Abstract
This study aims at investigating heavy metal mobility and bioavailability in sediments from the Mexicana and Jaralito streams, Northern Mexico. A chemical partition analysis (sequential extraction) was performed to determine geochemical phases in which metals are found. Geoaccumulation index (Igeo) and enrichment factor values were obtained from analytical results and geochemical baseline data. Sediments showed high concentrations (mg/kg) of Cd (below detection limit, BDL-3.50), Cr (3–41), Cu (238–1090), Fe (41267–61033), Mn (678–1143), Ni (18–35), Pb (51–124), and Zn (116–356). Metal concentrations in geochemical phases exhibited the following order: residual > interchangeable > Fe/Mn oxide > carbonate >organic matter/sulfide. Both streams presented high degree of enrichment for Cu, Fe, Mn, Ni, Pb, and Zn, indicating anthropic origin of these metals. Metal mobilities in Jaralito and the Mexicana were Fe > Cu > Mn > Pb > Zn > Ni > Cr and Fe > Cu > Mn > Zn > Ni > Pb > Cr > Cd, respectively. Jaralito and the Mexicana sediments exhibit a mostly gravel-sandy texture with higher metal contents than in fine fractions. Sediment Geoaccumulation index values suggest that Jaralito features moderate to strong contamination by Ni, Pb, and Cu, whereas the Mexicana features strong contamination by Cd, Cu, Pb, and moderate contamination by Ni, Pb, and Zn. The quality criteria comparisons (LEL and SEL) indicate these areas are contaminated by metals and represent a substantial environmental risk because of high metal mobility and availability. Future studies on water chemistry and biota are needed to fully assess pollution impact in the Jaralito and Mexicana streams. The probability of adverse biological effects from high metal levels in those streams confirms the urgency of implementing effective environmental management practices.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aquatic sediments contaminated with heavy metals have attracted considerable public attention in recent years. Heavy metals are introduced into an aquatic ecosystem through natural or anthropogenic sources (Idriss and Ahmad 2013). Some heavy metals are considered pollutants because of their high toxicity, persistence, and to the fact that they are likely to accumulate in aquatic organisms (Ghrefat et al. 2011). Sediments are capable of accumulating heavy metals that enter the aquatic systems. Changes in the physicochemical conditions of the sediments can mobilize and release the metals into the stream water. The study of sediments in an aquatic ecosystem allows the complete evaluation of a polluted site because it is the endpoint of most of the deposited contaminants from the water column by precipitation (Montalvo et al. 2014). Mining activities are considered to be one of the main anthropogenic sources of heavy metals in aquatic environments. The extraction and processing of minerals, as well as the mining waste with remaining metal contents, are regarded as major sources of pollution with respect to aquatic ecosystems. Most of the contaminants generated by mining activity, such as metals, are harmful to flora and fauna (both terrestrial and aquatic). Leachates and mining waste in particular cause deterioration of ecosystems (Smouni et al. 2010).
In Mexico, the mining industry is one of the economic activities with greatest tradition. The mining is mostly metalliferous and primarily dedicated to the production of Cu, Zn, Ag, and Pb. The Cananea Mining District is located in the state of Sonora, Mexico, and is considered one of the largest deposits of copper ore in the world. The mine waste with the associated district covers approximately 1000 ha and has been generated over the course of many decades of copper ore exploitation. Mining wastes have severely affected water quality in the San Pedro, Sonora, and Bacanuchi rivers due to the introduction of ferrous-cupric acid wastes with high levels of toxic metals and acidity (Gómez-Álvarez et al. 2007). The streams Mexicana and Jaralito are located in the area of Cananea, Sonora, and they are affected by the mining activities. The Mexicana stream empties into one of the tailings dams, and the Jaralito stream constitutes one of the many tributaries flowing into the Bacanuchi river. Previous studies (Gómez-Álvarez et al. 2009) reported that there are metal pollution problems in the Bacanuchi river, which is a tributary of the Sonora river and is considered a source of water for different populations located along the river channel, including the city of Hermosillo, the capital of the state of Sonora. Currently, several studies have attempted to assess the quality of water and sediments affected by the mining activity in the region of Cananea, Sonora. However, these have been mainly focused on evaluating the content of total metals in the sediment, which can provide misleading information with regard to measuring pollution levels.
In the sediments, metals can be found in different chemical forms and can exhibit diverse physical and chemical behaviors with respect to their mobility, bioavailability, and toxicity. Therefore, it is important to quantify the different chemical species of a metal bound to the sediment. This in order to obtain information regarding the possible transport, deposition, and release into the environment processes the metal may undergo downstream (Xiangdong et al. 2000). Sediments concentrate metals from aquatic systems, and they represent an appropriate and strategic medium for monitoring contamination. Studies of major and trace elements in the sediments and assessments of sediment quality are consequently critical steps in evaluating environmental pollution (Giuliani et al. 2011). Determining the total content of metals in the sediment may be useful for the characterization of contamination. However, identifying the chemical speciation of the metals by use of selective extraction agents provides further information about the behavior of metals in the sediment and helps to assess the environmental impact of contaminated sediment (Ogunfowokan et al. 2013). The sequential extraction technique has been used to study the different chemical forms and the possible associations between metals and components of the sediment. This method is based on a process known as fractionation, in which a sequential series of extractants with increasing extraction power are used to selectively dissolve different forms in the solid phase or mineralogical fractions (Hongrui et al. 2011).
The existing sequential extraction techniques include the methods created by Tessier et al. (1979) and the Community Bureau of Reference (BCR). The differences between these methods are minimal, with variations mainly in the chemical extractants and the operation conditions (Hongrui et al. 2011). The sequential extraction method provides more thorough information on the distribution of inorganic constituents (metals) among different forms of sediment and allows the prediction of their behavior under certain changing physicochemical conditions (Xiangdong et al. 2001). Geochemical parameters, such as the enrichment factor and the geochemical index, have been successfully used to estimate the anthropogenic impact on sediment (Cevik et al. 2009; Mayuri and Nema 2012). The main objectives of this paper are (i) to study metal distribution in different geochemical fractions of surface sediments from streams connected to mining areas, (ii) to estimate the degree of contamination on the basis of geoaccumulation and pollution indexes, (iii) to evaluate and quantify the metals that may be available to biota and humans (bioavailability and potential mobility), and (iv) to contribute to the study of aquatic ecosystems contaminated by mining activities in arid and semiarid regions in Mexico.
2 Materials and Methods
2.1 Study Area
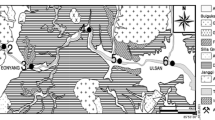
The Cananea Mining District is located in the state of Sonora, Mexico. It is considered one of the largest copper ore deposits in the world and has produced more than 1.4 × 109-kg Cu and smaller quantities of Au, Ag, Pb, and Zn through several years of mining operations. The main deposits contain pyrite, chalcopyrite, and bornite, as well as smaller quantities of galena. The area of the current mine covers approximately 12 to 16 km2. The resulting mine waste covers approximately 1000 ha and has been generated through the exploitation of copper ore in the Cananea Mining District. The municipality of Cananea is located in the north of the state of Sonora, Mexico, at an elevation of 1654 m above sea level (Fig. 1) in a mountainous area. The largest mountains are El Manzanal, Los Ajos, La Elenita, and La Mariquita. The Jaralito and Mexicana streams originate near the town of Cananea, Sonora, at an elevation of 1600 m above sea level. The Mexican sampling site is located approximately 6 km from the center of mine operations, while for the Jaralito sampling site that distance is 9 km.
The Mexicana flows east, and the Jaralito first flows east then south. The Mexicana stream belongs to the Sonora river-Arizpe basin. It is considered an intermittent stream of water, and its length is approximately 5.6 km. It receives input from other intermittent currents, such as “La Bombita,” and flows into the tailings management facility (INEGI 2010). The Jaralito stream is situated in Bacanuchi river basin, which has a catchment area of 1431 km2, and is the first tributary of the Sonora river. The Jaralito receives various streams and empties into “La Bellota” river, “La Bellota river is one of the many streams that drain the western flank of the “Sierra de Manzanal” mountain range that are tributaries of the Bacanuchi river (INEGI 1985).
The climate in the region is semi-arid, and vegetation is sparse. The annual maximum, minimum, and mean temperatures in the area are 25, 10, and 18 °C, respectively. Previous studies documented that there are pollution problems associated with metals in the Bacanuchi river, a tributary of the Sonora river, which is one of the most important sources of water to many small towns and to the city of Hermosillo, the largest city in the state of Sonora (Gómez-Álvarez et al. 2009).
2.2 Sample Collection and Analysis
Sampling was conducted in June 2012. In total, 18 samples were collected by use of a polyethylene corer. Each of the sediment samples was divided into three sub-samples, which were taken from the top 10 cm of sediment from the Mexicana and Jaralito streams. Hydrogen potential (pH) and electric conductivity (EC) were measured using a portable pHmeter and conductometer (Thermo Scientific Orion 3-star benchtop pH meter) according to the methodology recommended by Mudroch and Azcue (1995). Sediment samples were transported on ice to the laboratory. Sediment samples were dried at room temperature for 48 h and ground to a less than 100 mesh size using a porcelain mortar. Textural studies were conducted using the methodology recommended by Folk (1980). The sediment samples were separated into fractions with respect to particle size: (1) >2 mm, (2) 2–0.063 mm, (3) 0.063–0.004 mm, (4) <0.004 mm. The four fractions represent gravel, sand, silt, and clay. The sulfate content was determined through precipitation using BaCl2 (AOAC International 1999; Method 980.02). Sequential extraction was used to study the chemical partitioning of the sediment. The procedure involved preparing a composite sample per zone by taking 25 % by weight of each of the stations for each of the zones separately. This was performed to obtain a representative sample for each zone and to carry out the chemical partition study.
2.3 Chemical Speciation
One of the most used sequential extraction methods for assessing metal partitioning in sediments was developed by Tessier et al. (1979) (Ramos-Gómez et al. 2012; Liu et al. 2014; Mortatti et al. 2015). Such a method was used in this study to achieve the metal partition into the following fractions: (1) fraction I (interchangeable): sediment was extracted at a pH of 7.0 using 1 M MgCl2 for 1 h at room temperature; (2) fraction II (bound to carbonates): residue from step (1) was extracted using 1 M CH3COONa at a pH of 5 for 6 h at room temperature; (3) fraction III (Fe/Mn oxides): residue from step (2) was extracted with 0.04 M NH2OH.HCl in 25 % (v/v) acetic acid at 96 ± 3 °C for 6 h; (4) fraction IV (organic matter and sulfides): residue from step (3) was extracted with 30 % H2O2 and 0.02 M HNO3 at 85 ± 2 °C for 2 h with occasional agitation. Then, 30 % H2O2 and 0.02 M HNO3 were added and heated again to 85 ± 2 °C for 3 h. Later, it was cooled down to room temperature, 3.2 M ammonium acetate in 20 % (v/v) HNO3 was added and it was continuously agitated for 30 min; (5) fraction V (residual phase): A total digestion was carried out using a mixture of HF-HClO4 (5:1). After each extraction, the sample was centrifuged to 10,000 rpm for 30 min at room temperature. Supernatant was separated using a pipette, transferred to a volumetric flask and then the volume was made up to 50 mL with deionized (DI) water. The sediment was washed with DI water and centrifuged once more; the washing water was thrown away. The determination of the metal concentrations for the five fractions was carried out using flame atomic absorption spectroscopy (Perkin Elmer Model AAnalyst 400). The total concentration of each metal was calculated by adding the concentration obtained in each of the fractions: exchangeable, carbonates, Fe and Mn oxides, organic matter/sulfides, and residual.
2.4 Quality Control
Sediment samples were analyzed in duplicate. The agreement of the results is within ±5 %. In every case, blanks reactive were run in triplicate. Results obtained were below the detection limit for all metals. The certified reference material NIST 2702 (Inorganic in Marine Sediment) was digested and analyzed along with the sediment samples to validate the accuracy of sample preparation and analysis, and to verify the sequential extraction method. Recovery observed for metal concentrations in reference material fell in the range of 91–101 %, which can be considered acceptable (Vasyukova et al. 2010). For analysis by the atomic absorption technique, five standards were employed to obtain a calibration curve for each one of the metals, and a correlation coefficient (r > 0.9990) was obtained. Standards and blanks were treated similarly to the sediment samples to reduce matrix interferences and contamination during analysis. The detection limits (DL) (mg/kg) for each metal fraction are shown in Table 1. All reagents were of analytical grade or Merck quality. Deionized water (DI) was used in all the experiments. The equipment used to collect the sediment samples and the glassware were cleaned by soaking in a 20 % (v/v) HNO3 solution for 3 days and then rinsed with DI water to minimize potential contamination.
2.5 Normalized Enrichment Factor and Geo-accumulation Index
The normalized enrichment factor (EF) to establish metal concentration above uncontaminated background levels is used to evaluate the anthropogenic impact on a sediment (Dickinson et al. 1996). Elements that are most often used as reference are Al and Fe (Chabukdhara and Nema 2012). In this study, the metal concentrations were normalized to the textural characteristic of sediments with respect to Al. The advantage of using Al instead of Fe is that the former is mostly associated with the aluminosilicate fraction, which is the carrier phase of the dominant metals in the sediment. Al is highly refractory and its concentration is not usually influenced by anthropogenic forces (Schropp and Windom 1988). The EF is calculated using the following formula (Vuković et al. 2011; Asma et al. 2013):
where (Me/Al)sample is the metal to Al ratio in the samples of interest and (Me/Al)Background is the geochemical background value of the metal to Al ratio. The regional geochemical background values for the metals are as follows (S.G.M. 2013): Al (6.17 %), Fe (3.98 %), Cu (78.6 mg/kg), Cd (0.77 mg/kg), Cr (74.17 mg/kg), Mn (896.6 mg/kg), Ni (18.9 mg/kg), Pb (65.28 mg/kg), and Zn (153.7 mg/kg). An EF value close to 1 indicates that a given metal may be entirely from background materials or natural weathering processes, whereas an EF values greater than 1 denotes that a significant portion of trace metal is delivered from non-crustal materials and, consequently, anthropogenic sources are likely an important contributor (Szefer et al. 1996).
The geoaccumulation index (Igeo), a widely used empirical relationship for evaluating the degree of metal contamination in sediment in aquatic environments (Ogunfowokan et al. 2013), was applied to evaluate the contamination status of the studied area. The Igeo, originally defined by Müller (1969), is a quantitative measure of the metal pollution in aquatic sediments. The method assesses the degree of metal pollution in terms of seven enrichment classes based on increasing numerical values of the index. This index is calculated as follows:
where Cn is the measured concentration in mg kg−1 of the element in the sample, and Bn is the geochemical background value of the element in mg kg−1. The factor 1.5 is the factor used for lithological variations of metals in the sediments (Chabukdhara and Nema 2012). Müller (1969) evaluated the level of metal contamination on the basis of Igeo numerical value by using seven different grades: if Igeo ≤ 0 not contaminated; 0 < Igeo <1, unpolluted to moderately polluted; 1 < Igeo <2, moderately polluted; 2 < Igeo <3, moderate to heavily polluted; 3 < Igeo <4, heavily polluted; 4 < Igeo <5, largely very heavily polluted; and Igeo ≥ 5, very heavily polluted, respectively.
3 Results and Discussion
3.1 Sediment Characteristics
Sediments collected in Jaralito and Mexicana streams have a mostly gravel-sandy texture, and, to a lesser extent, a fine texture (silt and clay). The latter fraction (<0.002 mm) represents less than 4 % of the total. Gravel and sand fractions predominate in most sampling stations, comprising between 36.60 to 80 % and 19.79 to 62.85 wt%, respectively. It has been reported that variations in particle size of different types of sediment are directly related to water motion patterns (Baptista Neto et al. 2006). The observed difference in textural parameters of the Jaralito and the Mexican sediments shows that such parameters are mainly dependent on the dynamic processes affecting the region under study.
The distribution of metals among the different particle size fractions indicates that gravel fraction plays a very important role as a store of metals. In this fraction, metal content fluctuated according to the following percentages: Cu (40–78 %), Ni (48–84 %), Cr (36–78 %), Cd (38–66 %), Mn (33–83 %), Pb (27–82 %), Zn (40–81 %), and Fe (36–77 %). Significant amounts of metals were also observed in the sand fraction: Cu (22–53 %), Ni (15–51 %), Cr (22–58 %), Cd (32–57 %), Mn (17–62 %), Pb (17–64 %), Zn (19–54 %), and Fe (22–50 %). It can be seen that the content of metals in the gravel and sand fractions are greater than the fine fractions for all samples of sediment from the Jaralito and Mexicana streams. Other studies have reported similar behaviors (Combest 1991; Meza-Montenegro et al. 2012.). These exceptions show that the metal concentrations were not controlled solely by particle size, but other parameters such as chemical and mineralogical composition of the sediment (De Gregori et al. 1996). This behavior is contrary to that reported in other studies, where the contents of metals in soils and sediments tend to be higher with decreasing particle size (Wei and Yang 2010; Han et al. 2012; Gonzales et al. 2014).
3.2 Physical and Chemical Parameters
Table 2 presents the results of the physical-chemical analysis of the sediment in the Jaralito and Mexicana streams. In regard to the pH, the sediment in the Mexicana stream presented lower values (2.38 to 3.22) in comparison with the Jaralito stream (4.18 to 7.84). The Mexicana stream directly receives acidic infiltration originating from mining. An acidic pH can encourage mobilization of metals due to the high solubility, whereas the slightly alkaline pH values, such as those detected in the Jaralito (J1 and J2), severely limit the mobility of metals due to the low solubility (Pérez-González 2005). The pH of a system is the most influential factor in the mobilization of metals due to hydrolysis reactions of the cations, which increases the solubility in such a way that at a neutral or basic pH, minerals are poorly soluble. Thus, metal mobility is inversely proportional to the pH of the sediment (Ramos-Gómez et al. 2012). Acidic conditions favor the residence of metals in the solution, which amplifies the water pollution, and can cause the dissolution of other minerals that contain metals that are highly toxic to biota.
The highest values of electrical conductivity (E.C.) were detected in the Mexicana stream at the M2 and M3 stations (36120 to 94200 μS/cm) (Table 2). The lowest values were observed in the Jaralito stream (J1, J2), with values of 367 and 494 μS/cm. The high values of E.C. are due to the high activity of the hydrogen ion (low pH value), which is a major component in the acid waste. A high E.C. also favors the complexation of metals because it involves a high salt content, therefore involving anions. The overall concentration of sulfates ranged from 44 to 849 mg/L. The highest value was observed in the Mexicana stream (849 mg/L). The high values of sulfates are due primarily to the mining activity in areas adjacent to the Mexicana stream. The most important deposits located in the region of Cananea, Sonora, consist of copper porphyry and volcanic rocks, which contain abundant sulfurous minerals (Cendejas-Cruz et al. 1998). Pagnanelli et al. (2004) reported that the metallurgical processes for the production of copper (as is the case in the region of Cananea, Sonora) from sulfurous mineral deposits generate large piles of sulfide-rich waste rock. This may be the source of the acid mine drainage (AMD), which is characterized by high concentrations of sulfate and iron, low pH and high concentrations of metals. Therefore, AMD potentially represents a risk to the environment (Lu et al. 2005).
3.3 Total Concentrations of Metals in the Sediment
Sediments are considered a latent deposit of metals and can release metals into the water column when the physical and chemical conditions change. The total concentrations of the eight metals in the sediments are listed in Table 2. The concentration values (mg/kg) in the two streams fluctuated within the following ranges: Cd (<BDL-3.50), Cr (3.1–41), Cu (238–1090), Fe (41267–61033), Mn (678–1143), Ni (18–35), Pb (51–124), and Zn (116–356). The sediments in the Mexicana stream yielded the highest values (mg/kg) of most of the metals (except Cr and Fe): Cd 3.50; Cu 1090; Mn 1143; Ni 35; Pb 124, and Zn 356. The Jaralito stream yielded the highest values for Cr and Fe, with values of 41 and 61033 mg/kg, respectively. The behavior of the total metal concentrations in the Mexicana stream was as follows: Fe > Mn > Cu > Zn > Pb > Ni > Cr > Cd. The Jaralito stream presented the following order: Fe > Mn > Cu > Zn > Pb > Cr > Ni > Cd. These patterns were similar to sediments from other regions, but Cr and Ni were generally lower (Table 3).
The spatial behavior of the concentration levels of metals does not show a definite pattern with the pollution source (the mine). This may be due to the fact that the sampling stations in this study are structured so that they are chemically and physically different but perhaps not spaced far enough to identify different behavior that gradually decreases away from the source of contamination. However, analyzing the data between the two effluents in this study shows that Mexicana, due to the structure of the channels, is most clearly influenced by the mining activities. This is observed in the highly acidic pH condition (2.38 to 3.22), the high concentrations of metals (Cd, Cu, Fe, Mn, Ni, Pb, Zn) and sulfates, and the high electrical conductivity (Table 2). In the Jaralito, it can be observed that the J3 station is significantly influenced by the contaminant source (mining) because it featured a low pH (4.18) and high levels of metals (Cu, Fe, Mn, Pb, Zn), sulfates and electrical conductivity with respect to the J1 and J2 stations. Moreover, the variation in the concentration of total metals can be due to differences in the source, the prevailing physical and chemical conditions, and the chemical reactions (adsorption, precipitation, and redox conditions) that may occur in the sediment (Jain et al. 2008).
3.4 Metals on Geochemical Fractions of Sediments
The individual speciation fractions of Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn expressed as percentages of their total concentrations are shown in Fig. 2. Fraction I. Exchangeable. This fraction represents highly bioavailable metals by virtue that metal adsorption relates to changes in water ionic composition that may affect adsorption-desorption processes and mobility of metals (Fuentes et al. 2008). Therefore, the availability index is high because the metal can be solubilized with only slight changes in the environmental conditions of the sediment, such as a reduction in pH or Eh (Dang et al. 2002). It is therefore of great importance to provide very special attention to the exchangeable fraction because metals in this fraction can be toxic to aquatic organisms (Davutluoglu et al. 2011). In the Jaralito stream, the minimum and maximum concentrations (mg/kg) of the metals were as follows: Cd and Pb (BDL), Cr (0.20–0.30), Cu (BDL-2.0), Fe (6–11), Mn (15–79), Ni (BDL-3.60), and Zn (1.80–25). The highest metal values by sampling station were in the following order: J3 > J2 > J1, and the percentages of Mn, Zn, Cu, Cr, and Ni were 7.0, 17.0, 0.58, 5.48, and 12.31 with respect to the totals (Fig. 2).
The mobility behavior was the following: Mn > Zn > Fe > Ni > Cu. In the Mexicana stream, the minimum and maximum concentrations (mg/kg) were Pb (BDL), Cd (BDL-4), Cr (0.20–5), Cu (24–879), Fe (8–1200), Mn (78–532), Ni (BDL-19), and Zn (9.25–248). Station M3 featured the highest values of Cd, Cr, Cu, Fe, Mn, Ni, and Zn, representing percentage values of 100, 61.12, 80.68, 2.89, 78.40, 64.13, and 72.57 with respect to the totals. With regard to the non-detectable values of Pb (BDL), it is possible Pb may bound in an insoluble compound, such as sulfate. However, it can be observed that the Mexicana stream exhibited higher values of metals in comparison with the Jaralito stream, and it also featured high mobility, with the following order: Fe > Cu > Mn > Zn > Ni > Cr > Cd. However, it is important to emphasize that both streams contain a major burden of metals in the exchangeable fraction; this behavior has been observed in other studies in aquatic ecosystems contaminated by anthropogenic activity (Jain et al. 2008; Khorasanipour et al. 2011).
Fraction II. Carbonates
It has been reported that the fractions introduced by human activity include the exchangeable fraction and the fraction coupled with carbonates (Singh et al. 2005). Dang et al. (2002) reported that in the fraction of carbonates, the metals can precipitate or co-precipitate and are susceptible to pH changes. Metals bound to carbonates are sensitive to changes in pH, where the lowering of pH is associated with the release of metal cations. The minimum and maximum concentrations (mg/kg) in the Jaralito stream were as follows: Cd and Ni (BDL), Cr (0.10–1.0), Cu (11–19), Fe (11–111), Mn (18–143), Pb (BDL-4.65), and Zn (3–4). The Cr, Cu, Mn, Pb, and Zn values presented the highest percentages: 2.56, 5.89, 13.93, 9.13, and 3.0 with respect to the totals (Fig. 2). In the Mexicana stream, the minimum and maximum concentrations (mg/kg) were as follows: Cd and Ni (BDL), Cr (BDL-0.20), Cu (7.0–32), Fe (20–220), Mn (8–16), Pb (BDL-10), and Zn (2.0–8.0). The M3 and M2 stations had the highest levels of Cu, Mn, Pb, and Zn, featuring percentage values of 1.83, 2.34, 8.06, and 2.11 with respect to the totals. This behavior is similar to those that have been observed in other contaminated aquatic ecosystems (Chandra et al. 2003; Jain et al. 2007).
It is important to highlight that in the Jaralito stream there is a high mobility of potentially harmful metals in the following order: Mn > Fe > Cu > Pb > Zn > Cr. In the Mexicana stream, much higher levels of metals with regard to Jaralito were observed. The mobility of the metals was as follows: Fe > Cu > Mn > Pb > Zn > Cr. It was found that there is a high mobility of all the metals in the carbonate fraction in both streams and that the mobility was much higher in the Mexicana stream. The Cd and Ni featured lower values than the BDL, which means that they present in other geochemical forms (for example, oxides and silicates) and are not available. However, high values of metals were detected in this fraction, and due to its high mobility, this fraction can be considered a significant source of metals because they can return to the surface waters of the studied streams.
Fraction III. Oxides of Iron and Manganese
The metals that are adsorbed or co-precipitated in the oxides of the Fe/Mn fraction are unstable under reducing conditions; thus, they can redissolve into the water column and be highly mobile (Jain et al. 2008). Consequently, this fraction is also considered a potential source of harmful metals in the sediment of the Jaralito and the Mexicana streams. The minimum and maximum concentrations (mg/kg) in the Jaralito stream were as follows: Cd (BDL), Cr (1.0–1.60), Cu (73–160), Fe (3825–6350), Mn (313–429), Ni (1–3), Pb (5–13), and Zn (13–23) (Fig. 2). The percentage values were as follows: Cr (43), Cu (47), Fe (10), Mn (47), Ni (10), Pb (21), and Zn (15) with respect to the totals. In the Mexicana stream, the minimum and maximum concentrations (mg/kg) were as follows: Cd (BDL), Cr (1.5–1.9), Cu (59–69), Fe (6375–10500), Mn (22–775), Ni (0.5–2), Pb (8–8), and Zn (11–21). The M3 and M2 stations had the highest values of Cu, Cr, Fe, Mn, Pb, and Zn, with percentage values of 24, 49, 24, 68, 9, and 10 with respect to the totals. Cd was not present at detectable levels (<BDL) in either stream. A majority of the studied metals were present at higher values in this fraction than in the exchangeable and carbonate fractions. This is because the Mexicana stream features ferrous-cupric acid wastes, in which iron precipitation can occur as hydroxide by increasing the pH (>3), which causes the adsorption and co-precipitation of other metals as well (Hudson-Edwards et al. 1999). Consequently, this process increases the metal levels in the sediment. Metals such as Cu, Pb, Zn, and Mn can form stable complexes with Fe and Mn oxides (Bibi et al. 2007). In both streams, there is a high mobility of metals in this fraction, and their behavior is as follows: Fe > Mn > Cu > Zn > Pb > Ni > Cr. The Fe-Mn oxide fraction has a scavenging effect and may provide a sink for heavy metals. The release of metals from the matrix is most likely to be affected by the pH and redox potential (Jain et al. 2008).
Fraction IV. Organic Matter/Sulfides
The minimum and maximum concentrations (mg/kg) in the Jaralito stream were as follows: Cd and Pb (BDL), Cr (0.6–1.4), Cu (20–24), Fe (16–429), Mn (25–154), Ni (4–5), and Zn (4–6). In the Mexicana stream, the minimum and maximum concentrations (mg/kg) were as follows: Cd and Pb (BDL), Cr (1–2), Cu (15–34), Fe (75–276), Mn (5–116), Ni (3–4), and Zn (3–6) (Fig. 2). The levels of metals in this fraction in both streams were lower than those detected in the Fe/Mn oxide fraction. Similar results have been observed in other studies as well (Chandra et al. 2003; Khorasanipour et al. 2011). With regard to mobility, both streams featured similar behaviors (Fe > Mn > Cu > Zn > Pb > Cr > Ni). The Fe/Mn oxide and organic/sulfide fractions are considered among the most important components in the adsorption of metals in sediments. Some studies have reported high concentrations of metals associated with organic matter (Ramos et al. 1999; Fan et al. 2002), such as Xiangdong et al. (2001), who reported that Cu could easily form complexes with organic matter due to the high stability of Cu-organic compounds.
Fraction V. Residual
The residual fraction contains metals that are inert and contained within the crystalline structure of some primary and secondary minerals (Ramos-Gómez et al. 2012). Metals associated with this fraction are stable and are not soluble under normal environmental conditions. Therefore, they cannot have an impact on the surface water quality, nor are they biologically available (Idriss and Ahmad 2013). Consequently, it is expected that residual metals act as an inert phase. In general, the highest concentration levels of the eight metals in both streams were detected in this fraction (Fig. 2). The Jaralito stream exhibited the following percentages: Cr (13–93), Cu (42–57), Fe (89–92), Mn (37–51), Ni (65–77), Pb (70–90), and Zn (67–79). The Mexicana stream exhibited the following percentages: Cd (100), Cr (2–8), Cu (9–57), Fe (72–87), Mn (14–18), Ni (21–67), Pb (85–93), and Zn (21–82). Similar results have been reported in other studies (Galán et al. 2003; Alvarenga et al. 2008).
3.5 Metals in the Non-residual Fraction (I, II, III, IV)
The non-residual metal fraction was analyzed because this fraction is more bioavailable than the residual one (González et al. 1998; Ryan et al. 2002). In the Jaralito stream, the percentages of the eight metals in the non-residual fraction were as follows: Cr (7–86), Cu (43–58), Fe (8–11), Mn (49–63), Ni (23–34), Pb (9–30), and Zn (20–32). The proportion of metals (except for Cd) that are highly bioavailable is significant. In the Mexicana stream, the percentages were as follows: Cd (BDL-100), Cr (91–98), Cu (42–91), Fe (12–28), Mn (82–85), Ni (32–78), Pb (7–14.5), and Zn (18–78). Cd presented percentage levels of 100 % in fraction I (interchangeable) in the Mexicana stream (stations M1, M2, and M3). The potential mobilities of the metals in the non-residual fraction in the Jaralito stream and in the Mexicana stream were as follows: Fe > Cu > Mn > Pb > Zn > Ni > Cr and Fe > Cu > Mn > Zn > Ni > Pb > Cr > Cd, respectively. In both streams, there is a significant contribution of anthropogenic metals that are highly mobile and thus bioavailable. These metals therefore can have a detrimental effect on the biota.
3.6 Normalized Enrichment Factor and Geo-accumulation Index
The calculation of EF values normalized to the average crustal value of the Earth has been severely criticized because the sediments have been affected by geogenic variations resulting from the different formation processes of the rocks and the sediments, which alter the ratio of the elements in the crust (Reimann and De Caritat 2000; Bourennane et al. 2010). To obtain reliable results that can be interpreted in a more realistic manner in the present study, the composition of the elements present in the crust of the study area was considered (S.G.M. 2013). The results indicate that the Jaralito stream exhibited a high degree of enrichment in Cu (2.78–4.44), Fe (0.99–1.53), Mn (0.77–1.24), Ni (1.0–1.44), Pb (0.74–1.26), and Zn (0.72–1.08) at a majority of the sampling stations (Table 4). Therefore, enrichment is anthropogenic in origin. The low values for Cd and Cr (EF < 1) suggest that they are related to a lithogenic source. In the Mexicana stream, the results indicate high degrees of enrichment in Cd (4.29–5.26), Cu (2.93–16.04), Cr (0.34–0.47), Fe (1.20–1.22), Mn (0.88–1.21), Ni (0.90–1.78), Pb (1.33–1.67), and Zn (1.27–2.51) in a majority of the sampling stations and are also anthropogenic in origin.
The enrichment factor (EF) results obtained in this study were compared to those reported in the sediments of the Wuding River (Longjiang et al. 2011). The Wuding River has been enriched with Cd (2.56–4.64), Cr (1.79–2.53), Ni (1.09–2.68), Zn (1.05–1.64), Pb (0.82–1.05), Cu (0.76–1.05) and Mn (0.74–0.96). The results indicate that the Mexican presented higher EF values for Cd, Cu, Mn, Pb and Zn. However, EF values for Cr and Ni, were higher for the Wuding River. In the case of the Jaralito, EF values for Cu, Mn, and Pb were higher than those reported for the Wuding River. Wang et al. (2008) reported EF for the Pearl River Estuary: Cu (0.75–1.98), Cr (1.21–6.06), Pb (0.93–4.39), Ni (0.88–1.84), and Zn (0.61–1.97). For the Mexican, the values of EF of Cu and Zn were higher than those reported for the Pearl River Estuary. However, EF values for Cr, Pb and Ni were higher in the Pearl River Estuary. In the case of the Jaralito, EF values (except Cu), were higher in the Pearl River Estuary. Ghrefat et al. (2011), reported enrichment factors in sediments of Kafrain Dam: Cd (48.5), Cu (1.65), Cr (2.24), Mn (1.09), Pb (9.25), Ni (1.93), and Zn (1.58). EF values for Cu and Mn in the sediments of the Jaralito and the Mexican are higher than those reported in the sediments of Kafrain Dam.
The Igeo values obtained in the present study are shown in Table 4. Based on the classification system developed by Müller (1969), the sediments of the Jaralito stream do not show contamination by Cd, Cr, Fe, Mn, and Zn. However, there is a moderate to strong contamination by Ni and Pb, and a strong contamination by Cu. The Mexicana stream features significant contamination by Cd, Cu, and Pb, and moderate contamination by Ni, Pb, and Zn. However, the sediments vary between not contaminated and moderately contaminated with respect to Fe. Similar results were reported in the Wuding River for Cd, Cr, and Ni (Longjiang et al. 2011); In the sediments of Kafrain Dam for Pb, Cd, and Zn (Ghrefat et al. 2011); as well as in the sediments of the Hindon River (Chabukdhara and Nema 2012). Jaralito and the Mexicana streams do not show contamination by Cr (values < 0).
3.7 Correlation Analysis
In order to analyze the relationship between particle size and metals, a correlation analysis was performed. Because most parameters are not normally distributed, the Spearman’s correlation coefficient of was used. The results indicate that there is an association of Fe and Mn with gravel fraction (r = 0.37 and 0.71) (Table 5). While for the sand fraction, Cu, Pb, and Zn (r = 0.43 to 0.66), Fe and Mn (r −0.37 and −0.71). Cu, Pb, and Zn had a negative correlation with gravel fraction (−0.43 to −0.66). With the exception of Cr, Fe, and Mn, Cu, Pb, and Zn showed negative correlations with pH (r = −0.77 to −0.94). Cu, Ni, Pb, and Zn showed a positive correlation with each other (r = 0.54 to 0.81). Positive correlations between metals may be an indication of the common sources of these metals. From this, it could be said that the main source can be derived from the mining activity that exists in the region. Fe and Mn showed very poor correlations with respect to sulfates, as well as with Cr, Cu, Ni, Pb, and Zn. Negative correlations between metals indicate that these metals are derived from different sources or dissimilar sedimentological properties. Metals that showed a positive correlation with the E.C. were Cu, Ni, Pb, and Zn (r = 0.49 to 0.89) (except for Cr, Fe and Mn). Cr, Cu, Pb, and Zn showed very marked correlations with sulfates (r = 0.52 to 1.00). Most metals have a strong positive correlation with sulfate, suggesting that the main chemical association is as sulfates. About the Cd was not possible to determine this correlation indicator because most of the data are below the detection limit.
3.8 Sediment Quality Standards
Sediment quality standards are very important for the protection of organisms in aquatic ecosystems and can be used to assess sediment ecosystem health. In the present study, a comparison was conducted with the quality criteria for the metals in the contaminated sediments (low effect level, LEL) and highly contaminated sediments (severe effect level, SEL) that are considered highly toxic to aquatic life, as reported by Long and Morgan (1990) and Persaud et al. (1993). If the LEL is exceeded, the metal can moderately impact the health of biota, and if the SEL is exceeded, the metal can severely impact the health of biota (Table 6). The results of the metal concentrations (except Cd) in the Jaralito stream exceeded the LEL criteria values, meaning that they can moderately impact the health of biota. Furthermore, the concentrations of Cu, Fe, and Mn exceeded the SEL criteria; consequently, they can severely impact the health of biota (Bibi et al. 2007) (Table 6). In the Mexicana stream, the observed metal levels (except Cr) exceeded the values defined in the LEL criteria, therefore moderately impacting the health of biota. In the Mexicana stream, the metals Cu, Fe, and Mn exceeded the SEL criteria, suggesting severe effects on the biota.
4 Conclusions
High concentrations of total metals were present in the following order: (i) the Mexicana stream: Fe > Mn > Cu > Zn > Pb > Ni > Cr > Cd; (ii) the Jaralito stream: Fe > Mn > Cu > Zn > Pb > Cr > Ni > Cd. Jaralito and the Mexicana sediments exhibit a mostly gravel-sandy texture with higher metal contents than in fine fractions for those sampling sites.
The study of chemical partitioning (via sequential extraction) revealed that the order of the geochemical phases were as follows: residual > interchangeable > Fe/Mn oxides > carbonates > organic matter/sulfides. The residual fraction presented the highest values of all the metals in the studied sediment. However, a significant percentage of metals was associated with the non-residual fraction, representing anthropogenic contributions due to mining activity in the region.
The mobility potentials of metals in the non-residual fraction in the Jaralito stream and in the Mexicana stream were as follows: Fe > Cu > Mn > Pb > Zn > Ni > Cr and Fe > Cu > Mn > Zn > Ni > Pb > Cr > Cd, respectively. Therefore, they should be evaluated in terms of the levels of metal contamination in the sediments. The Jaralito exhibited significant enrichments in Cu, Fe, Mn, Ni, Pb, and Zn, and the Mexicana exhibited significant enrichments in Cd, Cu, Fe, Mn, Ni, Pb, and Zn. The results indicate that both streams exceeded the reference values (EF < 1), indicating that these enrichments are of anthropogenic origin.
The values of the geoaccumulation index (Igeo) showed that sediments in the Jaralito stream are not contaminated by Cr, Fe, Mn, and Zn, which come from a lithogenic source. Nonetheless, there is moderate to strong contamination by Ni and Pb, and also a strong contamination by Cu. In the Mexicana, there is strong contamination by Cd, Cu, Pb, and moderate contamination by Ni, Pb, and Zn.
A comparison to sediment quality criteria (LEL and SEL) indicates the sediments from the Jaralito and the Mexicana streams can be regarded as contaminated by potentially harmful metals, which likely has a detrimental effect on the biota.
The information generated in this study represents the beginning of the establishment of reference metal levels for sediment in semi-arid ecosystems in the state of Sonora, Mexico. The obtained information will ideally help the regional and federal authorities cope with the current environmental regulations and may be useful in planning remediation activities, such as proper disposal and management of mining waste, at mine sites that pollute aquatic ecosystems. Similarly, based on this study, it is expected that the local and federal authorities will recognize the importance of introducing reclamation plans and measures into the mine life planning. This, for preventing future environmental and societal risks associated with improper mines and mine facilities closure practices, like the ones actually observed across Mexico.
Future studies on water chemistry, biota, and other factors are needed to fully assess the pollution in the Mexicana and the Jaralito streams. Moreover, the possibility of adverse biological effects from Cd, Cu, Fe, Mn, Ni, Pb, and Zn implied by the observed levels of contamination in parts of the Mexicana and Jaralito streams confirms the urgency for implementing effective environmental management practices and regulations. These studies are of special importance due to the recent acid waste leak that occurred on August 6, 2014, in the Buenavista Copper Mining property located near Cananea, Sonora, Mexico. The effects of this incident have not yet been fully evaluated. This study establishes a precedent in the region and can serve as a starting point to evaluate the actual impact of the acid breach.
References
Alvarenga, P., Palma, P., Goncalves, A. P., Fernandes, R. M., Varennes, A., Vallini, G., Duarte, E., & Cunha-Queda, A. C. (2008). Evaluation of tests to assess the quality of mine-contaminated soils. Environmental Geochemistry and Health, 30, 95–99.
Asma, B. H., Sohail, K., Selim Reza, A. H. M., Mohammad, N. Z., Aminul, A., & Mamunur, R. (2013). Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary–Kumira), Chittagong, Bangladesh. Journal of Geochemical Exploration, 125, 130–137.
Baptista Neto, J. A., Gingele Franz, X., Thomas, L., & Brehme, I. (2006). Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environmental Geology, 49, 1051–1063.
Bibi, M. H., Faruque, A., & Hiroaki, I. (2007). Assessment of metal concentrations in lake sediments of southwest Japan based on sediment quality guidelines. Environmental Geology, 52(4), 1–15.
Bourennane, H., Douay, F., Sterckeman, T., Villanneau, E., Ciesielski, H., King, D., & Baize, D. (2010). Mapping of anthropogenic trace elements inputs in agricultural topsoil from Northern France using enrichment factors. Geoderma, 157, 165–174.
Cendejas-Cruz, R., Vázquez, M., & García-Cortés, J. A. (1998). Geología y yacimientos minerales de la Carta Cananea, Estado de Sonora, Clave H12-5, Escala 1:250,000. Compendios de Geología y Minería. México: Consejo de Recursos Minerales.
Cevik, F., Münir, Z., Osman, B., & Özlem, F. (2009). An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geoaccumulation index and statistical analyses. Environmental Monitoring and Assessment, 152, 309–317.
Chabukdhara, M., & Nema, A. K. (2012). Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere, 87, 945–953.
Chandra, S. K., Chary, N. S., Kamala, C. T., Suman, Raj, D. S., & Sreenivasa, R. A. (2003). Fractionation studies and bioaccumulation of sediment bound heavy metals in Kolleru Lake by edible fish. Environmental International, 29, 1001–1008.
Combest, K.B. (1991). Trace metals in sediments: Spatial trends and sorption processes. Water Resources Bulletin 27: 19-28
Dang, Z., Liu, C., & Haigh, M. (2002). Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environmental Pollution, 118, 419–426.
Davutluoglu, O. I., Seckin, G., Ersu, C. B., Yilmaz, T., & Sari, B. (2011). Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. Journal of Environmental Management, 92, 2250–2259.
De Gregori, I., Pinochet, H., Arancibia, M., & Vidal, A. (1996). Grain size effect on trace metals distribution in sediments from two coastal areas of Chile. Bulletin of Environmental Contamination Toxicology, 57(1), 163–170.
Dickinson, W. W., Dunbar, G. B., & McLeod, H. (1996). Heavy metal history from cores in Wellington Harbour, New Zealand. Environmental Geology, 27, 59–69.
Fan, W., Wang, W., Chen, J., Li, X., & Yen, Y.-F. (2002). Cu, Ni and Pb speciation surface sediments from a contaminated bay of northern China. Baseline/Marine Pollution Bulletin, 44, 816–832.
Folk, R. L. (1980). Petrology of sedimentary rocks. Austin: Hemphill Publishing Company.
Fuentes, A., Lloréns, M., Sáez, J., Aguilar, M. I., Ortuño, J. F., & Meseguer, V. F. (2008). Comparative study of six different sludges by sequential speciation of heavy metals. Bioresource Technology, 99, 517–525.
Galán, E., Gómez-Ariza, J. L., González, I., Fernández-Caliani, J. C., Morales, E., & Giráldez, I. (2003). Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Applied Geochemistry, 18, 409–421.
Ghrefat, H. A., Abu-Rukah, & Rosen, M. A. (2011). Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environmental Monitoring Assessment, 178(1–4), 95–109.
Giuliani, S., Romano, S., Turetta, C., Cu, N. H., Bellucci, L. G., Capodaglio, G., Mugnai, C., Nhon, D. H., & Frignani, M. (2011). Soils and sediments of the Thua Thien-Hue Province (central Vietnam): recognizing trace element sources and the likely influence of natural events. Journal of Environmental Monitoring, 13, 1383–1392.
Gómez-Álvarez, A., Valenzuela-García, J. L., Aguayo-Salinas, S., Meza-Figueroa, D., Ramírez-Hernández, J., & Ochoa-Ortega, G. (2007). Chemical partitioning of sediment contamination by heavy metals in the San Pedro river, Sonora, México. Chemical Speciation and Bioavailability, 9(1), 25–35.
Gómez-Álvarez, A., Meza-Figueroa, D., Villalba-Atondo, A. I., Valenzuela-García, J. L., Ramírez-Hernández, J., & Almendariz-Tapia, F. J. (2009). Estimation of potential pollution from mine tailings in the San Pedro River (1993–2005), México-U.S. Border. Environmental Geology, 57, 1469–1479.
Gómez-Álvarez, A., Valenzuela-García, J. L., Villalba-Atondo, A. I., Meza-Figueroa, D., Almendariz-Tapia, F. J., Whitaker-Bojórquez, T. O., Martínez-Morales, F., Valenzuela-Corral, M., & Ochoa-Valenzuela, L. E. (2011). Distribution of heavy metals and their chemical speciation from the Abelardo L. Rodríguez Dam, Sonora, México. Chemical Speciation and Bioavailability, 23(4), 201–212.
Gonzales, P., Felix, O., Caitlin, A., Lutz, E., Wendell, E., & Sáez, E. (2014). Laboratory dust generation and size-dependent characterization of metal and metalloid-contaminated mine tailings deposits. Journal of Hazardous Materials, 280, 619–626.
González, F., Silva, M., Schalscha, E., & Alay, F. (1998). Cadmium and lead in a trophic marine chain. Bulletin of Environmental Contamination Toxicology, 60, 112–118.
González, A. E., Rodríguez, M. T., Sánchez, J. C. J., Espinosa, A. J. F., & De La Rosa, F. J. B. (2000). Assessment of metals in sediments in a tributary of Guadalquivir river (Spain). Heavy metal partitioning and relation between the water and sediment system. Water Air, and Soil Pollution, 121(1–4), 11–29.
Han, Y. M., Cao, J. J., Wu, F., Zhang, B. C., Zhan, C. L., Wei, C., & Zhao, Z. Z. (2012). Geochemistry and environmental assessment of major and trace elements in the surface sediments of the Wei River, China. Journal of Environmental Monitoring, 14, 2762–2771.
Hongrui, M., Hua, L., & Ji, J. (2011). Speciation and phytoavailability of heavy metals in sediments in Nanjing section of Changjiang River. Environment and Earth Sciences, 64, 185–192.
Hudson-Edwards, K. A., Schell, C., & Macklin, M. G. (1999). Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Applied Geochemistry, 14, 1015–1030.
I.N.E.G.I. (Instituto Nacional de Estadística Geografia e Informática), (2010). Simulador de Corrientes Superficiales de la República Mexicana. http://antares.inegi.org.mx/analisis/red_hidro/SIATL/#.
Idriss, A. A., & Ahmad, A. K. (2013). Heavy metals nickel and chromiumin sediments in the Juru River, Penang, Malaysia. Journal of Environmental Protection, 4, 1245–1250.
INEGI (Instituto Nacional de Estadística Geografia e Informática). (1985). Cartas Hidrológicas de Aguas Superficiales: CANANEA H12-2 y H12-5, Escala 1:250,000. México: Primera Edición.
AOAC International (1999). Official methods of analysis of AOAC International; method 980.02, sulfur in fertilizers. Gravimetric method, chapter 2. 16Th edition, volume 1. USA.
Jain, C. K., Malik, D. S., & Yadav, R. (2007). Metal fractionation study on bed sediments of Lake Naintal, Uttaranchal, India. Environmental Monitoring and Assessment, 130(1–3), 129–139.
Jain, C. K., Gupta, H., & Chakrapani, G. J. (2008). Enrichment and fractionation of heavy metals in bed sediments of River Narmada, India. Environmental Monitoring Assessment, 141, 35–47.
Khorasanipour, M., Tangestani, M. H., Reza, N., & Hajmohammadi, H. (2011). Hydrochemistry, mineralogy and chemical fractionation of mine and processing wastes associated with porphyry copper mines: a case study from the Sarcheshmeh mine, SE Iran. Applied Geochemistry, 26, 714–730.
Liu, Y., Bao, A., & Pan, X. (2014). Depth variability and fractionation of Fe, Mn, Cu and Pb in sediments from Bosten Lake in Xinjiang, Northwest China. Advanced Materials Research, 864–867, 1036–1041.
Long, E. R., & Morgan, L. G. (1990). The potential for biological effects of sediment-sorbed contaminants tested in the National States and Trends Program. U.S.A: National Oceanic Atmospheric Administration (NOAA).
Longjiang, M., Qiang, F., Duowen, M., Ke, H., & Jinghong, Y. (2011). Contamination assessment of heavy metal in surface sediments of the Wuding River, northern China. Journal of Radioanalytical and Nuclear Chemistry, 290, 409–414.
López, D. L., Gierlowski-Kordesch, E., & Hollenkamp, C. (2010). Geochemical mobility and bioavailability of heavy metals in a lake affected by acid mine drainage: Lake Hope, Vinton County, Ohio. Water Air, and Soil Pollution. doi:10.1007/s11270-010-0364-6.
Lu, L., Wang, R., Chen, F., Xue, J., Zhang, P., & Lu, J. (2005). Element mobility during pyrite weathering: implications for acid and heavy metal pollution at mining-impacted sites. Environmental Geology, 49, 82–89.
Mayuri, C. H., & Nema, A. K. (2012). Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere, 87, 945–953.
Meza-Montenegro, M. M., Gandolfi, J., Santana-Alcántar, M. A., Klimecki, W. T., Aguilar-Apodaca, M. G., Del Río-Salas, R., De la O-Villanueva, M., Gómez-Alvarez, A., Mendivil-Quijada, H., Valencia, M., & Meza-Figueroa, D. (2012). Metals in residential soils and cumulative risk assessment in Yaqui and Mayo agricultural valleys, northern Mexico. Science of the Total Environment, 433, 472–481.
Montalvo, C., Aguilar, C. A., Amador, L. E., Cerón, J. G., Ceron, R. M., Anguebes, F., & Cordova, A. V. (2014). Metal contents in sediments (Cd, Cu, Mg, Fe, Mn) as indicators of pollution of Palizada River, Mexico. Environmental and Pollution, 3(4), 89–98.
Mortatti, J., de Oliveira, H., Meneghel de Moraesa, G., Vendraminia, G., & Martins, F. A. (2015). Distribution of heavy metals in the geochemical phases of sediments from the Tietê River, Brazil. Chemical Speciation and Bioavailability, 25(3), 194–200.
Mudroch, A., & Azcue, J. M. (1995). Manual of aquatic sediment sampling. USA: Lewis Publishers.
Müller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Ogunfowokan, A. O., Oyekunle, J. A. O., Olutona, G. O., Atoyebi, A. O., & Lawal, A. (2013). Speciation study of heavy metals in water and sediments from Asunle River of the Obafemi Awolowo University, Ile-Ife, Nigeria. International Journal of Environmental Protection, 3(3), 6–16.
Pagnanelli, F., Moscardini, E., Guiliano, V., & Toro, L. (2004). Sequential extraction of heavy metals in river sediments of an abandoned pyrite mining area: pollution detection and affinity series. Environmental Pollution, 132, 189–201.
Pérez-González, G. (2005). Disponibilidad de metales tóxicos en sitios contaminados. Aplicaciones y limitaciones de la fraccionacion en la determinación de gradientes de polución. Tesis de Doctorado. Universitad Autónoma de Barcelona, España.
Persaud, D., Jaagumagi, R., & A. Hayton (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario. Toronto, Canadá.
Ramos, L., González, M. J., & Hernández, L. M. (1999). Sequential extraction of copper, lead, cadmium, and zinc in sediments from Ebro Riber (Spain): relationship with levels detected in earthworms. Bulletin of Environmental Contamination Toxicology, 62, 301–308.
Ramos-Gómez, M., Avelar, J., Medel-Reyes, A., Yamamoto, L., Godinez, L., Ramírez, M., Guerra, R., & Rodríguez, F. (2012). Movilidad de metales en jales procedentes del Distrito Minero de Guanajuato, México. Revista Internacional de Contaminación Ambiental, 28(1), 49–52.
Reimann, C., & De Caritat, P. (2000). Intrinsic flaws of element enrichment (EFs) in environmental geochemistry. Environmental Science and Technology, 34, 5084–5091.
Ryan, P. C., Wall, A. J., Hillier, S., & Clark, L. (2002). Insights into sequential chemical extraction procedures from quantitative XRD: a study of trace metal partitioning in sediments related to frog malformities. Environmental Geology, 184, 337–357.
S.G.M. (Servicio Geológico Mexicano) (2013). Resultados de Análisis Geoquímicos de la carta Cananea H12-B52. México.
Schropp, S.J. & Windom, H.L. (1988). A guide to the interpretation of metal concentrations in estuarine sediments. Florida Department of Environmental Regulation.
Singh, K. P., Mohan, D., Singh, V. K., & Malik, A. (2005). Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges. Journal of Hidrology, 312, 14–27.
Smouni, A., Ater, M., Laplaze, L., El Mzibri, M., Berhada, F., Filali-Maltouf, A., & Doumas, P. (2010). Évaluation de la contamination par les éléments-traces métalliques dans une zone minière du Maroc oriental. Cahiers Agricultures, 19, 1–7.
Szefer, P., Glasby, G. P., Szefer, K., Pempkowiak, J., & Kaliszan, R. (1996). Heavy metal pollution in superficial sediments from the southern Baltic Sea off Poland. Journal of Environmental Science and Health, 31A, 2723–2754.
Tejeda, S., Zarazúa-Ortega, G., Ávila-Pérez, P., García-Mejía, A., Carapia-Morales, L., & Díaz-Delgado, C. (2006). Major and trace elements in sediments of the upper course of Lerma River. Journal of Radioanalytical and Nuclear Chemistry, 270(1), 9–14.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Vasyukova, E. V., Pokrovsky, O. S., Viers, J., Oliva, P., Dupré, B., Martin, F., & Candaudap, F. (2010). Trace elements in organic- and iron-rich surficial fluids of the boreal zone: assessing colloidal forms via dialysis and ultrafiltration. Geochimica et Cosmochimica Acta, 74(2), 449–468.
Vuković, Z., Radenković, M., Stanković, S. J., & Vuković, D. (2011). Distribution and accumulation of heavy metals in the water and sediments of the River Sava. Journal of the Serbian Chemical Society, 76(5), 795–803.
Wang, S., Cao, Z., Lan, D., Zheng, Z., & Li, G. (2008). Concentration distribution and assessment of several heavy metals in sediments of west-four Pearl River Estuary. Environmental Geology, 55, 963–975.
Wei, B., & Yang, L. (2010). A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Review article. Microchemical Journal, 94, 99–107.
Xiangdong, I., Zhenguo, S., Onyx, W. H. W., & Yok-Sheung, L. (2000). Chemical partitioning of heavy metal contaminants in sediments of the Pearl River Estuary. Chemical Speciation Bioavailability, 12(1), 17–25.
Xiangdong, I., Zhenguo, S., Onyx, W. H. W., & Yok-Sheung, L. (2001). Chemical forms of Pb, Zn and Cu in the sediments profiles of the Pearl River Estuary. Marine Pollution Bulletin, 42(3), 215–223.
Acknowledgments
The authors would like to acknowledge the Departments of Chemical Engineering and Metallurgy and the Direction of Research and Graduate Studies of the University of Sonora for their support in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• High levels of bioavailable metals in sediments near acid spill affected area are reported.
• A significant environmental risk near major water supply in northern Mexico is identified.
Rights and permissions
About this article
Cite this article
Aguilar-Hinojosa, Y., Meza-Figueroa, D., Villalba-Atondo, A.I. et al. Mobility and Bioavailability of Metals in Stream Sediments Impacted by Mining Activities: the Jaralito and the Mexicana in Sonora, Mexico. Water Air Soil Pollut 227, 345 (2016). https://doi.org/10.1007/s11270-016-3046-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3046-1