Abstract
In this study, the distribution of heavy metals (Co, Cr, Cu, Ni, Zn and Pb) in a sediment core from the Ganga (Hooghly) River estuary has been investigated. The data on concentrations of heavy metals, together with the concentrations of Al, Fe, Mn, carbonate, organic carbon (Corg) and nitrogen (Norg), have been used to understand (1) the phase association of metals, (2) their geochemical cycling in the sediment column and (3) if metals are enriched by anthropogenic contributions. The metals show significant positive correlations with Fe and Mn, indicating that Fe–Mn oxyhydroxide phases are the major host of metals in the sediment core. This observation together with significant negative correlations of Fe, Mn and the heavy metals with (C/N)org provides the evidence for diagenetic redistribution of metals in the sediment column and their partitioning into the Fe–Mn oxyhydroxides. The increasing upward exchangeable Mn concentrations and association of metals with Fe and/or Mn in the exchangeable phases provide the supporting evidence for digenetic remobilization of the metals and their cycling with the Fe–Mn oxyhydroxides. Down-core variations of the metal concentrations generally match with that of Mn and Fe. The results of calculations for enrichment factors (EF) show that EF > 1.5 for Pb in the sediment core, suggestive of non-crustal sources. The elevated concentrations of Pb with EF > 1.5 in the sediment samples of the effluent water that drain into the estuary suggest that industries are an important source of Pb to the Hooghly River estuary sediments. The results and observations of this study suggest that an assessment of temporal variation of metal pollution in estuaries will require understanding of phase association and cycling of metals in the sediment column.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries provide an environment where the compositions of the dissolved and particulate loads are modified through a number of biogeochemical processes. Specifically, the metal concentrations of the riverine suspended sediments are amended in the estuary via processes such as ion-exchange, adsorption–desorption and formation of organometallic complexes (Lion et al. 1982; Hatje et al. 2003; Abrahim and Parker 2008; Du Laing et al. 2009). In the sediment column, the metals may undergo further redistribution through post-depositional processes such as suboxic diagenesis (Robert et al. 2004; Audry et al. 2006; Lesven et al. 2008). The imprints of all these changes may be modified again by physical mixing via bioturbation and sediment re-suspension (Krantzberg 1985; Widdows et al. 2004; Arzayus and Canuel 2005). Anthropogenic activities also bring about changes in the cycling and distribution of trace metals in estuaries, given that the industrial effluents and urban wastewaters often encroach into the rivers and estuaries (Benjamin and Honeyman 2000; Bianchi 2007 and references therein). The estuarine sediment cores, therefore, can provide records of both natural and anthropogenic supply of metals to estuaries. Deciphering of the sources and processes contributing to the distribution of the metals, however, requires proper understanding of both their phase association as well as cycling in the estuary.
This study forms a part of our ongoing investigation of nutrient and trace metal cycling in the Ganga (Hooghly) River estuary. The specific objectives of the present study are (1) evaluation of the association of metals with various phases in the sediment core, (2) understanding the geochemical cycling of metals and (3) exploring if the down-core metal concentrations are unaltered and could be used to assess the impact of anthropogenic activity on the estuary as a function of time. Determination of phase association of metals not only provides an understanding of sources and cycling of metals in the estuary but also has implication for their mobility, bio-availability and potential for environmental degradation. In particular, an effort has been made to determine the relative role of clay minerals, organic matter and Fe–Mn oxyhydroxides on the cycling of metals in sediments. While the data on the concentrations of metals in the sediment cores of the Hooghly estuary have been reported earlier (Chatterjee et al. 2007; Banerjee et al. 2012), understanding of metal cycling in the sediment column and assessment of the role of post-depositional processes on the distribution of metals in the sediment column are lacking. These issues are addressed here based on the data on concentrations of Fe, Mn, Corg and Norg in the sediment core. Considering that the Hooghly River estuary receives considerable amount of discharge from the industrial effluents and urban wastewater (Basu et al. 1970; Gopalakrishnan et al. 1973; Bhattacharya et al. 1994; Ghatak and Konar 1994; Sadhuram et al. 2005), it is imperative to examine whether the concentrations of metals in the sediment column are influenced by anthropogenic activities. This issue has been dealt with after careful evaluation of phase association of metals and their cycling particularly during diagenetic mobilization.
Materials and methods
The study area
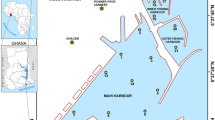
The Ganga River originates in the Gangotri glacier that is located in the western Himalaya. In the alluvial plains, the Ganga River is fed by several tributaries that take their course through the Himalaya. After flowing for more than 2000 km from its origin, the Ganga River bifurcates near Rajmahal at Farakka (Fig. 1a). The main distributary of the Ganga, named Hooghly, flows toward the south and drains into the Bay of Bengal (BoB). The annual rainfall during the years 2009–2013 in areas around the Hooghly River estuary varied from ~1310 to ~2300 mm (IMD 2013). The southwest monsoon period (June–September) accounts for about 80% of the annual rainfall in the catchment area. On its way from Farakka to the BoB, the Hooghly River receives 1154 million L/day of industrial effluents and urban wastewater (Sadhuram et al. 2005) from the highly populated and industrial cities such as Kolkata, Haldia and Howrah. The Hooghly River estuary is used as the navigable waterway for two major ports (Kolkata and Haldia) and for the Sundarbans islands. The estuary is likely to have several potential sources of metals such as port activity at Haldia and Kolkata, effluent discharges from a variety of industries located adjacent to the estuary, e.g., tanneries, jute mills, pulp and paper mills, pesticide-manufacturing plants, thermal power plants, small scale steel industries, kilns, rubber factories, fertilizer plants, soap factories, antibiotic plants and oil refineries (Sarkar et al. 2004; Saha et al. 2006; Banerjee et al. 2012). Pb can be supplied to the estuary from the thermal power plant located in the bank of the Rupnarayan River (Fig. 1b), oil refineries in Haldia and vehicular emission from large cities such as Kolkata (Chatterjee et al. 2007; Stephen-Pichaimani et al. 2008). Leaching of paints from the boats, trawlers and ships that operate in the Hooghly River can also supply Pb and Zn. Jute mills and rubber factories are known to discharge significant amount of Cu (Rice et al. 2002). Steel and chemical industries have been reported to be the potential sources of Co and Ni (Jonathan and Ram Mohan 2003).
a Map of the Ganga River system and the Hooghly estuary. b The Hooghly River estuary, the locations of the core and of the surface sediment samples from the channels carrying industrial effluents. The locations of the cores studied by Banerjee et al. (2012) and Chatterjee et al. (2007) are also shown
Location of the sediment core, sampling and analysis
A gravity core (C1) of ~1.6 m length was raised during the post-monsoon season (December 2013) from the depositional meander of the Hooghly River channel. The coring station (22°9′34.5″N and 88°8′49.6″E) is located on the right bank of the channel that is opposite to Diamond Harbour (Fig. 1b). The coring was carried out during the low-tide period when the river bed was partially exposed. The core was collected in a polyvinyl chloride (PVC) pipe that was attached to the coring assembly and was subsampled at every 3-cm depth interval. In addition to the sediment core, two samples of surface sediments from the channels that carry industrial effluent waters were also collected near Haldia (Fig. 1b). The discharges from industries located in and around Haldia flow through these channels and eventually drain into the Hooghly River estuary. Based on data on 210Pb excess activity, the rate of sedimentation has been reported to be 3.2 mm/year at a location adjacent to the coring location of this study (Core H1, Banerjee et al. 2012; Fig. 1b). Considering that the core H1 was collected from the eroding meander, whereas the core C1 investigated in this study was collected from the accreting meander, the maximum age of deposition for 1.6-m-long core C1 is about 500 years. The surface sediments at Diamond Harbour are characterized by clayey silt texture having ~90% silt + clay and ~10% sand (Sarkar et al. 2004). In the sediments of the core S1 (Fig. 1b) that was raised south of the core C1, silt + clay was also dominant (~70%), but the fraction of sand (29%) was higher than that in the surface sediment (Chatterjee et al. 2007). Based on the XRD patterns (Sarkar et al. 2004), the surface sediments were reported to comprise of quartz (>30%), feldspars and mica (5–10%) and chlorite (<5%).
In the core C1, 18 samples were processed for analysis. The sediment samples were dried at 70 °C in a hot air oven and powdered to <100 mesh using an agate planetary disk mill (Retsch RS200). About 1 g powder of each sample was washed with 18.2 M-Ω Milli-Q water to remove the sea salts. Out of this, ~60 mg was processed for the acid digestion, using either a microwave digestion system (Anton Paar Multiwave 3000) or the Savillex® vials that were kept heated on an Analab® hot block. The samples were treated with 0.5 mL Merck suprapur® H2O2 to oxidize the organic matter before digesting them in an acid mixture of HNO3, HCl and HF in the proportion of 3:1:3. The certified reference materials of shale (SBC-1) and granodiorite (GSP-2) and the procedural blanks were processed in every batch of digestion. The digestion procedure was replicated for two sediment samples.
Selective extraction of the exchangeable fractions of the sediment samples was carried out following the established procedures (Gupta and Chen 1975; Kersten and Forstner 1986; Idriss and Ahmed 2013). Briefly, 10 ml 1 N ammonium acetate (Sigma Aldrich® >99.99% purity) was added to ~0.5 g sample powder in the pre-cleaned centrifuge tubes, which were subsequently placed over a reciprocating shaker at 400 rpm for 16 h. The slurry was centrifuged at 4000 rpm, and the supernatant was decanted. The residue was washed with Milli-Q water; the washing (water) was collected and added to the supernatant. Prior to analysis on ICP-MS, the supernatant was dried and dissolved in 5% double distilled HNO3. The procedural blanks and sample replicates were also processed during selective extraction of sediments samples.
The concentrations of major elements (Al, Fe, Mn) and the heavy metals (Cr, Co, Cu, Ni, Zn, Pb) were measured on a quadrupole ICP-MS (Thermoscientific X Series 2) facility at IISER Kolkata. An internal standard of indium (In) was used to correct for the instrumental drift during the analysis. The average accuracy of the measurement was determined by analyzing the certified reference material GSP-2 (for major elements) and SBC-1 (for heavy metals). The average measurement accuracy was within 7% for Al, Fe and Mn and was 10–12% for Cu, Pb and Zn; and 15–20% for Co, Cr and Ni. Based on analyses of two samples in replicates, the analytical precision was better than 7% both for major elements and heavy metals except Co and Cr, for which precision was better than 10%. The concentrations of the metals based on analysis of the standard SBC-1, dissolved both in the microwave digestion and in the Savillex® vials, agreed within 8%.
The exchangeable concentrations of Fe, Mn, Co, Cu and Ni were measured on a quadrupole ICP-MS. Based on the analysis of the reference standards IV-stock 1643 (Inorganic Ventures®), BCR-610 (groundwater) and BCR-617 (artificial groundwater), the average measurement accuracy was better than 12% for Fe and better than 7% for Mn, Co, Ni and Cu. The results of replicate analyses show that the average analytical precision was better than 8% for the exchangeable concentrations. The exchangeable concentrations of Cr, Zn and Pb could not be determined due to higher levels of these metals in the ammonium acetate that was used for selective extraction.
Carbonate content of the samples were estimated by leaching ~3 g of powder with 40 ml of 1.5 N HCl for 72 h. Processing of four samples in replicates yielded the analytical precision of carbonate content that was better than 6%. Organic carbon (Corg) and organic nitrogen (Norg) were measured in the decarbonated samples on an elemental analyzer (Flash 2000) attached to an isotope ratio mass spectrometer (MAT 253, Thermo Scientific) via the conflo interface at Physical Research Laboratory, Ahmedabad. The mass spectrometer was calibrated by combusting a known quantity of the standards. Cellulose (IAEA-CH-3; C content ~44.4%) and ammonium sulfate (IAEA-N-2; N content ~21.2%) were used as the standards for Corg and Norg, respectively. The average precision of measurements based on analysis of duplicate samples was better than 10%.
Results
The data on the concentrations of Al, Fe, Mn, carbonate content, Corg, Norg and heavy metals (Co, Cu, Ni, Cr, Pb and Zn) are provided in Table 1. The mean concentrations (with 1 SD) were 6.71 ± 0.58 wt% for Al, 3.43 ± 0.30 wt% for Fe, 657 ± 62 μg/g for Mn, 0.70 ± 0.03 wt% for Corg, 0.033 ± 0.005 wt% for Norg and 14 ± 2.9 wt% for the carbonate content. The average metal concentrations were 39 ± 4 μg/g for Cr, 13.8 ± 1.6 μg/g for Co, 33 ± 5 μg/g for Ni, 27 ± 4 μg/g for Cu, 69 ± 8 μg/g for Zn and 28 ± 3 μg/g for Pb. The highest concentrations of Al, Fe, Mn, Co, Ni, Cu, Zn and Pb were observed in the surface section (0–3 cm) of the core. The variability of the major and trace elements in the sediment core was assessed (Table 1) in terms of the relative range (RR) in percent and the coefficients of variation (CV). The variability of the major components, expressed as the relative range, was 33–72%. The values of RR varied from 29 to 48% for the heavy metals. The values of CV are <10% for Al, Fe, Mn and Corg, and ≤15% for the heavy metals.
The concentrations of exchangeable Fe, Mn, Co, Ni and Cu are given in Table 2. The mean Fe and Mn concentrations of the exchangeable phase were 70 ± 10 μg/g and 32 ± 5 μg/g, respectively. The exchangeable concentrations of Co, Ni and Cu were in the range of 39–69 ng/g, 220–319 ng/g and 213–407 ng/g, respectively. The exchangeable Mn concentrations constitute up to ~6% of the bulk Mn concentrations, whereas the exchangeable Fe, Co, Ni and Cu account for up to 1.5% of their respective bulk concentrations.
Discussion
In this section, the level and variability of the metal concentrations in core C1 are first compared with the reported data in the upper continental crust (UCC) and average shale. In addition, comparisons are also made with the reported data in the sediment cores at locations that are adjacent to the study area. Considering that the metal concentrations are influenced by the major host phases such as the clay minerals and the Fe–Mn oxyhydroxides, the variability of the metal concentrations are discussed in conjunction with that of Al, Fe and Mn. The mean concentrations of Pb and Ni in core C1 are higher than the respective average values in the UCC (Wedepohl 1995), whereas for the rest of the trace metals, the measured concentrations in C1 are the same as the UCC values within errors. The metal concentrations in the average shale (Turekian and Wedepohl 1961, Table 1) are 38–131% higher than the corresponding average metal concentrations in core C1. However, the mean concentration of Pb in the core C1 (28 μg/g) is higher than that in the average shale (20 μg/g). The observation of higher metal concentrations in the average shale than in the core C1 is not surprising given that in the average shale, the concentrations of Al (8.83 wt%), Fe (4.72 wt%) and Mn (850 mg/g) are higher than those in the core C1. The concentrations of Cu, Ni, Cr and Zn observed in this study, on average, are similar to those reported by Banerjee et al. (2012) for the sediment core H1 collected near Diamond Harbour (Cr: 40.1 ± 3 μg/g, Ni: 33.9 ± 7 μg/g, Cu: 21.6 ± 4.5 μg/g, and Pb: 23.5 ± 2 μg/g). However, average concentrations of Zn and Co in this study are ~25% higher and ~20% lower, respectively, compared to the average concentrations (Zn: 53.4 ± 5 μg/g, Co: 18 ± 1.9 μg/g) in the core H1 (Banerjee et al. 2012). Overall agreement of metal concentrations between these two studies is also consistent with the observation that the average concentrations of Fe (2.9 ± 0.4 wt%), Mn (502 ± 52 μg/g) and Corg (0.5 ± 0.3 wt%) reported by Banerjee et al. (2012) for the core H1 overlap within errors with the corresponding mean concentrations in this study (Table 1). It should be noted that the metal concentrations in the sediment cores C1 (Table 1) and H1 (Banerjee et al. 2012) are generally lower compared to those reported for the sediment cores (S1, S2 and S3) collected from the Sundarbans mangrove forest (41–49 μg/g for Cr, 20–26 μg/g for Co, 44–56 μg/g for Ni, 34–42 μg/g for Cu, 62–84 μg/g for Zn and 29–34 μg/g for Pb; Banerjee et al. 2012).
The values of CV for the metals concentrations in core C1 (Table 1) are similar to those reported by Banerjee et al. (2012) for the sediment core H1 (CV ≤ 20%). However, the values of the relative range for Cu and Ni (75–85%) were higher in the sediment core H1 (Banerjee et al. 2012). The concentrations of the metals in the present study (Table 1) are within the range of values reported by Chatterjee et al. (2007) for sediment cores collected in and around Kakdwip and Sagar Island (36–180 μg/g for Zn, 8–38 μg/g for Cu, 29–79 μg/g for Cr, 9–19 μg/g for Co, 12–50 μg/g for Ni and 14–34 μg/g for Pb).
The processes and factors that may be responsible for the observed concentrations and distribution of metals in the sediment column are: (1) the variation in the abundance of host phases such as the clay minerals, Fe–Mn oxyhydroxides, organic matter and carbonate minerals, (2) remobilization of one or more of these phases during post-depositional diagenesis of sediments, (3) contributions from the anthropogenic activity and (4) change in the rate of sedimentation. The last of the four factors mentioned above can be ignored given that the rate of sedimentation for the core H1 has been reported to be more or less constant, at least for the last ~110 years (Banerjee et al. 2012). The importance of the rest of the three parameters/processes is assessed in the following section by examining the relationship between the metal concentrations with Fe, Mn, Al, Corg and carbonate contents in the sediments. The inferences based on bulk concentrations are further corroborated by using the distribution of major elements and heavy metals in the exchangeable phases of the sediments.
Association and geochemical cycling of metals
It is observed that the heavy metals show strong correlations (r ≥ 0.90) with each other. Although some of the metals, e.g., Cu, Ni and Co, usually behave similarly in soil and sedimentary environments (Manta et al. 2002; Tume et al. 2006; Chatterjee et al. 2007), strong inter-element association of all the heavy metals indicate that the metals are cycled mostly with a common phase. As suggested by the results of this study and discussed later in this section, the common phase that is relevant to the metal cycling is inferred to be the Fe–Mn oxyhydroxides. Analogous to the results of this study, significant positive correlations were also observed among the metals in sediment cores from the Hooghly estuary (Chatterjee et al. 2007; Banerjee et al. 2012), suggesting similar mode of cycling of metals in the Hooghly estuary sediment columns.
The metals exhibit significant to strong positive correlation with Fe (r: 0.63–0.79, Fig. 2, B1–B6) and Mn (r: 0.63–0.79, Fig. 2, C1–C6). This is suggestive of an association of metals with the Fe–Mn oxyhydroxide phases. In order to assess whether the above-mentioned positive correlation is due to the presence of microparticles of Fe or other magnetic metals, a bar magnet was covered with a transparent plastic sheet and was placed on the sediment powders. The presence of magnetic particles was not observed. It is essential to ascertain whether the association of the heavy metals with the Fe–Mn oxyhydroxides did exist before sedimentation or was achieved during post-depositional redistribution process. The results on the suspended particulate matter indicate that Fe–Mn oxyhydroxides are more important than the clay minerals in hosting the metals, particularly Co, Cu and Ni (Samanta and Dalai 2016). This observation suggests that the association of the metals with the Fe–Mn oxyhydroxides was inherited, at least partially, in the estuarine water column. Together, the data of the suspended particulate matter and the sediment core suggest that the Fe–Mn oxyhydroxides play a more important role than any other phase(s) in the cycling of heavy metals in the Hooghly estuary. Redox-cycling of metals during post-depositional diagenesis (Zwolsman et al. 1993; Brown et al. 2000) may also have contributed to the association of metals with Fe–Mn oxyhydroxides. This aspect has been dealt in greater detail later in this section. All the metals investigated in this study show weak positive correlations (r ≤ 0.36) with Al, an indicator for the clay minerals (Fig. 2, A1–A6), thereby indicating that the clay minerals are relatively less important in hosting the metals in the sediments. Considering that all the metals, except Zn, in the bed sediments collected during the years 2012–2013 generally show significant to strong positive correlations with Al (r: 0.40–0.99, Samanta 2017), it can be inferred that previous association of the metals with the clay minerals, if any, has been modified by post-depositional diagenesis during which the metals seem to have partitioned into the Fe–Mn oxyhydroxide phases.
Covariation of heavy metal concentrations with Al, Fe, Mn, Corg and (C/N)org. Significant positive correlations of metals with Mn and Fe, and significant negative correlations of metals with (C/N)org are evident. Correlations of metals with Al and Corg are relatively weak and insignificant. The correlation coefficients (r) and significance levels (p) are also given
The carbonate concentrations do not show any significant correlation with the metals. This is in line with the knowledge that carbonate minerals are not a significant host of metals (Chuong and Obbard 2006; Passos et al. 2010) and act as a diluent for the metals owing to their very low metal concentrations (Loring 1991; Brumsack 2006). It is also noteworthy that the metals exhibit weak negative correlations with Corg (r: −0.07 to −0.27, Fig. 2, D1–D6), thereby indicating that they are unlikely to be associated with the organic matter in the sediments as well. Considering that the metals are usually cycled with the organic matter, particularly via formation of organometallic complexes (Tessier et al. 1996; Santschi et al. 1997), the lack of their association with Corg is intriguing. Low Corg concentrations in the Hooghly estuary core sediments has been attributed to efficient bioturbation and mixing (Banerjee et al. 2012), and high microbial activity which converts organic matter into CO2 (Canuel and Martens 1993; Subramanian et al. 2001; Chatterjee et al. 2007). It is thus likely that the original metal-Corg association, if any, has been lost due to bioturbation, sediment re-suspension and efficient mineralization of organic matter driven by microbial activity. This inference is in line with the results of Samanta et al. (2015) that brought out the importance of degradation of organic matter as one of the major processes in generating dissolved inorganic carbon (DIC) in the Hooghly River estuary. Low values of Corg concentrations and TN/TOC in the Hooghly estuary sediments prompted Ray et al. (2015) to infer that intense mineralization and transformation of organic matter (OM) in sediments resulted in sedimentary OM that is significantly different from those originally derived from their sources. Another potential cause for the observed lack of metal-Corg correlation could be post-depositional diagenesis. Both Corg and Norg are lost from the sediments during post-depositional suboxic diagenesis, with Norg experiencing a greater loss than Corg (Burdige and Martens 1988; Wakeham 2002; Rojas and Silva 2005). As a result, the concentrations of Corg and Norg decrease, but the (C/N)org ratio increases. The rather low Corg concentrations (≤0.76 wt%) and higher (C/N)org ratios in core C1, compared to those reported for the particulate matter and surface sediments of the Hooghly River estuary (Sarkar et al. 2004; Ray et al. 2015) lend credence to the inference that the sediments have been subjected to post-depositional diagenetic processes.
Effect of post-depositional processes
It is well established that in the sediment column, oxygen depletion results from oxidation of organic matter (Froelich et al. 1979; Berner 1980; Holmer 1999; Lehmann et al. 2002). In the absence of free oxygen, a number of sedimentary phases beginning with Mn oxides and followed by Fe oxyhydroxides undergo reductive dissolution during oxidation of organic carbon. The metals are consequently released from the Fe–Mn oxyhydroxide phases (Audry et al. 2006) and are re-precipitated in the zone where the mobilized Fe and Mn are fixed. As mentioned earlier, both Corg and Norg are lost from sediments during diagenetic degradation of organic matter, but their disproportionate loss results in increased (C/N)org during progressive diagenesis (Burdige and Martens 1988; Wakeham 2002; Rojas and Silva 2005). Thus, the loss of Mn (and Fe) via diagenetic mobilization and increase of (C/N)org would result in a negative correlation between them in sediments subjected to suboxic diagenesis. In this study, the observed significant negative correlation of Mn (and Fe) with (C/N)org (Fig. 3) provides evidence for the inference that mobilization of Fe and Mn in sediments is concomitant with the preferential loss of Norg over Corg, and increase of (C/N)org during diagenesis. In the bed sediments, however, weak positive correlation between Fe and (C/N)org is observed (r < 0.32, Samanta 2017). Together, these observations suggest that the observed inverse correlation of Mn and Fe with (C/N)org is a result of post-depositional diagenesis and is not inherited prior to sediment deposition. In this study, the lack of typical diagenetic profiles, characterized by systematic decrease of Corg and Norg with depth (Rojas and Silva 2005), is most likely due to bioturbation, mixing and re-suspension of sediments in the Hooghly estuary as reported in the earlier studies (Subramanian et al. 2001; Chatterjee et al. 2007; Banerjee et al. 2012).
As mentioned earlier, the concentrations of the metals exhibit significant negative correlations (r =−0.63 to −0.69) with (C/N)org (Fig. 2, E1–E6). This observation together with significant positive correlations of Fe and Mn with the metals (Fig. 2, B1–B6, C1–C6) allows us to infer that during diagenetic transformation of the organic matter, the metals associated with the organic phases have been mobilized and partitioned into the Fe–Mn oxyhydroxides in the sediment column via re-precipitation. The observation that the surface sample (0–3 cm) of the core C1 has the highest concentrations of Fe, Mn and all the metals except Cr (Table 1; Fig. 4) supports our contention that the observed distribution pattern of metals is a result of mobilization of metals during diagenesis and their re-precipitation in Fe–Mn oxyhydroxides. The signatures of diagenesis have been likely modified further by processes of mixing driven by bioturbation and sediment re-suspension.
Down-core variation of (C/N)org and the concentrations of Corg, Al, Fe, Mn and heavy metals. The patterns of variations of the metal concentrations are more or less similar to that of Mn and Fe. Increasing upward trends are evident for the concentrations of Mn, Fe and the heavy metals in the top ~50 cm
The results of selective extraction experiments (Table 2) provide evidence for remobilization of Fe–Mn oxyhydroxide phases during suboxic diagenesis and the resulting mobilization of the metals. First, the exchangeable concentrations of Mn are significant, accounting for up to ~6% of the bulk sediment concentrations, and show an increasing upward trend in the top 50 cm of the sediment core (Fig. 5). This observation suggests that a significant amount of Mn has been mobilized via reduction during suboxic diagenesis and precipitated in the upper section of the core. The exchangeable Mn concentrations are consistent with the knowledge that in the sedimentary redox sequence, Mn oxides are the first among the phases undergoing reductive mobilization (Stumm and Morgan 1981; Tromp et al. 1995; Emerson and Hedges 2003). Second, the exchangeable Cu and Ni show significant positive correlation with each other (r = 0.78) and are positively correlated with the exchangeable Mn (r = 0.40–0.63). In addition, the exchangeable Co shows significant positive correlation (r = 0.75) with Fe. Thus, the data on the composition of the exchangeable phase support our interpretation that the investigated section of the sediment core C1 has been subjected to mobilization of Fe, Mn and the metals driven by suboxic diagenesis. The results and inferences mentioned above are in agreement with the findings of Tessier et al. (1996) who observed that the trace metals bind onto Fe–Mn oxyhydroxides during diagenesis, and also with the observation of Robert et al. (2004) that re-suspension of sediments and mud result in the adsorption of metals onto Fe–Mn oxyhydroxides.
Down-core variation and anthropogenic enrichment of metals
The metal concentrations in the studied core section do not show systematic variation trends for any of the metals studied (Fig. 4). This lack of systematic variation trends could have been caused by either one or a combination of the following processes: (1) sediment mixing due to bioturbation and re-suspension of sediments and (2) post-depositional mobilization of elements. Thus, it is necessary to understand the cause of variation of metal concentrations before an assessment of source of metals can be made. While sediment re-suspension and bioturbation can modify the metal distribution patterns in the upper part of the core, the lower part of the core is more likely to be influenced by diagenetic mobilization. Interestingly, down-core variations of metal concentrations generally follow that of Mn and Fe with a few exceptions (Fig. 4). This observation is consistent with the inference made earlier that the metals are cycled mostly with the Fe–Mn oxyhydroxide phases during diagenesis.
It is noteworthy that analogous to Fe and Mn, the metal concentrations show increasing upward patterns in the top 50 cm of the core. However, when normalized with Mn and Fe, the observed increasing upward trends for the metal concentrations disappear (Fig. 6). Such an observation underscores the importance of understanding phase association and cycling of metals in the studies that are aimed at evaluation of metal pollution by anthropogenic activities. In the following, the influence of anthropogenic activity on metal concentrations in the core C1 has been assessed by taking into account the strong association of metals with Fe–Mn oxyhydroxide phases.
Down-core variation of metal concentrations normalized to Fe and Mn. Note the disappearance of the increasing upward trends for heavy metals that were observed in the top ~50 cm of the core (Fig. 4)
Enrichment factors (EF) help in assessing whether metals are enriched over natural levels. The calculation of EF generally involves normalization of metals concentrations with Fe or Al (Windom et al. 1989; Sharma et al. 1999; Bresline and Sanudo-Wilhelmy 1999), and comparing the normalized ratios in samples with those in the average shale or the upper continental crust (UCC). Considering that the metals are mostly cycled with Fe–Mn oxyhydroxides in the present study, the EF were calculated as follows:
where M stands for any particular metal. The results of calculations (Table 3) indicate that the values of EF fall in the range of 1.0–1.5 within the errors for all of the metals except for Pb for which EF > 1.5. Considering the uncertainties of data and natural variability, an EF value of 1.5 or more can be considered to be indicative of non-crustal sources (Zhang and Liu 2002). Thus, enrichment of Pb over crustal levels indicates contributions from anthropogenic source(s). The results of calculations of EF based on the reported data (Chatterjee et al. 2007; Banerjee et al. 2012) in the Hooghly estuary sediment cores (Table 3) also show that EF > 1.5 for Pb, whereas for other metals EF < 1.5. In order to evaluate the role of industrial supply of metals to the estuary, the surface sediments collected from the channels that carry industrial effluent was analyzed (Table 1). The effluent channel integrates discharges from the industries such as the oil refineries, chemical and automobile industries. Table 4 shows a compilation of data from this study and previously reported data for metal concentrations in sediments from channels of effluent water and urban wastewaters that drain to the Hooghly estuary. These data show that metals are generally enriched in the sediments of the effluent water and wastewater compared to the sediment cores. However, the calculation of enrichment factors indicates that EF > 1.5 only for Pb in the effluent sediments (Table 4). This observation is consistent with those from the sediment cores.
Thus, the results of this study and reported data together suggest that Pb is supplied by non-crustal sources to the Hooghly estuary sediment core. The value of EF > 1.5 for Pb in the effluent sediments indicates the industries in the region could be a potential source of Pb. As mentioned earlier (“The study area” section), Pb can be supplied to the estuary from a number of sources such as the oil refineries, thermal power plants, vehicular emission, leaching of the paints on the ships, trawlers and boats used for navigations and fishing activity in the Hooghly estuary (Chatterjee et al. 2007; Stephen-Pichaimani et al. 2008).
Conclusions
Detailed investigation of major elements, Corg, Norg and heavy metals in a ~1.6 m long sediment core from the Hooghly estuary has provided the following results and observations.
The heavy metals show strong positive inter-element correlations, suggestive of their cycling with a common phase. Strong positive correlations of metals with Mn and Fe, together with significant negative correlations of (C/N)org ratio with the heavy metals, Mn and Fe indicate that the metals have been mobilized and partitioned into the Fe–Mn oxyhydroxides during diagenetic redistribution. This inference draws strong support from positive correlations of the heavy metals with Fe and Mn in the exchangeable phases. The down-core variation of metal concentrations is inferred to be a result of collective influence of diagenetic mobilization and mixing driven by sediment re-suspension and bioturbation.
The increasing upward trends of the metal concentrations in the top 50 cm of the core disappear after normalization of metal concentrations with Fe and Mn. This observation emphasizes the importance of understanding phase association of metals in studies that are aimed at evaluation of anthropogenic contribution of metals to the estuarine sediments. The calculations of enrichment factors indicate that only Pb is enriched in the sediment core over crustal levels. Available information on the industries in the studied region and observation of EF > 1.5 for Pb in the effluent channel sediments suggest that industries could be an important supplier of Pb to the Hooghly estuary sediments.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Adhikari S, Ghosh L, Rai SP, Ayyappan S (2009) Metal concentrations in water, sediment and fish from sewage-fed aquaculture ponds of Kolkata, India. Environ Monit Assess 159:217–230
Arzayus KM, Canuel EA (2005) Organic matter degradation in sediments of the York River estuary: effects of biological vs. physical mixing. Geochim Cosmochim Acta 69:455–464
Audry S, Blanc G, Schfer J, Chaillou G, Robert S (2006) Early diagenesis of trace metals (Cd, Cu Co, Ni, U, Mo, and V) in the freshwater reaches of a macrotidal estuary. Geochim Cosmochim Acta 70:2264–2282
Banerjee K, Senthilkumar B, Purvaja R, Ramesh R (2012) Sedimentation and trace metal distribution in selected locations of Sundarbans mangroves and Hooghly estuary, northeast coast of India. Environ Geochem Health 34:27–42
Basu AK, Ghosh BB, Pal RN (1970) Comparison of the polluted Hooghly estuary with the unpolluted Matlah estuary, India. J Water Pollut Control Fed 42:1771–1781
Benjamin MM, Honeyman BD (2000) Trace Metals. In: Jacobson MC, Charlson RJ, Rodhe H, Orians GH (eds) Earth system science. Academic Press, New York, pp 377–411
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton Univ. Press, Princeton
Bhattacharya B, Sarkar SK, Maji PK (1994) Bioaccumulation of heavy metals in flora and fauna of Hooghly estuary, India. Toxicol Environ Chem 42:123–130
Bianchi TS (2007) Biogeochemistry of estuaries. Oxford University Press, New York
Bresline VT, Sanudo-Wilhelmy SA (1999) High spatial resolution sampling of metals in the sediment and water column in port Jefferson Harbour, New York. Estuaries 22:669–680
Brown ET, Callonnec LL, German CR (2000) Geochemical cycling of redox-sensitive metals in sediments from Lake Malawi: a diagnostic paleotracer for episodic changes in mixing depth. Geochim Cosmochim Acta 64:3515–3523
Brumsack HJ (2006) The trace metal content of recent organic carbon-rich sediments: implications for Cretaceous black shale formation. Palaeogeogr Palaeoclimatol Palaeoecol 232:344–361
Burdige DJ, Martens CS (1988) Biogeochemical cycling in an organic-rich coastal marine basin: the role of amino acids in sedimentary carbon and nitrogen cycling. Geochim Cosmochim Acta 52:1571–1584
Canuel EA, Martens CS (1993) Seasonal variations in the sources and alteration of organic matter associated with recently-deposited sediments. Org Geochem 20:563–577
Chatterjee M, Silva Filho EV, Sarkar SK, Sella SM, Bhattacharya A, Satpathy KK (2007) Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environ Int 33:346–356
Chuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21:1335–1346
de Andrade Passos E, Alves JC, dos Santos IS, JdoPH Alves, Garcia CAB, Costa ACS (2010) Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem J 96:50–57
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
Emerson S, Hedges J (2003) Sediment diagenesis and benthic flux. Treatise Geochem 6:293–319
Froelich PN, Klinkhammer ML, Bender ML, Luedtke NA, Health GR, Cullen D, Dauphin P, Hammond D, Hartman B (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090
Ghatak DB, Konar SK (1994) Field survey on the status of pollution by various industrial effluents on Hooghly Estuary ecosystem at Haldia, West Bengal. Environ Ecol 12:128–132
Gopalakrishnan V, Ray P, Ghosh BB (1973) Present status of pollution of the Hooghly estuary with special reference to the adverse effects observed on the fishery resources, India. In: Proceedings of symposium on environmental pollution, Nagpur, pp 1–8, 17–19 Jan
Gupta SK, Chen KY (1975) Partitioning of trace metals in selective chemical fractions of near shore sediments. Environ Lett 10:129–158
Hatje V, Payne TE, Hill DM, McOrist G, Birch GF, Szymczak R (2003) Kinetics of trace element uptake and release by particles in estuarine waters: effects of pH, salinity, and particle loading. Environ Int 29:619–629
Holmer M (1999) The effect of oxygen depletion on anaerobic organic matter degradation in marine sediments Estuarine. Coast Shelf Sci 48:383–390
Idriss AA, Ahmed AK (2013) Heavy metals nickel and chromium in sediments in the Juru River, Penang, Malaysia. J Environ Prot 4:1245–1250
IMD (2013) Indian Metrological Department. District wise rainfall data for last five years, South 24 Parganas. http://www.imd.gov.in/section/hydro/distrainfall/webrain/wb/south24parganas.txt. Accessed 13 Mar 2014
Jonathan MP, Ram Mohan V (2003) Heavy metals in sediments of the inner shelf off the Gulf of Mannar, Southeast coast of India. Mar Pollut Bull 46:263–268
Kersten M, Forstner U (1986) Chemical fractionation of heavy metals in anoxic estuarine and coastal sediments. Water Sci Technol 18:121–130
Krantzberg G (1985) The influence of bioturbation on physical, chemical and biological parameters in aquatic environments: a review. Environ Pollut Ser A 39:99–122
Kumar B, Shah R, Mukherjee D (2011) Geochemical distribution of heavy metals in sediments from sewage fed fish ponds from Kolkata Wetlands, India. Chem Speciat Bioavailab 23:24–32
Lehmann MF, Bernasconi SM, Barbieri A, McKenzie JA (2002) Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochim Cosmochim Acta 66:3573–3584
Lesven L, Gao Y, Billon G, Leermakers M, Ouddane B, Fischer JC, Baeyens W (2008) Early diagenetic processes aspects controlling the mobility of dissolved trace metals in three riverine sediment columns. Sci Total Environ 407:447–459
Lion LW, Altmann RS, Leckle JO (1982) Trace-metal adsorption characteristics of estuarine particulate matter: evaluation of contributions of iron/manganese oxide and organic surface coatings. Environ Sci Technol 16:660–666
Loring DH (1991) Normalization of heavy-metal data from estuarine and coastal sediments. ICES J Mar Sci J Conseil 48:101–115
Maiti P, Banerjee S (2012) Fate of metals in Fish under variable sewage input in fish pond. Int J Sci Res Publ 2:6
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300:229–243
Ray R, Rixen T, Baum A, Malik A, Gleixner G, Jana TK (2015) Distribution, sources and biogeochemistry of organic matter in a mangrove dominated estuarine system, Indian Sundarbans. during the pre-monsoon. Estuar Coast Shelf Sci 167:404–413
Rice KC, Conko KM, Hornberger GM (2002) Anthropogenic sources of arsenic and copper to sediments in a Suburban Lake, Northern Virginia. Environ Sci Technol 36:4962–4967
Robert S, Blanc G, Schafer J, Lavaux G, Abril G (2004) Metal mobilization in the Gironde Estuary (France): the role of the soft mud layer in the maximum turbidity zone. Mar Chem 87:1–13
Rojas N, Silva N (2005) Early diagenesis and vertical distribution of organic carbon and total nitrogen in recent sediments from southern Chilean fjords. Investig Mar 33:183–194
Sadhuram Y, Sarma VV, Ramana Murthy TV, Rao BP (2005) Seasonal variability of physico chemical characteristics of the Haldia channel of Hooghly estuary, India. J Earth Syst Sci 114:37–49
Saha M, Sarkar SK, Bhattacharya B (2006) Interspecific variation in heavy metal body concentrations in biota of Sundarban mangrove wetland, northeast India. Environ Int 32:203–207
Samanta S (2017) Isotope and trace element geochemistry of the Ganga (Hooghly) River estuary: Sources, cycling and fluxes. Ph.D. dissertation, Indian Institute of Science Education and Research Kolkata
Samanta S, Dalai TK (2016) Sources and cycling of metals in the Ganga (Hooghly) River estuary, India: Role of sediment re-suspension and solute-particle interactions. Goldschmidt conference abstract. 2716
Samanta S, Dalai TK, Pattanaik JK, Rai SK, Mazumdar A (2015) Dissolved inorganic carbon, DIC and its δ13C in the Ganga (Hooghly) River estuary, India: evidence of DIC generation via organic carbon degradation and carbonate dissolution. Geochim Cosmochim Acta 165:226–248
Santschi PH, Lenhart JJ, Honeyman BD (1997) Heterogeneous processes affecting trace contaminant distribution in estuaries: the role of natural organic matter. Mar Chem 58:99–125
Sarkar SK, Franciscovic-Bilinski S, Bhattacharya A, Saha M, Bilinski H (2004) Levels of elements in the surficial estuarine sediments of the Hugli river, northeast India and their environmental implications. Environ Int 30:1089–1098
Sharma VK, Rhudy KB, Koenig R, Baggett AT, Hollyfield S, Vazquez FG (1999) Metals in sediments of Texas estuaries, USA. J Environ Sci Health 34:2061–2073
Stephen-Pichaimani V, Jonathan MP, Srinivasalu S, Rajeshwara-Rao N, Mohan SP (2008) Enrichment of trace metals in surface sediments from the northern part of Point Calimere, SE coast of India. Environ Geol 55:1811–1819
Stumm W, Morgan JJ (1981) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York
Subramanian S, Eswaramoorthi S, Periakali P, Saravana Kumar K, Lakshmi Narasimhan C (2001) Major and trace elements (Fe, Mn, Al, Cu, and Hg) in Pichavaram Mangrove sediments, Tamil Nadu, east coast of India. J Appl Geochem 3:6–12
Tessier A, Fortin D, Belzile N, DeVitre RR, Leppard GG (1996) Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: narrowing the gap between field and laboratory measurements. Geochim Cosmochim Acta 60:387–404
Tromp TK, Van Cappellen P, Key RM (1995) A global model for early digenesis of organic carbon and organic phosphorus in marine sediment. Geochim Cosmochim Acta 59:1259–1284
Tume P, Bech J, Longan L, Tume L, Reverter F, Sepulveda B (2006) Trace elements in natural surface soils in Sant Climent, Catalonia, Spain. Ecol Eng 27:145–152
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Wakeham S (2002) Diagenesis of organic matter at the water-sediment interface. In: Gianguzza A, Pelizzetti E, Sammartano S (eds) Chemistry of marine water and sediments. Springer, Berlin Heidelberg, pp 147–164
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
Widdows J, Blauw A, Heip CHR, Herman PMJ, Lucas CH, Middelburg JJ, Schmidt S, Brinsley MD, Twisk F, Verbeek H (2004) Role of physical and biological processes in sediment dynamics of a tidal flat in Westerschelde Estuary, SW Netherlands. Mar Ecol Prog Ser 274:41–56
Windom HL, Smith RG Jr, Rawlinson C (1989) Particulate trace metal composition and flux across the south eastern US continental shelf. Mar Chem 27:283–297
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China—weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54:1051–1070
Zwolsman JJG, Berger GW, van Eck GTM (1993) Sediment accumulation rates, historical input, postdepositional mobility and retention of major elements and trace metals in salt marsh sediments of the Scheldt estuary, SW Netherlands. Mar Chem 44:73–94
Acknowledgements
Tarun K. Dalai acknowledges the financial support from the Ministry of Earth Sciences, Govt. of India through the GEOTRACES program. Saumik Samanta acknowledges a research fellowship from IISER Kolkata. Santosh Ch. Das is acknowledged for his help in the ICP-MS Laboratory. We are thankful to Indrajit Batabyal of Geosolutions Proservices Pvt. Ltd. for his help with the sediment coring. The comments of anonymous reviewers helped improve an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samanta, S., Amrutha, K., Dalai, T.K. et al. Heavy metals in the Ganga (Hooghly) River estuary sediment column: evaluation of association, geochemical cycling and anthropogenic enrichment. Environ Earth Sci 76, 140 (2017). https://doi.org/10.1007/s12665-017-6451-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6451-x