Abstract
Heavy metal contamination has become a world concern with the rapid industrialization and urbanization process. In this study, a mesophile consortium including Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferriphilum was applied in metals’ bioleaching with the assistance of isolated acid-tolerant microorganisms Rhodotorula and Aspergillus niger. The results showed that the bioleaching was totally inhibited in non-batch method system for the alkalinity and buffering capacity of the sediment. The inhibition on bioleaching can be effectively relieved with the batch method adopted. Dissolved organic matter hampered the substrate utilization and prolonged the bioleaching process. The toxic effect of dissolved organic matter to acidophile can be reduced by the isolated heterotrophic microorganism. A. thiooxidans was the dominant species in the early bioleaching stage, while the ratio of ferrous oxidation bacteria increased in the later stage. The introduction of heterotrophic microorganism to the system contributed to form a suitable ecological niche of each species. In the batch method adopted and heterotrophic microorganism inoculated system, the bioleaching efficiency of Mn, Cu, Zn and Cd reached 94, 90.9, 94.74 and 84.2 %, respectively. The main fractions of heavy metals after bioleaching are comparatively stable speciation. Heavy metals were reduced both in total content and bioavailability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In China, heavy metal pollution has become a major environmental concern with the rapid industrialization and urbanization process during the last two decades (Wei and Yang 2010; Xu et al. 2012). Most of the smelting slag and wastewater produced by mining and metallurgical activities have been discharged without sufficient treatment (Deng et al. 2013). Heavy metals cannot be chemically or biologically degraded and are very difficult to be removed from a contaminated environment (Beolchini et al. 2009). When accumulated in sediment, water or aquatic organisms, heavy metals are particularly dangerous because of their persistency and toxicity. They would ultimately be assimilated by human beings through drinking water or the food chain.
Given that the sediment can act as carriers and potential sources for metals in an aquatic environment, and parts of these fixed metals would re-enter into water with the variation of water conditions (Peng et al. 2009), there is an urgent need to explore an effective remediation technique. Two main strategies, stabilization and extraction, have been adopted to remedy the heavy metal-contaminated sediment (Gan et al. 2015a; Peng et al. 2009). Stabilization is aimed at increasing the combination stability between metals and sediment particles, while extraction focuses on separating metals from sediment. Specific techniques including amendment, sand cap, phytoremediation, washing, electrochemical remediation, flotation and immobilization are being applied now (Gan et al. 2015b; Guemiza et al. 2014). The bioleaching technique has been increasingly applied to the extraction and recovery of heavy metals from contaminated sediment, soil and sludge (Zhu et al. 2013b). It is an environmental cleaner process when compared with physical and chemical processes (Zhu and Zhang 2014). As a low-cost and environment-friendly technique, bioleaching has been already successfully applied in the mineral processing area. The dominant mesophiles used in bioleaching are Acidithiobacillus species including Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans (Wen et al. 2013). Both these chemolithotrophic bacteria can get energy by oxidizing the reduced sulfur compound. Moreover, the former also possesses ferrous oxidation ability. The application of Leptospirillum ferriphilum in heavy metal-contaminated sediment bioremediation is very limited for it can only oxidize the ferrous iron, and the acid production ability is inferior to that of others. However, L. ferriphilum was recognized as the critical bacteria in biometallurgy due to the resistance to high-concentration ferric ion and metals, and it was also more tolerant to higher temperature, lower pH and ferric ion (Watling 2006). In this study, L. ferriphilum was also introduced into the bioleaching system to accelerate the oxidation of pyrite. Heterotrophic microorganisms have also attracted much attentions in bioleaching because of the organic acid utilization ability (Wang et al. 2010). According to previous research, organic acids such as formic, acetic, propionic and hexanoic would inhibit the ferrous and sulfur oxidation ability of the Acidithiobacillus species (Fang and Zhou 2006). In this study, a mixed heterotrophic microorganism obtained from the sediment which can tolerate low pH and heavy metals was inoculated into the bioleaching system to diminish the toxicity of low molecular weight organic matter on acidophilic bacteria (Fang and Zhou 2006).
The Xiawan Port of Xiangjiang River in Hunan Province was the most seriously heavy metal-polluted areas in China, affected by the smelter and chemical industry (Chen et al. 2004). The pollution would become more serious if no further management measures are taken. Heavy metal pollution threatens human health, balance of aquatic ecosystems, economic development and social prosperity (Zhu et al. 2013a). The discharged effluent was treated by quick lime, which contributed to the alkaline environment of the sediment and inhibited the proliferation of acidophilic bacteria and the subsequent bioleaching process. High content of organic matter, strong buffer ability and multiple heavy metals led to the slow activation and long bioleaching period. Considering these reasons, a successive batch method coupled with the assistance of heterotrophic microorganism was adopted in this bioremediation experiment. The aim of this research was to explore an effective way to remediate multiple heavy metals-contaminated alkaline sediment with high content of dissolved organic matter.
Materials and method
Sediment
The sediment was sampled from the confluence of the sewage outlet and the Xiangjiang River (Zhuzhou, Hunan province, China; N27.8554401652, E113.0786195397) in October, 2013. The sediment sample was taken from the station at 50 cm water depth. The top layer sediments (30 cm thick) were collected with shovel and deposited in plastic bags. The samples were naturally air dried, sieved through 100 meshes, intensively mixed and stored at 4 °C in a refrigerator prior to its use. Part of the sample was dried for 3 h at 105 °C before measuring the total organic matter content. The total organic carbon (TOC) content was decided by the difference between the dry weight and residue weight after combustion for 2 h at 500 °C. Heavy metal content was measured by inductively coupled plasma-atomic emission spectrometry. Table 1 shows that the heavy metal content tremendously exceeded the secondary standard environmental quality of China.
Heterotrophic microorganism isolation and identification
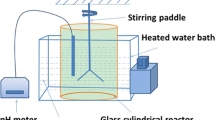
About 5 % (w v−1) undried fresh sediment was inoculated into the liquid LB medium (10 g L−1 tryptone, 5 g L−1 yeast extract, 10 g L−1 NaCl) to enrich the dissolved organic matter-degrading bacteria. It was cultured at 30 °C and 180 rpm for 72 h using the ZWY-2102c ZhiCheng incubator shaker (Fig. 1), and the initial pH of the medium was adjusted to 7.0 with diluted 5 % H2SO4. Then, 5 % obtained mixed consortium heterotrophic fungi were successively inoculated into a lower pH LB medium (6.0, 5.0, 4.0, 3.0, 2.0 and 1.5). The LB medium would become viscous under low pH, but the enrichment effect would not be affected. After a consortium of acid-tolerant heterotrophic microorganism was enriched, it was isolated through repeated plate streaking on solid LB medium and identified by molecular phylogenetic analysis based on 18S DNA. The specific heterotrophic microorganism identification procedure was referred to Wang et al. 2010. The specific procedure is summarized in Fig. 2.
The isolated acid-tolerant heterotrophic microorganism was cultured in 100 mL liquid LB medium at 30 °C, 180 rpm for 3 days, and then harvested at 12,000 rpm for 10 min. The heterotrophic microorganism was washed with distilled deionized water, pH 2.0, to remove the organic matter. The obtained heterotrophic microorganism was divided into two parts and added to the bioleaching system.
Bioleaching experiment
The bioleaching experiment was conducted in a 500 mL conical flask with 200 mL reaction volume. A consortium of A. ferrooxidans 23270, A. thiooxidans and L. ferriphilum was adopted in the bioleaching experiment. A. ferrooxidans 23270 and A. thiooxidans were preserved by the Key Laboratory of Biometallurgy of the Ministry of Education, China, and cultivated in 9K medium [3 g L−1 (NH4)2SO4, 0.1 g L−1 KCl, 0.5 g L−1 K2HPO4·3H2O, 0.5 g L−1 MgSO4·7H2O, 0.01 g L−1 Ca(NO3)2] with elemental sulfur (10 g/L), while L. ferriphilum got energy through oxidizing FeSO4.7H2O (44.7 g/L). The initial concentration of each strain of bacteria was \( 0.5 \times 10^{7} \) cell mL−1, which was determined by microscopic counting. All bioleaching experiment was performed at 30 °C, 180 rpm. 200 uL Leachate was sampled regularly, and the reduced leachate was replenished by corresponding pH deionized water.
In the batch method-adopted system, 1.0 g dry sediment was first added to 200 mL deionized water and adjusted to pH 4.0 with 5 % HCl (w v−1). The remaining 9.0 g sediment was divided into nine parts, and 1.0 g sediment was added to the system when its pH dropped to 2.0. While the bioleaching system adopted the non-batch method, the remaining 9.0 g sediment was added at once with no pH adjustment. The bioleaching system can be divided into four groups according to the batch method adopted and heterotrophic microorganism inoculated. Table 2 summarizes the design of the experiment. pH, ORP and SO4 2− were monitored every day; Fe and dissolved organic matter were monitored every 2 days. All experiments were performed in duplicate.
Analytical methods
pH and ORP were measured using a pHS-3C model digital pH-meter. The concentration of Fe(II) in solutions was determined using the 1, 10-phenanthroline method. Sulfate concentration was determined by BaSO4 spectrophotometric method. Speciation of heavy metals in the sediment before and after the bioleaching experiment was fractioned by a modified Tessier sequential extraction procedure (Tessier et al. 1979). DOM concentrations were determined by the TOC analyzer (TOC-L, Shimadzu). Titrations were conducted at 25 °C using an automated potentiometric titrator (ZDJ-5). The sediment sample (0.20 g) was added into 40 mL 0.01 M NaNO3 and then adjusted to approximately pH 2.3 with 0.1 M HNO3. Standardized CO2-free 0.01 M NaOH was used as the titrant. Microscopic counting was determined through Olympus CX21 microscope and the blood cell counting chamber (Qiujing): \( {\text{Cell density }}\left( {\text{cells/mL}} \right) {\text{ = Cell number in one hundred sub -box/100 }} \times 4 \times 1 0^{ 6} \).
DNA of the mixed culture was extracted in 6, 12 and 22 days. The leaching solution was allowed to settle for 10 min and then centrifuged at 12,000 rpm with Sigma centrifuge for 5 min to get the cells. The genomic DNA was extracted using TIANamp Bacteria DNA kit (Tiangen Biotech Co. Ltd., Beijing, China) in accordance with the manufacturer’s instructions. The real-time PCR was carried out with iCycler iQ Real-time PCR detection system (Bio-Rad Laboratories Inc., Hercules, USA). The reaction mixture contained 12.5 uL of SYBR Green Real-time PCR Master Mix (Toyobo Co. Ltd., Japan), 1 uL of 10 pmol of each primer, 2 uL of template DNA, and double-distilled water added to a total of 25 uL. The primers used for real-time polymerase chain reaction (real-time PCR) and the specific procedures were referenced to previous researches (Li et al. 2011; Wang et al. 2012). The CCA analysis was conducted with the Canoco for Windows software version 4.5.
Results and discussion
Isolation and identification of dissolved organic matter-degrading microorganism
Heterotrophic microorganisms capable of assimilating dissolved organic matter were isolated from the sediment through enrichment and isolation. Two isolates including fungus and yeast were obtained. For phylogenetic analysis, the species identified in this study were chosen to construct the phylogenetic tree based on 18S rRNA gene sequences with a bootstrap neighbor-joining method (Fig. 3). According to the 18S rRNA gene phylogenetic analysis, the isolated microorganisms belonged to the following two lineages: Aspergillus niger and Rhodotorula. The ferrous iron and sulfur oxidation ability was inhibited by the dissolved organic matter (Zheng et al. 2009). The coinoculation of biodegrading heterotrophic microorganisms can reduce the toxic effects of dissolved organic matter and elevate the concentration of dissolved carbon dioxide. The isolated A. niger and Rhodotorula possess dissolved organic matter degradation ability between pH 1.5 and 6.0, which were taken as integration introduced to the bioleaching system.
Dynamic changes of pH and redox potential during bioleaching
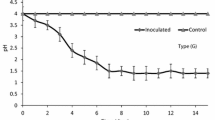
A remarkable pH dropping trend appeared in the successive batch method system (HM + B and C + B) in the initial period, followed by a relatively stable value with subsequent sediment addition (Fig. 4a), while the acidification efficiency was inconsistent between these two systems. The group which had heterotrophic microorganisms inoculated attained higher acidification efficiency and reached the bioleaching end point 8 days earlier. A previous study ascribed it to the organic acid in sewage sludge that the presence of 10.8 mM acetic and 9.88 mM propionic acid led to long lag periods of 6–7 days for sludge bioleaching (Wong and Gu 2004). A slightly rising trend was shown in the beginning stage of bioleaching in the HM + B group, which may be related to the assimilation of organic acid by the heterotrophic microorganisms. In the non-batch method system (HM + T and C + T), the bioleaching was totally inhibited because of the high initial pH and strong buffering ability of the sediment. The bacteria proliferation and substrate utilization were suppressed in such an adverse environment. As revealed by the oxidation reduction potential (ORP) (Fig. 4b), it shows a related variation trend with the pH change. In the batch method adopted coupled with heterotrophic microorganisms-inoculated system (HM + B), the ORP reached 500 mV, while the ORP of the system without heterotrophic microorganisms (C + B) was only 420 mV (Fig. 4b). This phenomenon can be due to the inhibition effect of dissolved organic matter on sulfur and ferrous oxidation. Higher substrate utilization efficiency and ferrous iron oxidation ratio contributed to a higher oxidation reduction potential.
The utilization of S and FeS2 during bioleaching
The component elements S and Fe of pyrite can both be utilized by the acidophilic bacteria and lead to the acidification effect in bioleaching. As shown in Reactions 1 and 2, proton produced with pyrite utilization and the acidification efficiency were higher than that of sulfur (Chandra and Gerson 2010). In addition, the huge reserves and low cost made it possible be industrially applied in bioleaching. The total iron concentration (ferrous and ferric iron) in group H + B and C + B was 2307.90 and 1633.73 mg L−1, respectively (Fig. 5a). Higher iron concentration showed that the heterotrophic microorganism promoted the pyrite utilization by acidophilic bacteria significantly. A similar trend was also observed in sulfate production (Fig. 5b). The promotion effect was also exhibited in the ferrous oxidation ratio; ferrous iron was completely oxidized to ferric iron in the heterotrophic microorganism co-inoculated system, while the ferrous iron oxidation ratio in the C + B system was less than 25 % during the first 12 days. Similar results were also reported in bioleaching systems co-inoculated with Acidithiobacillus species and acid-tolerant heterotrophic microorganism P. spartinae. The oxidation activities of A. ferrooxidans and A. thiooxidans enhanced by 33- and 12-fold, respectively, in P. spartinae co-inoculated sludge (Zheng et al. 2009). The main inhibitory substances in the sediment including formic and acetic acids inhibit the Fe and S oxidation ability of acidophilic bacteria by reacting abiologically with ferrous iron outside the bacteria cell (Wang et al. 2010). The results revealed that A. niger and Rhodotorula isolated in this study accelerated the utilization of sulfur and pyrite.
Removal of dissolved organic matter, cell density and community analysis
The CCA triplot shows the correlations between the microbial community and environmental parameters. S and C represent the H + B and C + B systems, respectively. Arrows on the graph represent environmental parameters. Triangles represent species. Circles 1, 5 and 9 represent samples from system H + B in 0, 6 and 12 days; Circles 2, 6, 10 and 13 represent samples from system C + B in 0, 6, 12 and 22 days.
Figure 6a indicates that heterotrophic microorganisms play a pivotal role in the removal of dissolved organic matter during bioleaching. At the initial phase, the concentration of DOM reached 85.83 mg DOC/L in the HM + T system and then declined afterward. By the ninth day, the concentration was below 47 mg DOC/L. The DOM concentration in the HM + B system was presented as a rising trend, for it adopted a batch method, while the concentration in the bioleaching end point was 43.68 mg DOC/L, which was much less than that in the C + B system (88.42 mg DOC/L). The DOM concentration in the group C + B was approximately twice higher than that of HM + B, while the bioleaching in C + B system was activated only when the end point was delayed. However, in the system that adopted the non-batch method but with the heterotrophic microorganism inoculated (HM + T), the bioleaching was totally inhibited. This phenomenon illustrated that dissolved organic matter in sediment was the inhibitor, while not the determining factor that led to inactivation in bioleaching. High alkalinity and strong buffer ability restrained bioleaching, which are the main reasons why bacteria cannot proliferate into the bioleaching system. Figure 6b shows the variation of bacterial cell density, from which it can be identified that the bacteria proliferation rate in the HM + B system is evidently faster than a system with a higher DOM concentration (C + B). Cells reached the highest density on the 10th and 14th day with a peak value of 1.50 × 109 and 1.07 × 109 ml−1 for systems HM + B and C + B, respectively. The difference in proliferation rate directly leads to the diversity in the acidification effect. In the non-batch method system, proliferation was not evident due to the adverse environment. In later stage, the cell density entered into a decline phase of the elevated metal concentration and lower pH. As shown in Fig. 6c, A. ferrooxidans, A. thiooxidans and L. ferriphilum are inoculated into both systems at equal ratios. On the sixth day, the ratio of A. thiooxidans in system H + B reached 63.27 %, while the ratio of A. thiooxidans in system C + B was 12 % less than the former. The dominant species in both systems H + B and C + B in this stage was A. thiooxidans, while the ratio of ferrous-oxidizing bacteria L. ferriphilum was relatively low. It illustrated that sulfur oxidation bacteria A. thiooxidans facilitated the acidification in the early stage. The oxidation of elemental sulfur can provide more growth energy to the sulfur-oxidizing microorganisms than ferrous iron to iron-oxidizing microorganisms. Also, the pure sulfur utilization ability of A. thiooxidans made it take a dominant position in a substrate abundant environment, but not A. ferrooxidans. Previous research has shown similar results in pure pyrite bioleaching (He et al. 2012). The high pH environment and lack of ferrous ion in the system inhibit the proliferation of L. ferriphilum in the initial period. With pH decline and pyrite mass utilization in the later stage, the ratios of L. ferriphilum and A. ferrooxidans increased in the later stage. This phenomenon was also confirmed by the elevated oxidation percentage of ferrous iron. Appropriate pH environment coupled with increase in ferrous iron was conducive to the proliferation. Canonical correspondence analysis (CCA) in Fig. 6d presents clearly the potential correlations between the microbial community and environmental factors. L. ferriphilum and A. ferrooxidans had higher tolerance to ferric ion, high ORP, multiple metals and low pH, contrary to A. thiooxidans. The introduction of heterotrophic microorganism to the bioleaching system contributes to a suitable ecological niche of each species formed during bioleaching.
Removal of heavy metals and fraction behavior
Figure 7 depicts the heavy metal solubilization efficiency in different treatments. The solubilization of heavy metals was not evident in non-batch system for the inhibition effect on bioleaching. In the HM + B system, the bioleaching efficiency of Zn, Mn, Cu and Cd reached 94.74, 94, 90.9 and 84.2 %, while Hg, As and Pb performed no more than 30 % which was determined by the low solubility product (Chen and Lin 2004). Correspondingly, the solubilization of Cd, Zn, Pb, Hg, As, Mn and Cu reached 85.03, 95.4, 18.5, 14.5, 24.7, 94.3 and 87.6 %, respectively, in system (C + B) with no heterotrophic microorganism inoculated. The bioleaching efficiency difference in both systems which adopted the batch method was insignificant for almost identical ultimate pH. The solubilization efficiency provides the evidence that the batch method is necessary for effective bioleaching of sediment with strong buffer ability.
The absolute content of heavy metal speciation in the batch method-adopted system (H + B and C + B) is shown in Fig. 8a, b. Geochemical characterization is of primary importance to assess the toxic effect of sediments; moreover, a detailed analysis of heavy metal speciation can provide important insights into the potential fate of each heavy metal (Pathak et al. 2014). After bioleaching, heavy metals not only reduced in total quantity, but also transformed in speciation. All five component speciation of Mn, Zn, Cu and Cd were significantly reduced, consistent with other research that metals can be ordered in terms of decreasing mobility as follows: Cu, Zn, Cd, Hg, Pb, Ni, As and Cr (Beolchini et al. 2009). While the speciation transformation of Pb, Hg, As is not significant due to the low mobility of these metals. Metals in exchangeable, carbonate and Fe/Mn oxide-bonded fraction are considered to be more mobile and bioavailable. Among them, exchangeable and carbonate form are acid soluble and easily affected by proton. The organic and residual fractions are more stable and non-bioavailable. Figure 8c, d shows that the acid-soluble fractions of Zn, Pb, Mn, Cd, Hg, As and Cu are 32.0, 25.5, 24.2, 59.1, 38.4, 23.9 and 15.3 %, respectively, in the original sediment. The results showed that the percentage of acid-soluble fraction was significantly decreased after bioleaching, while the major fractions after bioleaching existing in the sediment are comparatively stable: Fe–Mn oxide associated form,organic associated form and residual form. It implies that metals are not only reduced in total content, but also decreased in mobility and bioavailability.
Potentiometric titration and XRD analysis
Potentiometric titration technique was first applied to investigate the acid–base properties of the sediment sample before and after bioleaching. The potentiometric titration data are shown in Fig. 9a, b. The total proton concentration is calculated from the following equation:
where C a and C b are the respective molar concentrations of HNO3 and NaOH used, V o is the initial volume of the suspension, and V a and V b are the volumes of HNO3 and NaOH added. The consumed proton reached 0.04203 M when the sediment was titrated to pH 2.235 before bioleaching, while it was reduced to 0.0061 M after bioleaching. The distinct acid–base property can be attributed to the change in sediment composition. XRD analysis showed that the main component was silicon oxide (41.7 %) and acid consumption calcite (55.3) in the original sediment, while quartz (92.3 %) became the main component after the bioleaching (Table 3). It further confirmed the titration data. The diversity was also reflected in the titration process: two obvious plateaus were exhibited in Fig. 9a, while it was negligible in the sediment sample after bioleaching. Additionally, the transformation of sediment composition was further exhibited by the pHpzc values (the point of zero charge). As shown, the pHpzc of the sediment after bioleaching was ∼3.43, while the pHpzc of the sediment before bioleaching did not appear in the titration curve. The acid consumption of the sediment before bioleaching was 3.9 times more than that after bioleaching when the sediment was titrated from 2.24 to 10.08. The bioleaching significantly changed the acid–base property and composition of the sediment.
Conclusions
In this study, acid-tolerant dissolved organic matter-degrading microorganisms Rhodotorula HJM and A. niger HQM were isolated from the sediment. Acid-tolerant heterotrophic microorganisms contribute to suitable ecological niche of each species during bioleaching, which facilitated the oxidation of sulfur and pyrite. High alkalinity and strong buffering capacity of the sediment directly inhibited the activity of acidophilic bacteria and the subsequent bioleaching. The batch method can effectively relieve the inhibition effect. Dissolved organic matter hampered the substrate utilization and prolonged the bioleaching period. Its toxic effect on the acidophile can be reduced by the isolated heterotrophic microorganism. A. thiooxidans was the dominant species in the early stage, while the ratio of ferrous oxidation bacteria increased in the later stage. In the batch method and the heterotrophic microorganism-inoculated system, the bioleaching efficiency of Zn, Mn, Cu and Cd reached 94.74, 94, 90.9 and 84.2 %, respectively. The fraction of heavy metals mainly transformed to comparatively stable speciation after bioleaching. The acid consumed by original sediment is 3.9 times more than that after bioleaching.
References
Beolchini F, Dell’Anno A, De Propris L, Ubaldini S, Cerrone F, Danovaro R (2009) Auto- and heterotrophic acidophilic bacteria enhance the bioremediation efficiency of sediments contaminated by heavy metals. Chemosphere 74:1321–1326
Chandra A, Gerson A (2010) The mechanisms of pyrite oxidation and leaching: a fundamental perspective. Surf Sci Rep 65:293–315
Chen SY, Lin JG (2004) Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: effects of sulfur concentration. Water Res 38:3205–3214
Chen Y, Wu F, Lu H, Yao C (2004) Analysis on the water quality changes in the Xiangjiang River from 1981 to 2000. Resour Environ Yangtze Basin 13:508–512
Deng X, Chai L, Yang Z, Tang C, Wang Y, Shi Y (2013) Bioleaching mechanism of heavy metals in the mixture of contaminated soil and slag by using indigenous Penicillium chrysogenum strain F1. J Hazard Mater 248:107–114
Fang D, Zhou L (2006) Effect of sludge dissolved organic matter on oxidation of ferrous iron and sulfur by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Water Air Soil Pollut 171:81–94
Gan M, Jie S, Li M, Zhu J, Liu X (2015a) Bioleaching of multiple metals from contaminated sediment by moderate thermophiles. Mar Pollut Bull 97:47–55
Gan M, Zhou S, Li M, Zhu J, Liu X, Chai L (2015b) Bioleaching of multiple heavy metals from contaminated sediment by mesophile consortium. Environ Sci Pollut Res 22:5807–5816
Guemiza K, Mercier G, Blais JF (2014) Pilot-scale counter-current acid leaching process for Cu, Pb, Sb, and Zn from small-arms shooting range soil. J Soils Sediments 14:1359–1369
He Z, Yin Z, Wang X, Zhong H, Sun W (2012) Microbial community changes during the process of pyrite bioleaching. Hydrometallurgy 125–126:81–89
Li Q, Tian Y, Fu X, Yin H, Zhou Z, Liang Y, Qiu G, Liu J, Liu H, Liang Y, Shen L, Cong J, Liu X (2011) The community dynamics of major bioleaching microorganisms during chalcopyrite leaching under the effect of organics. Curr Microbiol 63:164–172
Pathak A, Srichandan H, Kim D-J (2014) Fractionation behavior of metals (Al, Ni, V, and Mo) during bioleaching and chemical leaching of spent petroleum refinery catalyst. Water Air Soil Pollut 225:1893–1903
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Wang S, Zheng G, Zhou L (2010) Heterotrophic microorganism Rhodotorula mucilaginosa R30 improves tannery sludge bioleaching through elevating dissolved CO2 and extracellular polymeric substances levels in bioleach solution as well as scavenging toxic DOM to Acidithiobacillus species. Water Res 44:5423–5431
Wang Y, Su L, Zhang L, Zeng W, Wu J, Wan L, Qiu G, Chen X, Zhou H (2012) Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium. Bioresour Technol 121:348–354
Watling H (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides—a review. Hydrometallurgy 84:81–108
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Wen YM, Cheng Y, Tang C, Chen ZL (2013) Bioleaching of heavy metals from sewage sludge using indigenous iron-oxidizing microorganisms. J Soils Sediments 13:166–175
Wong JWC, Gu XY (2004) Enhanced heavy metal bioleaching efficiencies from anaerobically digested sewage sludge with coinoculation of Acidithiobacillus ferrooxidans ANYL-1 and Blastoschizomyces capitatus Y5. Water Sci Technol 50:83–89
Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Lai C, Wei Z, Huang C, Xie GX (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Zheng G, Zhou L, Wang S (2009) An acid-tolerant heterotrophic microorganism role in improving tannery sludge bioleaching conducted in successive multibatch reaction systems. Environ Sci Technol 43:4151–4156
Zhu J, Zhang J, Li Q, Han T, Xie J, Hu Y, Chai L (2013) Phylogenetic analysis of bacterial community composition in sediment contaminated with multiple heavy metals from the Xiangjiang River in China. Mar Pollut Bull 70:134–139
Zhu J, Zhang J (2014) Bioleaching of heavy metals from contaminated alkaline sediment by auto-and heterotrophic bacteria in stirred tank reactor. Trans Nonferrous Metals Soc China 24:2969–2975
Zhu Y, Zeng G, Zhang P, Zhang C, Ren M, Zhang J, Chen M (2013) Feasibility of bioleaching combined with Fenton-like reaction to remove heavy metals from sewage sludge. Bioresour Technol 142:530–534
Acknowledgments
This research was supported by the National Natural Science Foundation of China (51174239), the Fundamental Research Funds for the Central Universities of Central South University (2015zzts089) and the Hunan provincial Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gan, M., Song, Z., Zhu, J. et al. Efficient bioleaching of heavy metals from contaminated sediment in batch method coupled with the assistance of heterotrophic microorganisms. Environ Earth Sci 75, 457 (2016). https://doi.org/10.1007/s12665-016-5307-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5307-0