Abstract
Soil contamination by heavy metals is a global problem that threatens human health and environmental sustainability. Biochar as an environmentally friendly soil amendment combined with bioremediation (heavy metals tolerance bacteria) as an effective and emerging strategy for sustainable remediation of heavy metals contaminated soil. Thus the objective of this research was to explore the potential of Alcaligenes Pakistanensis sp. Nov (NCCP-650) bacteria combined with biochar for remediation of cadmium (Cd) and lead (Pb) contaminated soil and its effect on lettuce growth. Biochar was applied at 0, 1, 2, 4% to Cd and Pb contaminated soil with and without bacteria in pots using lettuce as a test crop. The results of soil were recorded by analyzing different soil traits like pH, ECe, SOM, soil total N, P, K, Cd and Pb. The results revealed that, compared to single biochar amendment, biochar combined with bacteria inoculation at 4% significantly (p < 0.05) reduced the bioavailable soil AB-DTPA extractable Cd and Pb and improved lettuce growth. The highest immobilization index of Cd and Pb was 55 and 46% in biochar treatment at 4% combined with bacteria. Similarly, Cd, Pb content in shoot and root, bioaccumulation factors values of Cd, Pb were reduced with 4% biochar applied with bacteria inoculation. The results depicted that bacteria inoculated biochar at 4% not only reduced the bioavailable fractions of Cd and Pb in soil but also positively improved plant growth and soil properties. It is concluded that biochar combined with heavy metal tolerant bacterial inoculation Alcaligenes Pakistanensis sp. Nov (NCCP-650) was most effective in reducing Cd and Pb availability to plants and enhancing plant growth compared to sole application. Hence, the application of biochar at the rate of 4% combined with Alcaligenes Pakistanensis sp. Nov (NCCP-650) inoculation is recommended for sustainable remediation of heavy metals contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human health and environment have been adversely affected by the entrance of heavy metals into the soil (Naveed et al. 2020). The primary source of soil contamination is hazardous heavy metals and other organic contaminants, including pesticides, pharmaceuticals, petroleum, and surfactants. Although significant fractions of these contaminants were exhausted in soil environment by various ways like weathering, deposition of contaminants released from the eruption of volcanoes, sludge or wastewater from industries, and mining activities (Shah et al. 2010; Khan et al. 2008). The bioavailable fractions of heavy metals in plants can cause several human health disorders (Liu et al. 2018; Khan et al. 2013; Ali et al. 2013). These high-level toxic metals are unsafe for the environment because they are not decomposable and stay for a long time in nature (Radwan and Salama 2006; Muhammad et al. 2011; Khan et al. 2010). Mitigating these toxic elements is considered a target for achieving sustainable environmental goals (Naveed et al. 2020).
Heavy metals, for example, Cd and Pb, are one of the target metals; they are non-degradable, toxic, and nonessential elements for plants (Naveed et al. 2020). Because of their high toxicity and mobility, Cd and Pb are accumulated in crop grain from agricultural contaminated soil (Zhu et al. 2014), reducing the chlorophyll formation and adversely retarding the physiological and biochemical functions in plant, affecting plant height and crop yield (Rizwan et al. 2016; Kamran et al. 2019; Naveed et al. 2020). The continual uptake of theses heavy metals from food by humans can cause health problems (Alkorta et al. 2004; Huang et al. 2011). Heavy metals are persistent and difficult to remove or degrade once introduced into soils. For this reason, it is necessary to stabilize the Cd and Pb contaminates in the soils to recover the natural functioning of such soils by feasible measures (Naveed et al. 2020).
Among various organic amendments, biochar application is very prominent for heavy metals stabilization. Biochar is formed from the breakdown of carbon-rich biomass through high temperatures and a limited oxygen supply (Lehmann et al. 2006). From the last few decades biochar gained significant attention for heavy metals stabilization due to their unique properties like higher surface area, ion-complexion, ion-exchange and electrostatic interaction that help in metal fixation and surface adsorption (Abbas et al. 2017; Lee et al. 2013). Besides, soil type, biochar surface, temperature (pyrolysis), and applying rate play a significant role in heavy metals stabilization (Rehman et al. 2016; Shen et al. 2016). A glass house pot experiment demonstrated that biochar decreased cadmium and lead by 71% and 92%, respectively (Houben et al. 2013). Furthermore, biochar increased soil pH, EC, and cation exchange capacity (CEC), ultimately change the heavy metals bioavailability (Fellet et al. 2014).
Mixing biochar with different treatments, like heavy metal-tolerant microbial organisms such as bacteria can enhanced their efficiency, cost-effectiveness and ecological preservation (Naveed et al. 2020). Plant-developing bacteria are often used to improve crop yield and are appropriate to various agricultural settings (Mustafa et al. 2019). The rhizosphere soil along with metal-tolerant microbial organisms can be used as it is more conceivable and efficient in enhancing plant development because they are beneficial to plant tissues (Naveed et al. 2014). Besides growth promotion, these microorganisms may improve the immunity of plants against harmful organisms, dry spells, and hazardous heavy metals (Ryan et al. 2008). Such microorganisms (bacteria) make colonies with plant roots and improve their growth by numerous methods. When applied to polluted soils, these bacteria diminished bioavailability, resulting in low heavy metals ingestion in the root regions (Du et al. 2016; Ojuederie and Babalola 2017). The microorganisms (bacteria) may mitigate heavy metals by producing exopolysaccharides, siderophores, and metal phosphates or through improved rhizosphere acidification (Rajkumar et al. 2012; Ali et al. 2017).
In Pakistan, crops are primarily grown in regions where irrigation water is often contaminated with heavy metals (Qadir et al. 2000; Hossain et al. 2010). One plant species considered to be a bioindicator of heavy metals is lettuce (Lactuca sativa L.), which is used fresh by the population and it is in the edible part (leaves) where the highest concentration of these elements accumulates. The uptake of metals from soil into plants is affected by soil chemistry, metal speciation (i.e., inorganic and organic complexation), and molecular transport and storage processes in plants (Clemens et al. 2009). Some plants, such as lettuce can hyper-accumulate metal ions because of specialized mechanisms of absorption and transport of internal ions. These lettuce can tolerate high concentrations of toxic metals in soil, and may also have potential for phyto-remediation in contaminated soils (Clemens et al. 2009; Memon et al. 2009). However, edible plants grown in contaminated soils may also accumulate elevated levels of metals that may, when consumed, increase exposures to humans.
Therefore, there is a critical need to investigate procedures for limiting the bioavailability of heavy metals in plants, primarily edible crops (Naveed et al. 2020). There is preliminary work on the effect of biochar and metal-tolerant bacteria to alleviate the unfavorable effects of heavy metals on plants. Likewise, the impact of the collective use of bacteria and biochar on plants enhancement, physiochemical properties, and nutrient concentrations needs further examination.
Materials and Methods
Bulk Soil Sampling
A field previously utilized for growing different crops was selected in a research farm at The University of Agriculture, Peshawar, for bulk soil sampling. A bulk soil sample was taken from the field at 0–20 cm depth and was brought to the Soil and Environmental Sciences glass house for a pot experiment. The bulk soil was cleaned from crop residue, plastics, small pebbles, etc. After air drying, the soil was sieved through a 2 mm sieve. Before the pot experiment, the bulk soil was analyzed for various physicochemical characteristics.
Experimental Setup
A pot experiment in the glass house of the Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, was conducted for assessment of biochar applied alone or in combination with heavy metal tolerant bacteria on Cd and Pb remediation. Furthermore, the tolerance of lettuce plants was evaluated under biochar and bacteria application. The bulk soil used in the experiment was completely air dried and was passed through a 2 mm diameter sieve. All pots were filled with 10 kg of soil and were spiked with Cd and Pb at the rate of 20 and 30 mg kg−1 irrigated by achieving field capacity. Biochar was applied at the rate of 1, 2, and 4%, with and without bacteria. The pots were kept for two weeks in a controlled environment. During that time field capacity was maintained by applying water when needed. After two weeks, ten seeds of lettuce were sown in each pot. As a basal dose, 60 and 90 kg ha−1 N and P fertilizers were applied at sowing time. Another 60 kg ha−1 N was applied after 30 days. Irrigation was done using tape water according to the crops requirement. The experiment was carried out in a factorial design replicated three times. The crop was harvested from pots after 70 days of crop growth and plant samples were used for lab analysis. After crop harvesting, soil sample was taken from each pot for soil lab analysis.
Bacteria
The bacteria used for the pot experiment was Alcaligenes Pakistanensis sp. Nov (NCCP-650) is known to be a heavy-metal tolerant Novel Bacterium collected from National Agriculture Research Center (NARC), Islamabad. The strains are gram-negative, strictly aerobic, motile short rods, and tolerant to heavy metals (Cd, As, Pb and Cu) (Abbas et al. 2015). Lettuce seeds were treated with bacterial culture according to the standard procedure of Surette et al. (2003). Seeds were soaked adequately in bacterial solution and then air dried and sown in pots.
Biochar
The biochar used an experiment was prepared from wheat straw at 500 °C through pyrolysis process in a furnace. The biochar was grounded and sieved through 1 mm mesh.
Soil Analysis
After harvesting, soil samples were collected from each experimental unit and used for soil chemical analysis determination. The fraction of Cd, Pb, P and K in soil was analyzed by AB-DTPA extractable method porposed by Soltanpur and Schawab’s (1977). The pH of the soil was determined by the method of McLean (1983). The method of Richard (1954) was used for determination of soil electrical conductivity. The Bremner and Mulvaney (1996) standard procedure was used to find the total nitrogen concentration in soil. For the determination of soil organic matter, Nelson and Sommers’s (1996), method was followed.
Plant Analysis
Laboratory
The Cd and Pb content in plant leaves and roots were determined by AOAC method of wet digestion (1990).
Agronomic
After cutting the plants, shoot and roots were kept in open-air for 2–3 days to become dry. For complete drying, it was kept in an oven at 70 °C for 48 h. Data on shoot and root biomass was measured by taking the weight of five different plant shoots with a balance meter and the average was noted.
Phyto-Extraction Keys
Modified from (Lee et al. 2013)
Modified from (Shin et al. 2002; Arifin et al. 2012)
Statistical Analysis
A two-way analysis of variance was done for experimental treatment. The resulting data were expressed as the means, while the standard deviation was calculated as the means. The least significant test (LSD) was done using the statistical package Statistix 8.1 (Steel 1997).
Results
Physicochemical Properties of Soil Used in Pot Trail
The soil used in pot trial was analyzed for physicochemical properties before the experiment. The results are illustrated in Table 1. The soil texture class was silt loam, slightly alkaline (pH 7.51). The electrical conductivity (EC) was 1.16 dSm−1, organic matter was 0.50%, and total nitrogen content was 0.23%. The AB-DTPA extractable P, K, Cd, and Pb were 1.15, 42.5, 0.17, and 0.13 mg kg−1, respectively.
Plant Height
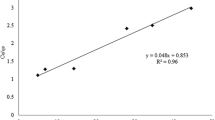
The data regarding plant height is shown in Fig. 1. Applying biochar with and without bacteria inoculation significantly increased plant height compared to control in Cd/Pb-contaminated soil. Sole biochar application at a 4% rate increased plant height from 14 cm (control) to 27 cm. In comparison, biochar combined with bacteria applied at a 4% rate increased plant height to 33 cm as compared to control. This indicates that the sole application of biochar and bacteria improved plant height. The lowest plant height in control showed that Cd and Pb stress adversely affected plant height.
Shoot Dry Weight
The data regarding lettuce shoot dry weight is presented in Fig. 2. The sole application of biochar and bacteria inoculation significantly enhanced the shoot’s dry weight. The dry shoot weight of lettuce plants was significantly increased to 11 g per pot compared to control with sole application of biochar at a 4% rate. While combined application of biochar and bacteria at 4% increased, shoot dry weight to 15 g compared to control. These results demonstrate that lettuce growth was improved using biochar or biochar combined with bacteria in Cd and Pb spiked soil. The significant improvement in lettuce growth compared to control by use of biochar or bacteria shows that Cd or Pb were stabilized in soil and unavailable to plant, adversely affecting the growth of lettuce.
AB-DTPA Extractable Cd and Pb
The application of biochar alone or combined with bacteria inoculation significantly decreased soil Cd compared to control (Fig. 3). The Cd concentration was reduced from 18.9 mg kg−1 to 8.5 mg kg−1 with the application of biochar combined with bacteria at a 4% rate. The results demonstrated that bacteria inoculation enhanced the efficiency of biochar to decrease Cd availability in the soil. Sole application of bacteria also significantly reduced Cd in soil, confirming its capability to minimize Cd concentration in soil. The complete application of biochar or in combination with bacteria inoculation significantly decreased Pb concentration in the soil compared to control (Fig. 4). The Pb was reduced from 28.9 mg kg−1 to 15.5 mg kg−1 with the application of biochar combined with bacteria at a 4% rate. The result shows that increasing levels of biochar without bacteria considerably decreased Pb content in the soil. However, bacteria also helped increase biochar’s efficiency in reducing Pb.
Immobilization Index of Cadmium and Lead
The immobilization index of Cadmium and Lead was increased by the use of sole applied of BC or in combination with heavy metal tolerant bacteria inoculation (Table 2). The highest immobilization index of Cd and Pb was 55.0 and 46.3% in biochar treatment at 4% combined with bacteria. The Cd immobilization index of sole biochar treatments (1, 2, and 4%) were 13.22, 32.8 and 37.5%, while the same application rates of biochar combined with bacteria resulted in 34.3, 50.8 and 55.0% immobilization index. Similarly, the Pb immobilization index of sole biochar treatments (2% and 4%) were 32.8 and 35.9% while the same application rates of biochar combined with bacteria resulted in 43.2 and 46.3% immobilization index. These results confirmed that biochar combined with bacteria was most effective in immobilizing Cd and Pb and reducing their accessibility to plants. The immobilization index results of Cd and Pb were confirmed by plant Cd/Pb data, which significantly decreased in shoot or root by combined application of biochar and bacteria.
Cd and Pb Concentration in Shoot
The sole application of biochar or in combination with bacteria inoculation significantly decreased Cd concentration in the shoot compared to the control (Fig. 5). The Cd concentration in the shoot was reduced from 21.1 mg kg−1 to 8.5 mg kg−1 when biochar at 4% rate was combined with bacterial strain. Similarly, the sole application of biochar or in combination with bacteria significantly decreased shoot Pb compared to control (Fig. 6). It was found that Pb in the shoot was reduced from 32.2 mg kg−1 to 12.5 mg kg−1 with biochar combined with bacteria at a 4% rate. In contrast, sole biochar at the rate of 4% reduced Pb from 32.2 mg kg−1 to 17 mg kg−1. The result shows that biochar, whether applied sole or in combination with bacteria, caused a considerable reduction in the concentrations of both Cd and Pb. While bacteria, when used in sole, slightly decreased Cd and Pb contents.
Cd and Pb Concentration in Root
The sole application of biochar or in combination with heavy metal tolerant bacteria inoculation significantly affected the Cd and Pb concentration in the root (Fig. 7). The Cd was reduced from 25.1 mg kg−1 (control) to 11.5 mg kg−1 with the application of BC combined with bacteria 4% at rate. Similarly, the Pb was reduced from 40.4 mg kg−1 (control) to 16.2 mg kg−1 with the application of BC combined with bacteria at a 4% rate (Fig. 8). Also, sole 4% biochar reduced Cd and Pb from 25.1 to 15.5 mg kg−1 and 40.4 to 24.5 mg kg−1, respectively. Results revealed that the highest reduction in Cd and Pb was caused by the combination of biochar and bacteria at the rates of 2% and 4%, while in control, the lowest reduction was noted. Besides, the application of bacteria sole caused a slight decrease in the concentrations of Cd and Pb.
Bioaccumulation Factor (BAF) of Cd and Pb
The bioaccumulation factor Cd and Pb are presented in Figs. 9 and 10. The bioaccumulation factor results stated that when biochar and bacteria were applied in combination to Cd and Pb, contaminated soil considerably reduced the concentration. Compared to the application of biochar alone, the combined application with bacteria highly reduced the Cd and Pb uptake by the plant, decreasing the Cd and Pb translocation and accumulation in plant tissue. The highest reduction in BAF of Cd and Pb was 1.01 and 0.81 in the treatment of biochar at 4% combined with bacteria. The Cd BAF of alone biochar treatments (1, 2, and 4%) were 1.06, 1.04 and 1.02, while the same application rates of biochar combined with bacteria resulted in 1.05, 1.03 and 1.01. Similarly, Pb BAF of alone biochar treatments (2% and 4%) were 0.97 and 0.95, while the same application rates of biochar combined with bacteria resulted in 0.83 and 0.81.
Soil pH, EC, and Organic Matter
The sole application of biochar combined with bacteria significantly affected soil pH compared to control (Fig. 11). The highest soil pH was recorded, which was amended with 4% biochar without bacteria strain. Compared to control, pH ranged from 7.51 to 7.87 at the highest rate of biochar (4%). The biochar with and without bacteria inoculation significantly affected soil EC. With the increase in the level of biochar, the soil EC was significantly increased (Fig. 12). The highest soil EC (0.51 dSm−1) was recorded at 4% biochar with and without bacteria compared to the control (0.32 dSm−1). The result revealed that the effect of biochar alone and in combination with bacteria was the same. The application of biochar with or without bacterial inoculation has significantly affected the SOM compared to control soil containing Cd and Pb (Fig. 13). The highest SOM was recorded where biochar was applied alone at the rate of 4% (1.26%) compared to control soil (0.62%). Inoculation of bacteria sole showed (0.72%) SOM compared to control. SOM was further increased when inoculation was combined with biochar; the increase was 1.62% at 4% biochar compared to control.
Soil N, P, and K Content
The sole application of biochar or in combination with bacteria has significantly affected the total soil nitrogen (Fig. 14). The application of 4% biochar without bacteria significantly increased total soil nitrogen to 0.49%, and the sole application of bacteria increased total soil nitrogen to 0.45% compared to control (0.43%). The results show that biochar has a significant nitrogen level, significantly increasing with bacterial inoculation. Bacterial inoculation helps make available the insoluble nitrogen to plants. The soil phosphorus has been considerably affected by applying biochar with and without bacteria (Fig. 15). Biochar applied alone in different doses has significantly increased the soil P compared to control. The highest soil P was noted at 4% biochar 5.1 mg kg−1 compared to control, which was 2.9 mg kg−1. While bacteria applied without biochar treatment has also significantly increased soil P compared to control. Further, compared to control, soil P was enhanced when biochar was used in combination with bacteria. The highest soil P was noted at 4% biochar (6.3 mg kg−1) in combination with bacteria. Applying biochar with and without bacterial inoculation considerably increased the soil potassium compared to control soil containing Cd and Pb (Fig. 16). The soil AB-DTPA K was improved from 60.3 mg kg−1 (control) to 95.6 mg kg−1 using biochar combined with bacteria.
Discussion
Our results showed that growth parameters such plant height and shoot dry weight were significantly improved by application of biochar along with metals tolerance bacteria in heavy metals stress condition. The lettuce can tolerate high concentrations of toxic metals in soil, and may also have potential for phyto-remediation in contaminated soils (Clemens et al. 2009; Memon et al. 2009). According to Abass et al. (2017) who reported that biochar application significantly increased the shoot length of plant compared to unamended soil. Glaser et al. (2002) and Yamato et al. (2006) suggested that biochar is essential for agricultural land that improved the degraded soil as it improves soil properties, releases sufficient amount of plant essential macronutrients and ultimately enhances plant growth. Studies have shown that biochar can improve soils physiochemical and biological properties, creating a suitable environment for plant roots, nutrient uptake, and plant growth (Erdem et al. 2017; Arif et al. 2015). Previous research showed that plant height and shoot, root, and grains dry weight significantly increased with increasing levels of biochar (Abass et al. 2017). Egamberdieva et al. (2020) found that maize straw biochar at a 2% rate significantly increased the dry shoot biomass of the lupin plant by 21 to 25%. Lua et al. (2014) found that shoot of lettuce plant (Lactuca sativa) was increased by 89% when oak wood-derived biochar was used. Jiang et al. (2008) reported that the shoot dry weight of maize and tomato plants was increased when the soil was inoculated with bacteria (Burkholderia sp. J62) by 30% and 54% compared to uninoculated soil. Naveed et al. (2020) reported that dry shoot weight and dry root weights were increased when biochar was used without bacteria (49%), while they were further enhanced when biochar was applied together with bacteria (57%). It has been widely documented that biochar has the potential to remediate soil contaminated with heavy metals (Naveed et al. 2020; Rehman et al. 2020; Muhammad et al., 2020). Park et al. (2011) reported that poultry manure-derived biochar notably decreased the Cd and Pb concentration by 88.4% and 93.5%, respectively, in artificially contaminated soil, similarly green waste-derived biochar significantly reduced Cd by 30.3% and Pb by 36.8% in spiked soil. Irfan et al. (2021) reported Pb concentration in soil was decreased by 89, 89, and 94% for 5, 10, and 50 mg kg−1 Pb spiked by the application of 6% biochar. Naveed et al. (2020) experimented that biochar and bacteria (Enterobacter sp. MN17) in combination reduced cadmium by 42%, while alone application of bacteria decreased Cd by 22% in soil. Kader et al. (2013) argued that the stability and survival of bacteria that immobilize heavy metals are further substantiated when combined with an immobilizing agent such as biochar. De et al. (2008) added to soil mercury-resistant bacteria, i.e., Alcaligenes faecalis and Bacillus pumilus to immobilize Cd and Pb in soil. It was found that Alcaligenes faecalis immobilized Cd by 75% and 306 Bacillus pumilus immobilized Pb by 88% in contaminated soil.
Biochar has been extensively recommended for diminishing metals obtainability to plants. Choppala et al. (2011) found that GW (Green waste-derived biochar) was very efficient in decreasing the Cd and Pb availability in the mustard shoot with increasing levels of GW biochar. The heavy metals (HMs) reduction in the mustard shoot was 72, 79, and 82% for Cd, and 74.4, 81.5, and 97.1% for Pb at different levels of BC (1, 5, 10%) addition, respectively. Lua et al. (2014) experiment results showed that the Cd contents in the shoot significantly decreased to 47% and 22%, respectively, using bamboo and rice straw biochar. Similarly, the Pb was reduced considerably with increasing rates of rice straw biochar (RSB), i.e., at 5%, the uptake of Pb in the shoot from 61 to 18 mg kg−1. Naveed et al. (2020) recently found that Cd contents in pea plant shoot decreased by 52% by applying biochar and bacteria (Enterobacter. MN17). Kamran et al. (2019) argued that decrease in the uptake of Cd in biochar and bacteria-amended soil might be due to the increased immobilization of Cd. According to Hamid et al. (2018), when the soil was amended with biochar in sole or combination with bacteria, the bioaccumulation of Cd in rice shoots was significantly reduced by 67% and 72%, respectively. Similarly, the biochar sole or in combination with bacteria, the Pb concentration in the shoot of rice plant decreased by 90% by biochar and by 89% for bacteria. These results revealed that integrated source of organic amendments with micro-organisms such as metals tolerance bacteria in reducing Cd and Pb bioaccumulation in rice, which may be owing to improved immobilization by biochar treatment and changes in soil HMs mobility.
An efficient and developing method for the long-term restoration of polluted soil is the combination of biochar and bioremediation using functional bacterial or fungal strains. Numerous microbial strains with significant metal tolerance or adsorption capacity were discovered and used as microbial agents in the soil to remove heavy metals, either by direct inoculation of free-living cells or by immobilizing cells with a specific carrier material. Biochar, activated sludge, zeolite, and diatomite are examples of natural materials that can be used as carriers for microbial cell immobilization. Synthetic macromolecular materials can also be used as carriers, such as polyvinyl alcohol, polyurethane, and acrylamide. Artificial inorganic materials can also be used as carriers, such as porous ceramics and activated carbon.
The Cd availability in biochar treatments may be reduced due to sorption, complexation and precipitation (Bolan et al. 2003; Beesley et al. 2010; Zhang et al. 2013). Zheng et al. (2012) reported that 5% straw biochar additions caused the greatest decrease in Cd and Pb concentrations in rice roots. Park et al. (2011) reported that poultry manure biochar (PMB) and green waste biochar (GWB) significantly reduced the Cd and Pb concentration in Indian mustard roots. The Cd concentration of roots was reduced by 53, 67 and 69% for PMB, and 28, 54 and 65% for GWB with 1, 5 and 15% of the application, respectively, compared to the control. Similarly, the effect of biochar addition on HMs concentration was more noticeable in the Pb case, showing 60, 84 and 88% reduced by PMB addition and GWB reduced Pb concentration in roots by 14, 29, and 63%, with 1, 5 and 15% application compared to control. Huang et al. (2016) reported that different types of bacteria significantly immobilize Cd in roots of green peas. The higher pH of biochar could be due to the separation of alkali salts from organic compounds during pyrolysis (Irfan et al. 2016). In 50 days incubation experiment, Shah et al. (2017) reported that biochar application caused a considerable increase in soil pH after its application. Yuan et al. (2011) reported that biochar made from leguminous plant materials amplified soil pH compared to biochar made from non-leguminous plant materials. The soil pH may increase or decrease by using biochar, mainly depending on the salt contents of applied biochar. The results agree with previous scholarly work that biochar increased soil electrical conductivity (EC) and pH (Liang et al. 2006; Warnock et al. 2007). The increase in soil EC with biochar amendments could be due to salts in biochar (Shah et al. 2017). It is also imperative to know the EC value of biochar before applying it to cropland to avoid creating a soil salt problem, which would adversely affect plant growth. The EC value of Conocarpus biochar produced at 600°C was 9.03 dSm−1; when applied to the soil, it may increase soil salinity and subsequently provide undesirable impacts on plant growth (Al-Wabel et al. 2013). Rashid et al. (2016) noted that using bacteria in combination with organic fertilizer (biochar) or without in combination significantly restored the fertility of soil and OM content than the sole use of bacteria. Biochar application increased SOM, phosphorus, potassium, sulfur 355 contents, and wheat’s shoot and root biomass (Bista et al. 2019). Biochar is a key source of many nutrients; its complex reaction with soil releases nutrients, making them available for plant uptake over time (Biederman and Harpole 2013; Lehmann et al. 2015). Biochar made from wheat straw can increase the content of soil total nitrogen (Feng et al. 2017). Biochar has the potential that can improve N recycling in agricultural soil-plant system (Gul et al. 2016). Previousstudies showed that N2O emission was reduced (Case et al. 2012), decreased nitrogen leaching (Güereña et al. 2013) and accessibility of soil nitrogen, built crop efficiency, and promoted the activity of soil microorganisms (Kim et al. 2014) by the application of biochar. Nelson et al. (2016) reported that N cycling was much promted by microorganisms activities. The total nitrogen was further increased when biochar was applied in combination with bacterial inoculation, which was 0.64% compared to control. Rashid et al. (2016) suggested that bacteria and fungi produce organic acids and siderophores; alternatively, they promote nutrient bioavailability, including N fixation, P and K mobilization. Chaer et al. (2011) further suggested that bacteria and organic amendments (biochar) might be a possible alternative use in integrated nutrient management approach for degraded soils. Also, Ahemad et al. (2014) and Owen et al. (2015) stated that introducing this inoculant can exploit, translocate, mineralize, and mobilize soil P, K reserves, increase soil organic matter or fix nitrogen from the atmosphere. Bacteria release various types of organic acids to solubilize K in soil through various processes such as acidolysis, chelation, complexolysis, and exchange reactions (Rashid et al. 2016). Biochar application increased soil organic matter (SOM), soil pH, phosphorus (P), potassium (K), sulfur (S) contents, shoot and root biomass of wheat (Bista et al. 2019). There is a synergistic impact between both, as evidenced by the fact that applying bacteria and biochar together generally had superior heavy metal remediation outcomes than applying them alone. According to this study, biochar combined with bacteria that immobilize heavy metals is a viable in situ technique for remediating soil that has been contaminated by heavy metals.
Conclusions
The inoculation of metals tolerant bacteria enhanced the efficiency of biochar to stabilize cadmium and lead in soil. The concentration of cadmium and lead in both shoot and root were significantly decreased by the application with bacteria inoculation as compared to the sole application of biochar. Plant growth was much improved by combining the application of biochar and bacteria as compared to the sole application of biochar. The availability of nutrients such as N, P, K, and SOM were enhanced by bacteria inoculation with BC as compared to the sole application of biochar. The inoculation of heavy metal tolerant bacteria with biochar is recommended to enhance the remediating efficiency of biochar. Field experiments on contaminated soil are needed to explore the potential of metal tolerant bacteria combined with biochar.
References
Abbas S, Ahmed I, Iida T, Lee YJ, Busse HJ, Fujiwara T, Ohkuma M (2015) A heavy-metal tolerant novel bacterium, Alcaligenes pakistanensis sp. nov., isolated from industrial effluent in Pakistan. Antonie Van Leeuwenhoek 108(4):859–870
Abbas T, Rizwan M, Ali S, Zia-ur-Rehman M, Qayyum MF, Abbas F, Hannan F, Rinklebe J, Ok YS (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Saf 140:37–47
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J King Saud Univ Sci 26(1):1–20
Al-Wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman AR (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour Technol 131:374–379
Ali MA, Naveed M, Mustafa A, Abbas A (2017) The good, the bad and the ugly of rhizosphere microbiome. In: Probiotics and plant health. Springer, Singapore, pp 253–290
Ali S, Duan J, Charles TC, Glick BR (2013) A bioinformatics approach to the determination of genes involved in endophytic behavior in Burkholderia spp. Theor Biol 343:193–198
Alkorta I, Hernandez-Allica J, Becerril JM, Amezaga I, Albizu I, Garbisu C (2004) Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Environ Sci Bio Technol 3:71–90
AOAC (1990) AOAC official methods of analysis, 15th edn. Association of Official Analytical Chemists, Arlington
Arif M, Jalal F, Jan MT, Muhammad D, Quilliam RS (2015) Incorporation of biochar and legumes into the summer gap: improving productivity of cereal-based cropping systems in Pakistan. Agroecol Sustain Food Syst 39(4):391–398
Arifin A, Parisa A, Hazandy AH, Mahmud TM, Junejo N, Fatemeh A, Majid NM (2012) Evaluation of cadmium bioaccumulation and translocation by Hopea odorata grown in a contaminated soil. Afr J Biotechnol 11(29):7472–7482
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158(6):2282–2287
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. Glob Change Biol Bioenergy 5:202–214
Bista P, Ghimire R, Machado S, Pritchett L (2019) Biochar effects on soil properties and wheat biomass vary with fertility management. Agronomy 9(10):623
Bolan N, Adriano D, Mani S, Khan A (2003) Adsorption, complexation, and phytoavailability of copper as influenced by organic manure. Environ Toxicol Chem 22(2):450–456
Bremmer JM, Mulvaney CS (1996) Kjeldhal Method. In method of soil analysis part-2: Chemical and microbiological properties. Amer Soc Agron, Madison, pp 903–948
Case SD, McNamara NP, Reay DS, Whitaker J (2012) The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil—the role of soil aeration. Soil Biol Biochem 51:125–134
Chaer GM, Resende AS, Campello EF, de Faria SM, Boddey RM (2011) Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol 31(2):139–149
Choppala GK, Bolan NZ, Chung JW, Park JH Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Clemens S, Palmgren MG, Krämer UA (2009) Long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7(7):309–315
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10(4):471–477
Du RY, Wen D, Zhao PH, Chen Y, Wang FH (2016) Effect of bacterial application on metal availability and plant growth in farmland-contaminated soils. J Bioremediat Biodegrad 7(02):341
Egamberdieva D, Shurigin V, Alaylar B, Ma H, Müller ME, Wirth S, Reckling M, Bellingrath-Kimura SD (2020) The effect of biochars and endophytic bacteria on growth and root rot disease incidence of Fusarium infested narrow-leafed lupin (Lupinus angustifolius L.). Microorganisms 8(4):496
Erdem H, Kınay A, Gunal E, Yaban H, Tutus Y (2017) The effects of biochar application on cadmium uptake of tobacco. Carpathian J Earth Environ Sci 12(2):447–456
Fellet G, Marmiroli M, Marchiol L (2014) Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci Total Environ 468:598–608
Feng CY, Meng J, Wang QX, Zhang WM, Cheng X, Chen WF (2017) Effects of straw and biochar addition on soil nitrogen, carbon, and super rice yield in cold waterlogged paddy soils of North China Cui. J. Integrat Agric 16(5):1064–1074
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35(4):219–230
Güereña D, Lehmann J, Hanley K, Enders A, Hyland C, Riha S (2013) Nitrogen dynamics following field application of biochar in a temperate North American maize-based production system. Plant Soil 365(1):239–254
Gul S, Whalen JK (2016) Biochemical cycling of nitrogen and phosphorus in biochar amended soils. Soil Biol Biochem 103:1–5
Hamid Y, Tang L, Wang X, Hussain B, Yaseen M, Aziz MZ, Yang X (2018) Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci Rep 8(1):1–0
Hossain MK, Strezov V, Chan KY, Nelson PF (2010) Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 78(9):1167–1171
Houben D, Evrard L, Sonnet P (2013) Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 57:196–204
Huang J, Liu Z, Li S, Xu B, Gong Y, Yang Y, Sun H (2016) Isolation and engineering of plant growth promoting rhizobacteria Pseudomonas aeruginosa for enhanced cadmium bioremediation. J Gen Appl Microbiol. https://doi.org/10.2323/jgam.2016.04.007
Huang SW, Lin YY, You EM, Liu TT, Shu HY, Wu KM, Tsai SF, Lo CF, Kou GH, Ma GC, Chen M (2011) Fosmid library end sequencing reveals a rarely known genome structure of marine shrimp Penaeus monodon. BMC Genomics 12(1):1–9
Irfan M, Chen Q, Yue Y, Pang R, Lin Q, Zhao X, Chen H (2016) Co-production of biochar, bio-oil and syngas from halophyte grass (Achnatherum splendens L.) under three different pyrolysis temperatures. Bioresour Technol 211:457–463
Irfan M, Ishaq F, Muhammad D, Khan MJ, Mian IA, Dawar KM, Muhammad A, Ahmad M, Anwar S, Ali S, Khan FU (2021) Effect of wheat straw derived biochar on the bioavailability of Pb, Cd and Cr using maize as test crop. J Saud Chem Soc 25(5):101232
Jiang CY, Sheng XF, Qian M, Wang QY (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72(2):157–164
Kader NA, Shahin RR, Khater HA (2013) Assessment of heavy metals immobilization in artificially contaminated soil using some local amendments. Open J Metal 3:68–76
Kamran M, Malik Z, Parveen A, Zong Y, Abbasi GH, Rafiq MT, Shaaban M, Mustafa A, Bashir S, Rafay M, Mehmood S (2019) Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J Environ Manag 250:109500
Khan K, Lu Y, Khan H, Ishtiaq M, Khan S, Waqas M, Wei L, Wang T (2013) Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem Toxicol 58:449–458
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152(3):686–692
Khan S, Rehman S, Khan AZ, Khan MA, Shah MT (2010) Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol Environ Saf 73(7):1820–1827
Kim P, Hensley D, Labbé N (2014) Nutrient release from switchgrass-derived biochar pellets embedded with fertilizers. Geoderma 232:341–351
Lee SS, Lim JE, Abd El-Azeem SA, Choi B, Oh SE, Moon DH, Ok YS (2013) Heavy metal immobilization in soil near abandoned mines using eggshell waste and rapeseed residue. Environ Sci Pollut Res 20(3):1719–1726
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Change 11(2):403–427
Lehmann J, Kuzyakov Y, Pan G, Ok YS (2015) Biochar and the plant-soil interface. Plant Soil 395:1–5
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill BJ, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70(5):1719–1730
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194:495–503
Lua K, Yang X, Shen J, Robinson B, Huang H, Liu D, Bolan N, Pei J, Wanga H (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric Ecosyst Environ 191:124–132
McLean EO (1983) Soil pH and lime requirement. In: Methods of soil analysis: Part 2 Chemical and microbiological properties, pp 199–224
Memon AR, Schröder P (2009) Metal Accumulation in Plants and Its Implication in Phytoremediation. Environ Sci Pollut 16(2):162–175
Muhammad N, Nafees M, Khan MH, Ge L, Lisak G (2020) Effect of biochars on bioaccumulation and human health risks of potentially toxic elements in wheat (Triticum aestivum L.) cultivated on industrially contaminated soil. Environ Pollut 260:113887
Muhammad S, Shah MT, Khan S (2011) Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Micro Chem J 98:334–343
Mustafa A, Naveed M, Saeed Q, Ashraf MN, Hussain A, Abbas T, Kamran M, Minggang X (2019) Application potentials of plant growth promoting rhizobacteria and fungi as an alternative to conventional weed control methods. Sustain Crop Prod 1:1–23
Naveed M, Mitter B, Yousaf S, Pastar M, Afzal M, Sessitsch A (2014) The endophyte Enterobacter sp. FD17: a maize growth enhancerselected based on rigorous testing of plant beneficial traits andcolonization characteristics. Biol Fertil Soils 50(2):249–262
Naveed M, Mustafa A, Majeed S, Naseem Z, Saeed Q, Khan A, Nawaz A, Baig KS, Chen JT (2020) Enhancing cadmium tolerance and pea plant health through Enterobacter sp. MN17 inoculation together with biochar and gravel sand. Plants 9(4):530
Nelson DW, Sommer LE (1996) Total C, organic C and organic matter. In: spark DL (ed) Method of soil analysis part 3. Am. Soc. Agron. 34, pp 961–1010
Nelson MB, Martiny AC, Martiny JB (2016) Global biogeography of microbial nitrogen cycling traits in soil. Proc Natl Acad Sci 113(29):8033–8040
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14(12):1504
Owen D, Williams AP, Griffith GW, Withers PJ (2015) Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl Soil Ecol 86:41–54
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348(1):439–451
Qadir M, Ghafoor A, Murtaza G, Murtaza G (2000) Cadmium concentration in vegetables grown on urban soils irrigated with untreated municipal sewage. Environ Dev Sustain 2(1):13–21
Radwan MA, Salama AK (2006) Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem Toxicol 44(8):1273–1278
Rajkumar M, Sandhya S, Prasad MN, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574
Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Ismail IM, Oves M (2016) Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res 183:26–41
Rehman MZ, Rizwan M, Ali S, Fatima N, Yousaf B, Naeem A, Sabir M, Ahmad HR, Ok YS (2016) Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol Environ Saf 133:218–225
Rehman MZU, Batool Z, Ayub MA, Hussaini KM, Murtaza G, Usman M, Naeem A, Khalid H, Rizwan M, Ali S (2020) Effect of acidified biochar on bioaccumulation of cadmium (Cd) and rice growth in contaminated soil. Environ Technol Innov 19:101015
Richard LA (1954) Diagnosis and improvement of saline and alkali soil. Agriculture Handbook No. 60. US Department of Agriculture, Washington, pp 1–160
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. Fems Microbiol Lett 278(1):1–9
Shah MT, Begum S, Khan S (2010) Pedo and biogeochemical studies of mafic and ultramfic rocks in the Mingora and Kabal areas, Swat, Pakistan. Environ Earth Sci 60(5):1091–1102
Shah T, Khan S, Shah Z (2017) Soil respiration, pH and EC as influenced by biochar. Soil Environ 36:77–83
Shen Z, Som AM, Wang F, Jin F, McMillan O, Al-Tabbaa A (2016) Long-term impact of biochar on the immobilisation of nickel (II) and zinc (II) and the revegetation of a contaminated site. Sci Total Environ 542:771–776
Shin HW, Sidharthan M, Young KS (2002) Forest fire ash impact on micro-and macroalgae in the receiving waters of the east coast of South Korea. Mar Pollut Bull 45(1–12):203–209
Soltanpour PN, Schwab AP (1977) Anew soil test for simultaneous extraction of macro and micronutrients in alkaline soils. Commun Soil Sci Plant Anal 8:195–207
Steel GR (1997) Principles and procedures of statistics of biometrical approach. Rep. No. 0070610282
Surette MA, Sturz AV, Lada RR, Nowak J (2003) Bacterial endophytes in processing carrots (Daucus carota L. var. sativus): their localization, population density, biodiversity and their effects on plant growth. Plant Soil 253(2):381–390
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300(1):9–20
Yamato M, Okimori Y, Wibowo IF, Anshiori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Yuan JH, Xu RK, Wang N, Li JY (2011) Amendment of acid soils with crop residues and biochars. Pedosphere 21(3):302–308
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20(12):8472–8483
Zheng RL, Cai C, Liang JH, Huang Q, Chen Z, Huang YZ, Arp HP, Sun GX (2012) The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 89(7):856–862
Zhu N, Qiang L, Guo X, Hui Z, Yu D (2014) Sequential extraction of anaerobic digestate sludge for the determination of partitioning of heavy metals. Ecotoxicol Environ Saf 102:18–24
Acknowledgements
The authors express their gratitude to the editorial board and reviewers for their suggestions and reviewing this paper which has greatly improved the overall quality of this paper. The authors also appreciate the editor for his cooperation during the review process.
Author information
Authors and Affiliations
Contributions
Manuscript writing and data analysis Usama Khan, Supervision and conceptualization Muhammad Irfan, Zaryab Murad Proofreading and reviewing Ijaz Ahmad, Muhammad Owais Khan, Imran Mehmood, Muhammad Waleed and Abid Kamal. All authors have read and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
U. Khan, M. Irfan, Z. Murad, I. Ahmad, M.O. Khan, I. Mehmood, M. Waleed and A. Kamal declare that they have no conflict of interests.
Additional information
Availability of data and materials
Data is available from the corresponding author with a formal request.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Khan, U., Irfan, M., Murad, Z. et al. Enhancing Lettuce Growth and Cadmium and Lead Tolerance Through Biochar and Bacteria. Gesunde Pflanzen 75, 2685–2696 (2023). https://doi.org/10.1007/s10343-023-00914-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-023-00914-4