2. According to the EF levels suggested by Wang et al. (2015), EF levels less than 2 exhibit minimal enrichments, and likely are of natural origin in the study area. However, Cd had higher EF value compared to the other metals and showed significant enrichment, suggesting possible anthropogenic sources in the study area.
The I geo values of surface sediments from Choghakhor Wetland were negative values, with the mean of −3.9, −3.9, −3.7, −8.2, and −2.2 for Fe, Pb, Mg, Cr, and Mn, respectively. The negative I geo values are the results of relatively low levels of contamination by metals (Zamani-Ahmadmahmoodi et al. 2013). According to scale proposed by Müller (1979), the I geo values for Fe, Pb, Mg, Cr, and Mn fell in the first class, unpolluted (I geo ≤ 0). These findings confirmed the results of EF for Fe, Pb, Mg, and Cr, which presented minimal enrichments and showed low levels of contaminations for the metals. In contrast, for Cd the mean I geo value was 1.23, positioning Cd in the third class, moderately polluted (1 < I geo ≤ 2). This result was similar to the result of EF for Cd, in that Cd had significant enrichment and probably had anthropogenic sources in the study area.
Ecological risk assessment based on SQGs
Metal concentrations in surface sediments of Choghakhor Wetland were evaluated with respect to sediment quality guidelines for metals in freshwater ecosystems proposed by MacDonald et al. (2000) and sediment quality guideline developed by the Ministry of Environment and Energy, Ontario, Canada (MOE 1993). The results of these comparisons are tabulated in Table 1. For Mg, Mn, Pb, and Cr, all measured values were below the values recommended for the occurrence of adverse biological effects, namely either ERL, TEL, or LEL; or ERM, PEL, or SEL. In contrast, the mean value of Cd was higher than TEL or LEL and lower than the values proposed for ERL, ERM, PEL, and SEL. These results indicated that the values of Fe, Mg, Mn, Pb, Cd, and Cr were lower than levels above which adverse biological effects are expected to occur and above which serious adverse effects on the majority of sediment-dwelling organisms are expected. Furthermore, with the exception Cd, almost all of the values of Fe, Mg, Mn, Pb, and Cr were lower than levels below which adverse biological effects are not expected to happen and below which no effects on the majority of sediment-dwelling organisms are expected. These findings indicated that only Cd concentration in sediments of Choghakhor Wetland is a threat for aquatic organisms of Choghakhor Wetland, and remediation programs should be designed to decrease the Cd concentration in sediments.
Source identification
In the present study, multivariate techniques such as PCA and CA have been used to identify possible sources of metals. The results of Kaiser–Meyer–Olkin (KMO = 0.61) and Bartlett’s tests (P < 0.05) confirmed the adequacy of the data set for PCA. The results of PCA appear in Table 3 and Fig. 2. The results of PCA show three principal components (PCs) explained 58.59% of the total variance in the original dataset. The rotated component matrix revealed that Cd, Fe, and Mn, associated with the first principal component, explained 37.59% of total variance with positive loading on Cd, Mn, and Fe. Mg and Cr, which had high strong positive and negative correlation with the second component, explained 21% of total variance. Pb was associated with the third component, explaining 15.42% of 74.01 of total variance (Table 3). Additionally, a loading plot was used to depict the results of PCA (Fig. 2). The lines that go in the same direction and are close to one another indicate how the variables may be grouped. The loading plot showed that Fe, Mn, and Cd had high positive association with the first component, while Mg and Cr had high strong positive and negative correlation with the second component, respectively; and Pb was associated with both components. The occurrence of Fe and Mn in the same PC might be due to strong association with the geochemical matrix between the two elements (Jonathan et al. 2010). In addition, a lithogenic control over the distribution of Fe and Mn has been reported by some authors (Han et al. 2006; Mico et al. 2006; Bai et al. 2011). Mico et al. (2006) stated that Fe and Mn are well known to be geogenic. Thus, these findings suggested that Fe and Mn concentrations in sediments of Choghakhor Wetland have a natural source and probably originated from parent rock materials. On the other hand, Cd is located in the same group with Fe and Mn. Cd is specifically adsorbed by crystalline and amorphous oxides of Al, Fe, and Mn (Rodriguez et al. 2006). The results of EF and I geo indices suggested that Cd concentrations in sediments of Choghakhor Wetland originated from anthropogenic sources. Cd concentrations in sediments can result from use of phosphate fertilizers and other chemical fertilizers (Sundaray et al. 2011; Zahra et al. 2014; Wang et al. 2015). Therefore, Cd occurring with Fe and Mn in the first principal component suggests that most likely Cd concentrations in sediments in Choghakhor Wetland had both natural and anthropogenic sources in the study area. In the present study, agricultural activities are concentrated south of Choghakhor Wetland, and because of the tendency of agricultural lands to slope toward the wetland, runoff originating from agricultural lands can be easily released into the wetland. The second component consists of Cr and Mg and explained 21% of total variance with negative loadings on Cr and Mg. Chromium pollution is commonly attributed to industrial activities such as mining, tanneries, electroplating, paint facilities, and other chemical facilities (Silva Filho et al. 2010; Davutluoglu et al. 2011; Qadir et al. 2013; Ali et al. 2015). The mean concentration of Cr in sediments of Choghakhor Wetland was considerably lower than those reported elsewhere and lower than the average shale of this metal (Cr = 90), reported by Turekian and Wedepohl (1961). In addition, there are no chromium industries around Choghakhor Wetland. Furthermore, the mean concentration of Mg (1674 µg/g dw) in sediments of Choghakhor Wetland is lower than other published data (Table 2) and the average shale of this metal (15,000 µg/g dw) (Turekian and Wedepohl 1961). Anthropogenic sources of magnesium include fertilizers and liming (Reimann and Caritat 1998). However, because of the low level of Mg in sediments of Choghakhor Wetland, the source of this metal is related to parent rock materials. Consequently, these findings suggest no clear influences from anthropogenic activities over the distribution of Cr and Mg in the study area. Cluster analysis was also applied to identify metal groups. Ward’s method was used as a linkage method and correlation as a distance measure. Usually, CA is coupled with PCA to check the analytical results and to group individual parameters and variables (Wang et al. 2014; Li et al. 2013b). Dendrogram showed three main groups of metals: (1) Cr–Mg, (2) Pb, and (3) Cd–Fe–Mn (Fig. 3). The results of CA were in a good agreement with PCA. Similar to the results of PCA, Pb is positioned in the separate group and probably originated from natural sources because of its very low background level in Choghakhor Wetland (Table 1). In two other groups, Cr–Mg and Cd–Fe–Mn, clustering of metals was similar to the results of PCA, and a similar interpretation can be reached about possible sources of metals.
Conclusion
Implementation of multivariate analyses, such as PCA and CA, and indices, such as EF, I geo, and SQGs, was useful to explore possible sources of heavy metals in Choghakhor Wetland. The results of the present study revealed that Choghakhor Wetland is not heavily polluted compared to other wetlands. Overall, the results of the present study demonstrate that with the exception of Cd concentrations in the sediments of Choghakhor Wetland, other metals present no concern in the study area. Regardless, regular biomonitoring programs should be designed and implemented to control inputting pollution sources, such as agriculture and wastewater runoffs. For many people around Choghakhor Wetland, their most important jobs are in fishing. Since the demand for fish consumption is increasing, as are agricultural and industrial activities, monitoring of metals in fish species in terms of environment and human health is essential. Based on the results of the present study, we assume that because the level of metals in sediment is low, the levels of metals in aquatic organisms such as fish are low; however, more analyses should be performed to confirm this hypothesis. To our knowledge, this is the first study about metal concentrations in the sediments of Choghakhor Wetland, and because of the low levels of these metals, these concentrations can be considered as background levels for future investigation. To date, little information has been developed about the examination of heavy metals in biota of Choghakhor Wetland. Rahimi and Raissy (2009) investigated the level of lead and cadmium in some fish species caught in Choghakhor Wetland. They found that the mean level of lead was lower than threshold limit recommended by the European Union, but the mean level of cadmium was higher than that acceptable to the European Union. Overall, information is lacking about the level of other important metals in Choghakhor Wetland, such as mercury, arsenic, and copper, which are important to ecotoxicology and should be investigated in future studies.
References
Alhashemi A, Sekhavatjou MS, Hassanzadeh Kiabi B, Karbassi AR (2012) Bioaccumulation of trace elements in water, sediment, and six fish species from a freshwater wetland, Iran. Microchem J 104:1–6
Ali Z, Malik RN, Shinwari ZK, Qadir A (2015) Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int J Environ Sci Technol 12:537–550
An YJ, Kampbell DH (2003) Total, dissolved, and bioavailable metals at Lake Texoma marinas. Environ Pollut 122:253–259
Arain MB, Kazi TG, Jamali MK, Jalbani N, Afridi HI, Shah A (2008) Total dissolved and bioavailable elements in water and sediment samples and their accumulation in Oreochromis mossambicus of polluted Manchar Lake. Chemosphere 70:1845–1856
Bai J, Xiao R, Cui B, Zhang K, Wang Q, Liu X, Gao H, Huang L (2011) Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River Estuary, South China. Environ Pollut 159:817–824
Benson NU, Essien JP, Bassey D (2007) Hydrobiological constraints of trace metals in surface water, coastal sediment and water lily of Calabar River, Nigeria. Afr J Biotechnol 6:2358–2362
Caeiro S, Costa MH, Ramos TB, Fernandes F, Silveira N, CoimbraA Medeiros G, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Indic 5:151–169
Chatterjee M, Massolo S, Sarkar SK, Bhattacharya AK, Bhattacharya BD, Satpathy KK, Saha S (2009) An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland, India for evaluating sediment quality guidelines. Environ Monit Assess 150:307–322
Chaudhary MZ, Ahmad N, Mashiatullah A, Ahmad N, Ghaffar A (2013) Geochemical assessment of metal concentrations in sediment core of Korangi Creek along Karachi Coast, Pakistan. Environ Monit Assess 185:6677–6691
Davutluoglu OI, Seckin G, Ersu CB, Yilmaz T, Sari B (2011) Assessment of Metal Pollution in Water and Surface Sediments of the Seyhan River, Turkey, Using Different Indexes. Clean Soil Air Water 39:185–194
Ennouri R, Chouba L, Magni P, Kraiem MM (2010) Spatial distribution of trace metals (Cd, Pb, Hg, Cu, Zn, Fe and Mn) and oligo-elements (Mg, Ca, Na and K) in surface sediments of the Gulf of Tunis (Northern Tunisia). Environ Monit Assess 163:229–239
Fu J, Zhao C, Luo Y, Liu C, Kyzas GZ, Luo Y et al (2014) Heavy metals in surface sediments of the Jialu River, China: their relations to environmental factors. J Hazard Mater 270:102–109
Gao H, Bai J, Xiao R, Liu P, Jiang W, Wang J (2013) Levels, sources and risk assessment of trace elements in wetland soils of a typical shallow freshwater lake, China. Stoch Env Res Risk A 27:275–284
Ghrefat HA, Abu-Rukah Y, Rosen MA (2011) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environ Monit Assess 178:95–109
Han Y, Du P, Cao J, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ 355:176–186
Harikumar PS, Jisha TS (2010) Distribution pattern of trace metal pollutants in the sediments of an urban wetland in the southwest coast of India. Int J Eng Sci Technol 2:840–850
Hou D, He J, Lü C, Ren L, Fan Q, Wang J, Xie Z (2013) Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in water and sediments from Lake Dalinouer, China. Ecotoxicol Environ Saf 93:135–144
Hu B, Cui R, Li J, Wei H, Zhao J, Bai F et al (2013) Occurrence and distribution of heavy metals in surface sediments of the Changhua River Estuary and adjacent shelf (Hainan Island). Mar Pollut Bull 76:400–405
Jonathan MP, Sarkar SK, Roy PD, Alam MA, Chatterjee M, Bhattacharya BD, Bhattacharya A, Satpathy KK (2010) Acid leachable trace metals in sediment cores from Sunderban Mangrove Wetland, India: an approach towards regular monitoring. Ecotoxicology 19:405–418
Katip A, Karaer F, Ileri S, Sarmasik S, Aydogan N, Zenginay S (2012) Analysis and assessment of trace elements pollution in sediments of Lake Uluabat, Turkey. J Environ Biol 33(5):961–968
Kibera G (2016) Trace metals/heavy metals and its impact on environment, biodiversity and human health—a short review. doi:10.13140/RG.2.1.3102.2568/1
Li F, Huang J, Zeng G, Yuan X, Li X, Liang J et al (2013a) Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J Geochem Explor 132:75–83
Li G, Hu B, Bi J, Leng Q, Xiao C, Yang Z (2013b) Heavy metals distribution and contamination in surface sediments of the coastal Shandong Peninsula (Yellow Sea). Mar Pollut Bull 76(1–2):420–426
MacDonald DD, Ingersoll CG, Berge TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39(1):20–31
Massolo S, Bignasca A, Sarkar SK, Chatterjee M, Bhattacharya BD, Alam A (2012) Geochemical fractionation of trace elements in sediments of Hugli River (Ganges) and Sundarban wetland (West Bengal, India). Environ Monit Assess 184(12):7561–7577
Mico C, Recatala L, Peris M, Sanchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872
Müller G (1979) Schwermetalle in den sedimenten des Rheins-veranderungen seit 1971. Umschau 79:778–783
Müller G (1981) Die schwermetellbelastung der sediment des Neckars und Seiner Nebenflusse: eine Bestand saufnahme. Chem Ztg 105:157–164
Nemr AE, Khaled A, Sikaily AE (2006) Distribution and statistical analysis of leachable and total heavy metals in the sediments of the suez gulf. Environ Monit Assess 118(1–3):89–112
Ontario Ministry of the Environment (MOE) (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Queen’s Printer for Ontario, Ottawa
Qadir A, Malik RN, Feroze A, Jamil N, Mukhtar K (2013) Spatiotemporal distribution of contaminants in Nullah Palkhu-highly polluted stream of Pakistan. J Environ Sci Water Resour 2(10):342–353
Rahimi E, Raissy M (2009) Determination of lead and cadmium in the fishes caught from Choghakhor Lagoon in Chaharmahal and Bakhtyari Province, Iran. Iran J Vet Sci 4:79–83
Ramsar Sites Information Service (RSIS). (2010).Choghakhor Wetland. https://rsis.ramsar.org/ris/1939?language=en. Accessed 4 Aug 2015
Reimann C, Caritat PD (1998) Chemical elements in the environment: fact sheets for the geochemist and environmental scientist. Springer, Heidelberg
Rodrıguez A, Lo M, Corbı MG (2006) Heavy metals contents in agricultural topsoils in the Ebro basin (Spain). Application of the multivariate geoestatistical methods to study spatial variations. Environ Pollut 144:1001–1012
Ruhong L, Kun S, Yueying L, Yong S (2010) Assessment of heavy metal pollution in estuarine surface sediments of Tangxi River in Chaohu Lake Basin. Chin Geogr Sci 20(1):009–017
Silva Filho EV, Jonathan MP, Chatterjee M, Sarkar SK, Sella SM, Bhattacharya A, Satpathy KK (2010) Ecological consideration of trace element contamination in sediment cores from Sundarban wetland, India. Environ Earth Sci 63(6):1213–1225
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-A case study: Mahanadi basin, India. J Hazard Mater 186(2–3):1837–1846
Sungur A, Özcan H (2014) Chemometric and geochemical study of the heavy metal accumulation in the soils of a salt marsh area (Kavak Delta, NW Turkey). J Soils Sediments 15:323–331
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Wang LF, Yang LY, Kong LH, Li S, Zhu JR, Wang YQ (2014) Spatial distribution, source identification and pollution assessment of metal content in the surface sediments of Nansi Lake, China. J Geochem Explor 140:87–95
Wang Y, Yang L, Kong L, Liu E, Wang L, Zhu J (2015) Spatial distribution, ecological risk assessment and source identification for heavy metals in surface sediments from Dongping Lake, Shandong, East China. Catena 125:200–205
Xiao R, Bai J, Wang Q, Gao H, Huang L, Liu X (2011) Assessment of Heavy Metal Contamination of Wetland Soils from a typical aquatic-terrestrial ecotone in Haihe River Basin, North China. Clean Soil Air Water 39(19):612–618
Yaroshevsky AA (2006) Abundances of chemical elements in the Earth’s crust. Geochem Int 44(1):48–55
Zahra A, Hashmi MZ, Malik RN, Ahmed Z (2014) Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah-Feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci Total Environ 470–471:925–933
Zamani-Ahmadmahmoodi R, Esmaili-Sari A, Mohammadi J, Bakhtiari AR, Savabieasfahani M (2013) Spatial distribution of cadmium and lead in the sediments of the western Anzali wetlands on the coast of the Caspian Sea (Iran). Mar Pollut Bull 74(1):464–470
Acknowledgements
This research was funded by Shahrekord University, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
For this type of study, formal consent is not required. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Zamani-Ahmadmahmoodi, R., Jafari, A. & Alibeygi-beni, H. Potential ecological risk assessment, enrichment, geoaccumulation, and source identification of metals in the surface sediments of Choghakhor Wetland, Iran. Environ Earth Sci 76, 398 (2017). https://doi.org/10.1007/s12665-017-6718-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6718-2