Abstract
Sediment core from Korangi Creek, one of the polluted coastal locations along the Karachi Coast Pakistan, was collected to trace the history of marine pollution and to determine the impact of industrial activity in the area. Down core variation of metals such as Ca, K, Mg, Al, S, Ti, V, Cr, Mn, Fe, Ni, Cu and Zn was studied in the 72.0 cm core. Nuclear analytical techniques, proton induced X-rays emission (PIXE), was employed to ascertain the chemical composition in sediment core. Grain size analysis and sediment composition of cored samples indicated that Korangi creek sediments are clayey in nature. Correlation matrix revealed a strong association of Ni, Cu, Cr and Zn with Fe and Mn. To infer anthropogenic input, enrichment factor (EF), degree of contamination and pollution load index were calculated. EF showed severe enrichment in surface sediment for Ni, Cu, Cr and Zn, indicating increased industrial effluents discharge in recent years. The study suggests that heavy metal discharge in the area should be regulated. If the present trend of enrichment is allowed to continue unabated, it is most likely that the local food web complexes in the creek might be at highest risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine pollution due to urbanization and industrial development is a major concern worldwide (Heyvaert et al. 2000; Alemdaroglu et al. 2003; Nadia et al. 2009; Liu et al. 2010). Among the components of the marine environment, sediments play a critical role in distribution and circulation of toxic compounds. Marine sediments confiscate hydrophobic chemical pollutants entering marine environments and act as sensitive indicators for monitoring contaminants in water bodies (Cohen 2003). The sediments can be polluted with various kinds of hazardous and toxic substances, including metals which get accumulated in sediments. Concentration of metals in coastal estuaries can be elevated due to high inputs from natural, as well as anthropogenic sources. Metals derived from lithogenous and non-lithogenous sources tend to accumulate in upper few centimeters of the coastal sediments (Karbassi and Amirnezhad 2004). Accumulations of metals are governed by biological and geological machanisms. The favorable physicochemical conditions of the sediments can remobilize and release the metals to the water column causing potential adverse effects to the ecosystem. Moreover, metals can be directly taken up by the benthic organisms which in turn increase chances of entering these metals in marine food chain (Chen et al. 2007). In recent years, metal pollution studies, especially in coastal regions near large industrial and urban areas, have attracted significant attention as it is linked to the deterioration of marine ecosystems (Mudroch et al. 1988). It has been reported that pollution problems in such areas lead to increased metals levels by five or ten times than those characteristic of 50 to 100 years ago (Cardoso et al. 2001). Sediment cores analysis provides useful information on the changes in the quality of the marine environment from a past period as well as historical record of various influences on aquatic system by providing information about anthropogenic input of metals over an extended peroid of time (Kumar and Edward 2009). The environmental geochemistry of Karachi coast sediments has receieved little attention. This paper is an attempt to examine the content and spatial distribution of the chemical composition sediments core collected from Korangi Creek, which is a recipient of industrial effluents along Karachi Coast in order to provide fundamental data for understanding the natural behavior of metals in sediments core and their enrichment with time.
Study area
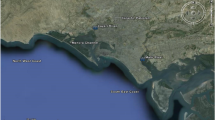
Karachi is the largest city of Pakistan and a hub of industrial activity. The coastal zone of Karachi, which extends up to 135 km, is exposed to heavy pollution load of both domestic and industrial origin (Mashiatullah et al. 2009; Munshi et al. 2004). With time, urbanization discharges of sewage and industrial effluent into aquatic and marine ecosystems is also on the rise. The organic load of sewage depletes oxygen levels in water and indirectly reduces the diversity of animal and plant life. Korangi creek is situated on the southeast coast of Karachi and depicts an environment which is subjected to a type of anthropogenic stress (Fig. 1). It receives industrial effluents from Korangi and Landhi Insdustrial state. In addition to these effluents, the untreated wastewater from metropolis of Karachi, and domestic effluents from smaller coastal settlements are also released into the creek. The Korangi creek area is dominated by mangroves that serve as a spawning and nursery ground for a number of commercially important marine fauna. The toxic pollutants from the Korangi Creek reach the mangrove areas which may pose serious and potential health hazards to fisheries and other fauna in the area (IUCN 2005; Shahzad et al. 2009). The impacts of these pollutants on commercial fin-fish and shrimp fisheries are unknown, but are likely to be significant (WWF 2000).
Materials and methods
Core collections
Sediment core was collected from Korangi creek (24°46′30″N and 67°13′18″E). The sampling location was determined by GARMIN III global positioning system (GPS). Core was collected from a boat with the help of a gravity corer (KC Kojak Demanmark A/S) having acrylic coring pipe (2 m long and 7 cm diameter). Acid-washed coring pipe was driven into the sediment until it reached a depth of 73 cm. The core was slowly retrieved, closed with its cover immediately and marked as to which is the upward direction. The core was transported in a vertical position and frozen for 2 days in vertical position after which it was sliced at 2.5-cm increments, and the sliced samples were stored in clean, labeled polythene bags. Prior to the analysis, all sediment samples were dried at 70–80 °C until constant weight. The shells in the sediments were handpicked. Raw samples were used to determine grain size and sediment composition. Sediment composition was determined following Krumbein and Pettijohn (1938) method. Grain size analysis was done using a set of five sieves ranging from 2 mm to 70 μm for 15 min using Rotop sieve shaker (Folk and Ward 1957). Nature of the sediments was found out the using GRADISTAT software. Enrichment factor (EF) was calculated as described by Mashiatullah et al. (2011) and Ghaffar et al. (2010).
Sample preparation for metal analysis by PIXE
The principal analytical technique used to ascertain the chemical composition in sediment core was proton induced X-rays emission (PIXE). Five milligrams of each sediment sample was mixed with 10 μl of Y(NO3)3 solution in double-distilled water containing 1.16 mg of yttrium as internal standard and mounted on a small piece of 20-μm-thick Mylar. The area covered by the sample on the Mylar was about 1.5 cm2. The samples were placed inside a desiccator and dried under a lamp. The samples were mounted on a wheel controlled by a stepper motor and placed inside the analytical work station for analysis (Ahmad et al. 2010).
The samples were irradiated by a proton beam of 3.8 MeV energy from the National Electrostatic Corporation tandem pelletron accelerator model 6SDH-2 installed at the Accelerator Laboratory of Centre for Advanced Studies in Physics, Government College University Lahore. The characteristic X-rays emitted by the target were detected by an energy- dispersive Si (Li) detector placed at right angle to the direction of proton beam at a distance of approximately 15 cm. The detector has an area of 30 mm2 and an energy resolution of 138 eV. The detector has 0.34-μm-thick aluminum-coated NORVAR window. The semiconductor detector was cooled to liquid nitrogen temperature for optimum result. Beam current used in the experiment was ten nano-amperes and each sample was irradiated for 900 s. The beam current was measured by a Faraday cup. The signal from the detector was processed by the usual PIXE electronics and displayed by multi channel analyzer as channel (elements) versus peak height (concentration) spectrum. The spectrum for each sample was recorded and stored in the computer. The detector was energy calibrated by the characteristic L X-rays emission from a gold target.The system was calibrated for concentration measurements by K X-rays using known salt mixture containing 12 elements Z = 11 to Z = 38 with Y (NO3)3 as internal standard and by L X-rays. To check the accuracy of calibration, the target was prepared from the International Atomic Energy Agency (IAEA) reference material SOIL-7 in a manner described above using Y as the internal standard. The results obtained for the IAEA SOIL-7 reference material are presented in Table 1. Error for the elements Al, K, Ca, and Fe is less than 7 % and for Mn, it is 19 %. The measured value of Mg differs from the IAEA value by a factor of 2; this could perhaps be due to the self-absorption of low energy X-rays of Mg. Concentration of all other elements are within or close to the range specified by IAEA.
Result and discussion
Grain size and sediment composition
Grain size distribution is one of the most important characteristics of sediments. It is the most fundamental property of sediment particles, affecting their entrainment, transport and deposition. Grain size analysis, therefore, provides important clues to the sediment provenance, transport history and depositional conditions. Grain size analysis was carried out only for the surface sediments of the core. GRADSTAT was applied for determination of grain size statistic. This software gives a range of cumulative percentile values (the grain size at which a specified percentage of the grains are coarser): D 10, D 50, D 90, D 90/D 10, D 90–D 10, D 75/D 25 and D 75 –D 25. Grain size analysis of the Korangi creek reveals that sedimemt is clayey in nature. Table 2 gives the detailed description of the sediments. The D 50 value was 2,257.3 μm. Figure 2 shows the variations in statistics of grain size.
Sediment composition was determined for all sub samples and the results are shown in Fig. 3. Clay was the dominating particle in all core slices. The top 10 cm is around 60 % clayey, 20 % sand and 20 % silt, while the remaining section comprises of around 50 % clay and 50 % silt + sand. Metals concentrations and grain size are, in general, inversely related, i.e., they increased with decreasing grain size of sediments. Large amounts of metals bound in the fine-grained fraction of the sediment, mainly because of its high surface area/grain size ratio and humic substance content (Horowitz and Elrick 1987; Moore et al. 1989).
pH
The accumulation of certain trace metals in sediments is directly or indirectly controlled by redox conditions through either a change in redox state and/or speciation (McKay et al. 2007). Variations in pH with depth in sediment core are shown in Fig. 4. pH in Korangi creek core was acidic (around 5.9–6.5) starting from a depth of 72.5 cm to subsurface. On a closer observation in core, it appeared that the top 10-cm slices have pH values closer to neutral where as the bottom sediments from a depth of 10–72.5 cm the pH values in the range of 6.3–5.7. This indicated that only the recent sediment deposits have a pH value of about 6.5, and if this trend continues, it will become neutral in nature with time.
Total organic carbon (TOC)
Organic matter plays an important role in accumulating metals in the sediments. When metals are released into the marine environment, they are absorbed by organic matters and transfered to the sediments. Organic ligands, formed as the consequence of organic matter decomposition, may extract metals from sediments and mobilized them in overlayer water thus increasing their concentration in water (Seshan et al. 2010). Figure 5 shows variation in TOC with depth. Highest TOC was recorded at 2.5 cm depth (4 %). Increasing trend of TOC has been observed throughout the core with very little fluctuation. Enrichment of organic matter at different intervals indicates incorporation of domestic oraganic material in Korangi creek via waste drains. Organic matter (OM) content in core layers depend upon soil type, i.e., more OM in clay particle than sand.
Down core profiles of metals
Sediments represent one of the ultimate sinks for metal discharge into the environment. A variety of processes lead to the association of metals with solid phases, such as direct adsorption by fine grained inorganic particles of clays, adsorption of hydrous ferric and manganic oxides which may in turn be associated with clays, adsorption on, or complexation with natural organic substances which may also be associated with inorganic particles, and direct precipitation as new solid phases (Gibbs 1977). Elemental ranges with means of the metal are (all values in μg/g): Mg 2,654–4,239 (mean 2,939), K 7,223–9,486 (mean 8076), Ca 3,190–3,987 (mean 3,446), Al 3,432–5,002 (mean 4,201), S 160–290 (mean 225),Ti 154–341 (mean 256, V 34–84 (mean 50), Cr 22–72 (mean 53), Mn 45–187 (mean 105), Fe 1,980–4,321 (mean 2,942), Ni 19–66 (mean 34), Cu 16–56 (mean 25), Zn 76–224 (mean 108). Figures 6, 7, 8 and 9 show the down core variations in the concentrations of metals in creek sediments. Mg, K and Ca behavior in the core was more or less uniform with its concentration varying from bottom to top (Fig. 6). Mg and Ca followed more or less similar pattern. Their concentration was almost constant from bottom to 10 cm and then increased. However, after 5 cm, their concentration exceeded 4,000 ppm at the surface. K concentration was constant up to 40 cm from bottom with very little variation, after which enrichment is observed. A detailed study is needed to explain the recent but uniform increase in the concentrations of K over the years.
Te downward profile of Al and Fe is shown in Fig. 7. The vertical profile shows enrichment of Fe in surface layer which may be attributed to early diagenetic process. A decrease in Fe concentration in subsurface is indicative of oxic/suboxic interface. The Fe oxyhydroxide get dissolved in partly reduced sediment and migrate upward in the sediment column and get precipated (Kumar and Edward 2009). Al contents followed an uneven pattern, and its concentration remained less than 4,500 ppm up to a depth of 12.5 cm; however, above this depth up to the surface, its concentration rrose to 5,000 ppm. Enrichment of Al in surface sediment samples suggests more industrial eflluent in recent years.
Depth-wise variations in the concentrations of V, Cr, Cu and Ni are shown in Fig. 8. Concentrations of these metals have similarities among them at different depth and indicate that main source of input to the creek the same. The range of increase was almost quite similar: V 34.00–84.00, Cr 22.0–72.0, Ni 18.9–66.0 and Cu 16.0–56.0 μg/g from bottom to surface. Such increases in these metal concentrations may be indicative of human influence on marine environment.
The down core profile of Mn, Zn, Ti and S is shown in Fig. 9. There is an enrichment of sulfur in surface layer which indicates that it is transported from bottom sediment layers to sediment water interface. The oxidation of Fe sulfide may be the cause of decrease in S content in bottom layers. Mn, Ti and Zn concentration varied in the examined samples over a broad range. The most significant differences were in Zn, from 76 in bottom most layers to 224 in surface layer. While Mn concentration increased from 76 ppm at the bottom to 187 ppm at the surface. Mn and Zn were found to be the highest occurring metals in the core as compared to V, Cr, Cu and Ni, and their contents elevated significantly in the upper layers. Mn and Zn values in bottom most layers are higher than V, Cr, Cu and Ni, which indicates that the background values of Mn and Zn are higher than others.
The increasing trends of the metals in surface samples show increased deposition of these metals due to industrial efflluents. Metals are released from specific local sources such as discharge from smelters (Cu, Ni), metal-based industries (Zn, and Cr from electroplating), paint and dye formulators (Cr, Cu, and Zn), petroleum refineries (Mn, Pb), as well as effluents from chemical manufacturing plants (Nadia et al. 2009). Once accumulated into the sediments, metals can be mobalized and find their way into overlying water column. Although these metals are essential for aquatic life but at elevated levels it is toxic to some species of aquatic life (WHO 2004). Inter-elemental association has also been evaluated by Pearson correlation coefficient (r), and the results are presented in Table 3. Elemental association may signify that each paired elements has identical source or common sink in the marine sediments. Results from Regression analysis showed significant corelation coefficient between metal concerntrations. Statistical analysis of inter-metal relationship shows high positive correlation among the metals. Mn, Fe and K are found to correlate well with each other (r = 0.95) and appear to control distribution of Cu, Ni, Cr and Zn. The significant correlation between Fe and Mn also indicate presence of Fe/Mn compounds. It has been reported that Fe and Mn oxide and hydroxide have high affinity with most of the trace element (Zabetoglou et al. 2002). Elements not significant correlations with each other suggest that these metals might have different anthropogenic and natural sources in sediments of the area of study.
Cluster analysis (CA) was performed on the data using Ward linkage and absolute correlation coefficient distance. Results of CA are shown in Fig. 10. Elements and physicochemical parameters were fused into clusters because sediments samples contain similar heavy metal elements. Three groups were distinguished in the dendrogram, performed using the Ward method, which used the squared (Fig. 10). The dendrogram clarifies the influence and association of the clusters or groupings by their relative elemental concentrations (Fig. 10). CA showed the association of pH with heavy metal elements in the third cluster. pH values in water are one of the most important factors that regulate the dissolved metals. Elements in the cluster II originated from terrigenous source. The domination of clay, silt, Cu, Ni, Zn and V with Mn indicates their association with the Fe oxides and fine particles.
Enrichment factor
EF was employed to assess the degree of contamination and to understand the distribution of the elements of anthropogenic origin from sites by individual elements in sediments. Al, Fe and Mn are commonly chosen as the normalizing element while determining EF values, as these of the widely used reference element (Baptista Neto et al. 2000; Mucha et al. 2003). In the present study, Al was used as reference element because Al is chemically inactive element with respect to other diver’s elements, so it is used as a normalization element to estimate the relative abundances of diverse element (Ghaffar et al. 2010). EF was calculated using the following equation.
where C n is the concentration of element n. The background value is that of average values of element in shale as reported by Turekian and Wedepohl 1961. EF values were interpreted as suggested by Ergin et al. (1991) and Birch (2003). According to their classification, EF of <1 indicates no enrichment, <3 is minor; 3–5 is moderate; 5–10 is moderately severe; 10–25 is severe; 25–50 is very severe; >50 is extremely severe. Table 4 shows metal EF in core sediments (EF of surface and mean of all sub samples). The order of average EF in the sediment core is summarized as:
The surface sediments showed moderate enrichment for Zn (avg. 3.29), Cu (avg. 3.17) and Ni (3.17), minor enrichment for Cr (avg. 2.34), and V (1.74) and no enrichment for other element (<1). Ti (avg. 0.17) exhibited lowest EF values. When EF values are observed for the entire length of the core, high values are seen in the upper portion of core compared to the lower portion of the core for all metals. The enhanced values of EF for Zn, Cu, Ni and Cr might have resulted from the increase in urban and industrial discharge in the creek in recent years.
Index of geoaccumulation (I geo)
Müller (1969) introduction Geo-accumulation index I geo to assess heavy metal pollution in sediments. The I geo was calculated using following equation:
where C n is the measured concentration of a heavy metal in sediments, B n is the geochemical background value in the average shale of element n, and 1.5 is the background matrix correction due to Terrigenous effects. Background value in average shale was taken from Wedepohl (1995). Muller (1969) has distinguished seven classes of geoaccumulation index: (1) I geo < 0: unpolluted; (2) 0 ≤ I geo < 1: unpolluted to moderately polluted; (3) 1 ≤ I geo < 2: moderately polluted; (4) 2 ≤ I geo < 3: moderately to strongly polluted; (5) 3 ≤ I geo < 4: strongly polluted; (6) 4 ≤ I geo < 5: strongly to extremely polluted; I geo > 5: extremely polluted.
I geo values are regarded as effective and meaningful in explaining the sediment quality (Karbassi et al. 2005; Lina et al. 2012). The I geo values for Korangi creek sediment shows that all the metals fall into class 1 and class 2 of Muller scale grade (Table 5).
According to the Muller scale, the Korangi creek surface sediment can be considered moderate polluted with respect to Cr, Ni Cu and Zn (1 ≤ I geo < 2) and unpolluted to moderately polluted with respect to unpolluted for rest of metals (Table 6). On the basis of the average I geo values, sediment is moderately polluted with respect to Cr and Zn unpolluted to moderately polluted for Ni and Cu. I geo plot with metal concentration is shown in Fig. 11, which also reveals moderate pollution with respect to Zn and Cr. Increased industrial effluent in the area is the main anthropogenic source for these metals.
Contamination degree (C d) and pollution load index (PLI)
Overall sediment contamination with metals was calculated based on the degree of contamination. Degree of contamination is calculated by adding contamination factor (C f) of all the metals examined (Lina et al. 2012)
where C f = C e/C b; C e is the concentration of element in the sediment and C b background value of element of shale as quoted by Wedepohl (1995). Four categories of contamination degree have been defined by Häkanson (1980):
-
(a)
C d < 7 = low degree of contamination
-
(b)
7 ≤ C d < 14 = moderate degree of contamination
-
(c)
14 ≤ C d < 28 = considerable degree of contamination
-
(d)
C d ≥ 28 = very high degree of contamination
Table 6 indicates a considerable degree of contamination in upper 15 cm for 12 metals, which may be attributed to increase industrial activity and its effluent in the Korangi creek. Tomlinson et al. (1980) introduced the concept of PLI to assess metal pollution in the sediment. PLI was calculated by using following equation (Tomlinson et al. 1980)
where CF is the contamination factor and n is the number of metals.
The PLI value >1 shows metal pollution, whereas PLI value <1 indicates no pollution. The PLI represents the number of times by which metal contents in the sediment exceeds the average natural background concentration gives a summative indication of overal level of metal toxicity in the core or sample. Calculated PLI values with depth is shown in Table 6, which indicate overall no pollution; however, PLI has an increasing trend from bottom to the surface. Increase in PLI in recent sediments may be due to the influence of anthrogenic sources.
Assessment of sediment pollution based on sediment quality guidelines
The accumulation of heavy metals in sediments can be a secondary source of water pollution, once environmental condition is changed (Chen et al. 1996; Cheung et al. 2003). Therefore, an assessment of heavy metal contamination in sediments is an indispensable tool to assess the risk of an aquatic environment. To assess metal concentrations in sediment, numerical sediment quality guidelines (SQGs) were applied. The primary purpose of SQGs is to protect aquatic life from adverse effects related to sediment bound contaminants (Seshan et al. 2010). Sediments were classified as non-polluted, moderately polluted and severely polluted, based on SQGs of USEPA (Perin et al. 1997). The SQGs include a threshold effect concentration (TEC) and a probable effect concentration (PEC) as shown in Table 7. If the metals in sediments are below the TEC, harmful effects are unlikely to be observed. If the metals are above the PEC, harmful effects are likely to be observed. MacDonald et al. (1996) noted in his studies that most of the TECs provide an accurate basis for predicting the absence of sediment toxicity, and most of the PECs provide an accurate basis for predicting sediment toxicity (MacDonald et al. 1996). In present study, Ni and Cu exceed TEL values, which can lead to adverse impact on the sediment dwelling fauna. The concentrations of Cr and Zn in sediment samples are lower than the proposed TECs indicated that there are no harmful effects from these metals.
Conclusion
The results of present investigation helped in understanding the extent of metal pollution in Korangi creek sediment. The different parameters of pollution, i.e., EF, degree of contamination and PLI indicated, are moderately polluted sediments with respect to Cr, Cu, and Ni. However, PLI indicated overall no pollution. The study also reveals significant positive correlations between Ni, Cu, Cr, Zn with Fe and Mn, indicating a significant role in remobalization of metals. Enrichment of Cr, Ni, Cu and Zn delineate increased industrial effluent discharge in the area. The enrichment trend of Ni, Cr, Cu and Zn poses a threat to the marine life of the Korangi creek, and it is most likely that the local food web complexes in creek might be at highest risk. The study also concludes that concentration of metals in bottom most layers can be used as baseline/background values for further study.
References
Ahmad, N., Akhtar, N. A., Shahnawaz, M., & Sadaat, S. (2010). Aerosole studies of urban areas of Lahore using PIXE. International Journal of PIXE, 20, 101–107.
Alemdaroglu, T., Onur, E., & Erkakan, F. (2003). Trace metal levels in surface sediments of lake Manyas, Turkey and tributary rivers. International Journal of Environmental Studies, 60, 287–298.
Baptista Neto, J. A., Smith, B. J., & McAllister, J. J. (2000). Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southeast Brazil. Environmental Pollution, 109, 1–9.
Birch, G. (2003). A scheme for assessing human impacts on costal aquatic environments using sediments. In: Woodcoffee, C.D., Furness, R.A. (Eds.), Costal GIS. Wollongong University papers in Center for MaritimePolicy,14, Australia. (http://www.ozestuaries.org/indicators/def_q-t.jsp).
Cardoso, A., Boaventura, G., Silva, E., & Brod, J. (2001). Metal distribution in sediments from the Ribiera bay, Rio de Janeiro, Brazil. Journal of the Brazilian Chemical Society, 12, 767–774.
Chen, W., Tan, S. K., & Tay, J. H. (1996). Distribution, fractional composition and release of sediment-boundheavy metals in tropical reservoirs. Water, Air, and Soil Pollution, 92, 273–287.
Chen, C. W., Kawo, C. M., Chen, C. F., & Dong, C. D. (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbour, Taiwan. Chemosphere, 66, 1431–1440.
Cheung, K. C., Poon, B. H. T., Lan, C. Y., & Wong, M. H. (2003). Assessment of metal and nutrient concentrations in river water and sediment collected from the cities in the Pearl River Delta, South China. Chemosphere, 52, 1431–1440.
Cohen, A. S. (2003). Paliolimnology. New York: Oxford University Press.
Ergin, M., Saydam, C., Basturk, O., Erdem, E., & Yoruk, R. (1991). Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Chemical Geology, 91, 269–285.
Folk, R. L., & Ward, W. C. (1957). Brazos river bar: a study in the significance of grain size parameters. Journal of Sedimentary Petrology, 27, 3–27.
Ghaffar, A., Tabata, M., & Nishimoto, J. (2010). A comparative metals profile of higashiyoka and kawazoe sediments of Ariake Bay, Japan. Electronic Journal of Environmental, Agricultural and Food Chemistry, 9, 1443–1459.
Gibbs, R. J. (1977). Transport phases of transition metals in the Amazon and Yukon Rivers. Geological Society of America Bulletin, 88, 829–843.
Häkanson, L. (1980). An ecological risk index for aquatic pollution control—a sedimentalogical approach. Water Research, 14, 975–1001.
Heyvaert, A. C., Reuter, J. E., Sloton, D. G., & Goldman, C. R. (2000). Paleo-limnological econstruction of historical atmospheric lead and mercury deposition at Lake Tahoe. California–Nevada. Environmental Science & Technology, 34, 3588–3597.
Horowitz, E., & Elrick, K. (1987). The relation of stream sediment surface area, grain size and surface area to trace element chemistry. Applied Geochemistry, 2, 437–451.
Karbassi, R., & Amirnezhad, R. (2004). Geochemistry of heavy metals and sedimentation rate in a bay adjacent to the Caspian Sea. International journal of Environmental Science and Technology, 1, 191–198.
Karbassi, A. R., Nabi-Bidhendi, G. R., & Bayati, I. (2005). Environmental geochemistry of heavy metals in a sediment core off Bushehr, Persian Gulf. Iran. Journal of Environmental Health Science & Engineering, 2, 255–260.
Krumbein, W. C., & Pettijohn, F. J. (1938). Manual of sedimentary petrography (pp. 549–551). New York: D. Appleton Century Co. Inc.
Kumar, S. P., & Edward, J. K. P. (2009). Assessment of metal concentration in the sediment cores of Manakudy estuary, south west coast of India. Indian Journal of Marine Sciences, 38, 235–248.
Lina, F., Nayak, G. N., & Ilangovan, D. (2012). Geochemical assessment of metal concentrations in mangrove sediments along Mumbai Coast India. World Academy of Science Engineering and Technology, 61, 258–263.
Liu, E., Shen, J., Yang, L., Zhang, E., & Meng, X. (2010). Assessment of heavy metal contamination in the sediments of Nansihu Lake Catchment, China. Journal of Environmental Monitoring and Assessment, 161, 217–227.
MacDonald, D. D., Car, R. S., Calder, F. D., Long, E. R., & Ingersoll, C. R. (1996). Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology, 5, 253–278.
Mashiatullah, A., Qureshi, R. M., Ahmad, N., Khalid, F., & Javed, T. (2009). Physico- chemical and biological water quality of Karachi coastal Water. The Nucleus, 46, 53–59.
Mashiatullah, A., Chaudhary, M. Z., Ahmad, N., Qureshi, R. M., Javed, T., Ghaffar, A., & Khan, M. S. (2011). Metal pollution assessment in sediments of Karachi Coast, Pakistan. The Nucleus, 48, 223–230.
McKay, J. L., Pedersen, T. F., & Mucci, A. (2007). Sedimentary redox conditions in continental margin sediments (N.E. Pacific) – influence on the accumulation of redox-sensitive trace metals. Chemical Geology, 238, 180–196.
Moore, P. A., Reddy, K. R., & Fisher, M. M. (1989). Phosphorus flux between sediment and overlying water in Lake Okeechobee, Florida: spatial and temporal variations. Journal of Environmental Quality, 27, 1428–1439.
Mucha, A. P., Vasconcelos, M. T. S. D., & Bordalo, A. A. (2003). Macrobenthic community in the Doura estuary, relations with trace metals and natural sediment characteristics. Environmental Pollution, 121, 169–180.
Mudroch, A., Sarazin, L., & Lomas, T. (1988). Summary of surface and background concentration of selected elements in Great Lake sediments. Journal of Great Lake Research, 14, 241–251.
Muller, G. (1969). Index of geo-accumulation in sediments of the Rhine River. Journal of Geology, 2, 108–118.
Munshi, A. B., Detlef, S. B., Schneider, R., & Zuberi, R. (2004). Geochemical assessment of metal concentrations in sediment core of Korangi Creek along Karachi Coast-Pakistan. Marine Pollution Bulletin, 49, 597.
Nadia, B. E., Anwar, B. A., Alaa, E. R., Mostafa, B., & Al-Mur, A. (2009). Metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia. Environmental Monitoring and Assessment, 155, 509–526.
IUCN Pakistan, Mangroves of Pakistan Status and management, (2005).
Perin, G., Bonardi, M., Fabris, R., Simoncini, B., Manente, S., Tosi, L., & Scotto, S. (1997). Heavy metal pollution in central Venice Lagoon bottom sediments, evaluation of the metal bioavailability by geochemical speciation procedure. Environmental Technology, 18, 593–604.
Seshan, B. R. R., Natesan, U., & Deepthi, K. (2010). Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. International journal of Environmental Science and Technology, 7, 291–306.
Shahzad, A., Khan, M. A., Shaukat, S. S., & Ahmad, W. (2009). Chemical pollution profile of Rehri creek area, Karachi (Sindh). Journal of the Chemical Society of Pakistan, 31, 592–600.
Tomlinson, D. C., Wilson, J. G., Harris, C. R., & Jeffery, D. W. (1980). Problems in the assessment of heavy metals levels in estuaries and the formation of a pollution index. Helgol Wiss Meeresunters, 33, 566–575.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s crust. Bulletin Geological Society of America, 72, 175–192.
Wedepohl, K. H. (1995). The composition of continental crust. Geochicam et Cosmochemica acta, 59, 1217–1233.
World Health Organization. (2004). Guidelines for drinking water quality. 3rd edn. Pakistan: World Health Organization, ISBN: 92-4-154638-7, 516.
WWF, 2000 World Wide Fund for Nature, Government of Pakistan, IUCN — The World Conservation Strategy, Biodiversity Action Plan for Pakistan, A Framework for Conserving Our Natural Wealth.
Zabetoglou, K., Voutsa, D., & Samara, C. (2002). Toxicity and heavy metal contamination of surficial sediments from the bay of Thessaloniki (Northwestern Aegean Sea) Greece. Chemosphere, 49, 17–26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhary, M.Z., Ahmad, N., Mashiatullah, A. et al. Geochemical assessment of metal concentrations in sediment core of Korangi Creek along Karachi Coast, Pakistan. Environ Monit Assess 185, 6677–6691 (2013). https://doi.org/10.1007/s10661-012-3056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-3056-4