Abstract
The quality of the Upper Cheliff groundwater, located in North West Algeria, has in recent years undergone serious deterioration due to uncontrolled discharge of urban wastewaters, intensive use of chemical fertilizers in agriculture as well as to overexploitation. This study aims at analyzing the flow pattern of the Upper Cheliff groundwater, determining its current hydrochemical status and understanding the mineralization processes involved in its chemical quality. Two piezometric and sampling campaigns were carried out in 2008 in high water (April) and low water (October) periods. The major chemical ions (Ca2+, Mg2+, Na+, K+, Cl−, HCO3 −, NO3 −, SO4 2−) were analyzed in all samples. The piezometric data were mapped and allowed to analyze the groundwater flow conditions, in particular at the boundaries of the aquifer. The interpretation of hydrochemical data was made using various methods (Piper diagram, Stabler classification, base exchanges index, bi-elements scatter diagrams, saturation indices, mapping and multivariate principal component analysis). The results provide a better understanding of this aquifer hydrogeology and hydrochemistry. Several hydrochemical types (chloride-calcium, chloride-sodium and bicarbonate-calcium) characterize the groundwater. Mineralization processes and the origin of salinity are determined by the lithology of the aquifer (dissolution, base exchanges), and by climatic (evaporation) and anthropogenic factors (agricultural and urban wastes). The groundwater in the Upper Cheliff is currently of poor quality. This status is worrying, as this groundwater is an important natural resource for the socio-economic development of this region. Urgent measures must be taken to preserve this resource.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is a significant and crucial resource in many countries, and it commonly plays a key role as a water supply both for drinking and irrigation. In the last decades, water demand has dramatically increased, especially in developing countries, driven by population growth, improvements in living standards, development of industry, agriculture and urbanization (World Water Assessment Programme 2009; Llamas and Martínez-Santos 2005). This has led to increasing pressures on groundwater resources. Excessive abstractions of groundwater over the past decades to meet these demands have resulted in serious troubles: water table decline, groundwater quality degradation and damage to ecosystems. It is evident that groundwater quality issue is as important as groundwater quantity for satisfying water needs (Karanth 1997; World Water Assessment Programme 2012; United Nations Environment Programme 2010). Poor groundwater quality may have a number of economic and social impacts (ecosystems degradation, health problems, treatment costs, impacts on agriculture, industry, tourism). This issue is thus becoming a global concern of increasing significance. Groundwater quality degradation risks are many and diverse. Untreated wastewaters of urban settlements and industries are main sources of groundwater point pollution. Diffuse pollution from agricultural land continues to be of critical concern throughout the world (Scanlon et al. 2007).
Sustainable groundwater resources management, paying due attention to quality, is thus vital (Narasimhan 2005; Esteller and Andreu 2004). Policy makers must make a concerted effort to better integrate issues of both groundwater quantity and quality in their decisions. In turn, the research community should provide them with consistent and credible water resources data and information to understand the groundwater systems and better quantify the problems. Without an appropriate level of knowledge of the problems at both quantity and quality levels, the impacts related to groundwater quality are expected to increase. Thus, understanding the groundwater characteristics and hydrochemistry is fundamental to determine the origin of chemical composition of groundwater and accordingly for sound groundwater management and decision making (Zaporozec 1972; Adams et al. 2000).
Groundwater plays a major role in many parts of Algeria both for drinking as well as irrigation purposes. However, groundwater resources are overexploited, vulnerable and exposed to various forms of pollution which may alter, sometimes irretrievably, their quality. The Upper Cheliff groundwater in North West Algeria has in recent years undergone serious deterioration due to uncontrolled discharge of urban wastewaters, intensive use of chemical fertilizers in agriculture, as well as due to overexploitation. These factors affect the chemistry of the groundwater and make it unsuitable for desired uses. So far the characteristics and geochemistry of the groundwater and its suitability to diverse purposes (drinking, agriculture) in the study area have not been addressed. Any attempt to sustainably manage this important resource faces this gap.
In this framework, the purpose of the present study is to determine the current hydrochemical status of the Upper Cheliff groundwater and understand the mineralization processes. Major chemical ions (Ca2+, Mg2+, Na+, K+, Cl−, HCO3 −, NO3 −, SO4 2−) were analyzed for this purpose. Samples were collected in 2008, during periods of high water (April) and low water (October).
The interpretation of hydrochemical data was made using various methods (Piper diagram, Stabler classification, base exchanges index, bi-elements scatter diagrams, saturation indices, mapping and multivariate principal component analysis).
Location of the study area
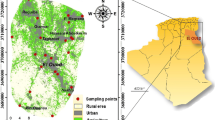
The study area corresponds to the Upper Cheliff basin, located 110 km South-West of Algiers, and is part of the Cheliff watershed (Fig. 1a). The Upper Cheliff plain is located between 36° 12′ and 36° 30′ North latitude and 02° 2′ and 2° 44′ East longitude. It is bordered to the North by the dolomitic limestone of Jebel Zaccar (1,578 m altitude) and the sandstones of Jebel Gantas, to the South by the first foothills of the clayey-marly and sandstone Ouarsenis massive. One enters the plain at the East by the Djendel threshold at 308 m a.s.l. (above sea level) and comes out through the West by the Doui threshold at 248 m a.s.l. (Mania and Djeda 1990).
This plain had an area of approximately 370 km2 and a population of 270,000 inhabitants in 2008. The groundwater resource is used to supply drinking water to cities (Djendel, Ain Sultan, Khemis-Miliana, Djelida Sidi Lakhdar and Arib), and also for industrial and irrigation usages.
The Plain of Upper Cheliff has an agricultural vocation requiring sprinkler irrigation due to a semi-arid continental climate with very dry summers and rainy winter causing sometimes dramatic floods of the wadi Cheliff. Interannual average temperature ranges from 13 to 19 °C, with a monthly maximum of more than 30 °C recorded in July. The construction of dams (Ghrib, Deurdeur, Harreza and Sidi Mhamed Ben Taiba) has regulated the wadis flows and provides irrigation water from April to September.
Interannual average rainfall varies between 200 and 700 mm. It is more concentrated in altitudes, about 700 mm recorded on the southern slopes of Zaccar and 500 mm on the northern slopes of the Ouarsenis. Across the Upper Cheliff basin, annual potential evapotranspiration ranges from 1,200 to 1,500 mm according to the map of potential evapotranspiration in northern Algeria (ANRH Agence Nationale des Ressources Hydrauliques 2004).
Geological setting
The Upper Cheliff plain is a large subsidence depressed zone of East–West axis where Miocene, Pliocene and Quaternary sediments accumulated (Fig. 1b). The stratigraphy of the formations from bottom to top is the following. The Primary consists of alternating black schists and quartzites beds and clays. It is surmounted by the Triassic which generally consists of dolomites and dolomitic limestones, exposed in the Doui and Zaccar massives (Mattauer 1958). The Jurassic in the Zaccar massive is formed by compact, fractured and karstified limestones, topped by sandstone schists and calcareous marls. The entire series reaches a thickness of about 700 m. In Jebel Doui, Jurassic is represented primarily by dolomitic limestones with a thickness around 80 m (Mattauer 1958; Meghraoui et al. 1986). Cretaceous outcrops on the side borders of the plain. It is represented by schistic clays, with a thickness of about 800 m to the North and West of Zaccar and marls with interbedded limestone beds in the Dahra massive. Miocene is up to 300 m thick. The Lower Miocene discords on the ante-Neogene bedrock and begins with a conglomerate series of about 220 m thick, then it ends with a marl series. The Miocene is marked by a new and progressive transgression. It begins with a blue marl series visible mainly in the North East of the plain interbedded with clays and small sandy beds (Boulaine 1957; Perrodon 1957). Rather coarse red sandstone and intercalated conglomerate beds appear quite frequently in the Gantas and terminate the Miocene cycle. The Mio-Pliocene consists of quartz pebbles, conglomerates, sandstones and detrital clays, and travertine deposited at the Zaccar springs.
The lower marine Pliocene begins with sandstone detrital levels, and sometimes sandy conglomerate with gradual sandy marls and clayey sands shift. The sandstones, with a thickness of a hundred meters, are a continuous layer from the southern slopes of Dahra. The sandstones often have a calcareous cement, and locally shift to calcareous sandstones. The Upper Pliocene is composed of conglomerate with sandstone and limestone elements and unconsolidated sands. Sometimes the Upper Pliocene conglomerates do not exist. To the South of Cheliff, at the border of Ouarsenis, the Pliocene disappears completely. At this level, the Quaternary sandstones directly overlie the Miocene. The old Quaternary is represented by alluvial conglomerate observed at the foot of the Zaccar, particularly in the east of Sidi Lakhdar. They form the vast hills on the southern border of the plain of Upper Cheliff. The recent Quaternary is formed by silt which thickness varies from 50 to 200 m.
Hydrogeology
The main aquifer in the plain of Upper Cheliff is formed by alluvial formations. This aquifer is characterized mainly by coarse alluvia silt and pebbles in the center of the valley with a thickness of 50–145 m. A layer of clay and silt covers the coarse alluvia to the south-west with a thickness of 7–20 m. This aquifer overlies the Mio-Pliocene sandstones, which are observed at the East of the plain at Jebel Gantas and can reach 200 m thick in the North.
The hydraulic continuity between these two formations may be locally disturbed by clay lenses. However, hydraulic heads of both Miocene sandstones and Quaternary alluvium reservoirs are identical. Thus, all two formations have been considered as a single aquifer system.
As groundwater is the mean for mineral and organic substances underground transport, determining its flow pattern provides information about its mineralization processes and origin of pollution. The piezometric map is illustrated on the basis of head data of two campaigns in 2008. The piezometric map surveyed in high water (Fig. 2a) shows that groundwater flows from the North and South of Upper Cheliff basin to the main East–West drainage axis, which coincides with the course of Wadi Cheliff. Groundwater generally supplies wadi Cheliff, in particular, over the downstream half of the plain where the wadi is in direct contact with the coarse alluvium.
At the North West and South borders, impervious Cretaceous schists constitute no-flow limits. However, the contour lines show probable infiltration from the Deurdeur massive and Harreza tributaries. The sandstone foothills of Zaccar in the North, which form the contact between the Zaccar limestones and the alluvial aquifer, supply the former.
To the North East, the piezometric contour lines are perpendicular to the Gantas massive, indicating a no-flow border. High hydraulic gradients of the order of 2 × 10−2 are found to the East on the foothills of the Gantas massive near Djendel and to the South-West near Djelida on the foothills of the Doui massive, because of reduced permeability. Low hydraulic gradients from 1.3 × 10−3 to 1.8 × 10−3 (between 260 and 280 m piezometric lines) are observed in the central part of the plain. These low values are related to the formation of gravel and pebbles dominant in this area and indicate better permeability of the aquifer.
The piezometric map of the low water period (not reported) has the same morphology as the high water map. However, some depressions are observed in the central and the North-western areas of the plain due to the intensive pumpings of groundwater for irrigation.
The depth of the water table from the soil surface varies between 2 m to the West and 30 m to the East. In the central part, the average depth is about 10 m. Given the semi-arid climate, the effect of evaporation is thus quite sensitive on the chemical quality of groundwater (Fig. 2b).
Materials and methods
Sampling and analytical procedure
A sampling network was set up to allow collection of representative data of the variability in space and time of the groundwater quality. This network consisted of 28 wells during high water period and 22 wells in low water period and covers the whole plain from East to West (Fig. 2b).
Water samples were collected by the National Agency of Hydraulic Resources (ANRH) in wells that form the observation network. Two surveys were conducted in 2008 during April (high water) and June (low water). Groundwater samples were collected after pumping the wells for a minimum time of 15 min. Samples were subsequently filtered and collected in polyethylene bottles. The samples were then analyzed in the laboratory of ANRH. The analyses focused on the most common and most abundant ions in groundwater. The analyzed parameters included four cations (Ca2+, Mg2+, Na+, K+) and four anions (Cl−, HCO3 −, NO3 −, SO4 2−), with TDS (Total Dissolved Solids). Temperature and pH were measured in the field.
Filtered and acidified (1 % v/v HNO3) samples were analyzed for major cations (calcium Ca2+, magnesium Mg2+, sodium Na+, potassium K+) by Atomic absorption spectroscopy (AAS). The methods used for anions analyses are the following: the mercuric thiocyanate method for chloride (Cl−), the turbidimetric method for sulfate (SO4 2−), the PDA (Phenol disulfonic acid) colorimetric method for nitrate (NO3 −). These methods conform to the US EPA-approved procedures (EPA 1983). The bicarbonate (HCO3 −) was determined by potentiometric method (Rodier et al. 2009).
The ion-balance-error computation, taking the relationship between the total cations (Ca2+, Mg2+, Na+, K+) and the total anions (NO3 −, SO4 2−, HCO3 − and Cl−) for each water sample, is observed to be within the range of acceptability (± 5 %) used in most laboratories (Domenico and Schwartz 1990), for 49 water samples, i.e., 98 % of the samples. The hydrochemical data are given in Table 1.
Principal components analysis
A Principal Components Analysis (PCA) was performed on the chemical data for better understanding of the groundwater hydrochemistry. Principal Component Analysis is useful for reducing and interpreting large multivariate data sets with underlying linear structures, and for discovering previously unsuspected relationships. Multivariate techniques have been applied to groundwater hydrochemistry by several authors and proved very efficient to understand a number of geochemical processes (Dawdy and Feth 1967; Hitchon et al. 1971; Ashley and Lloyd 1978; Lawrence and Upchurch 1976, 1983; Seyhan et al. 1985; Usunoff and Guzman 1989; Razack and Dazy 1990; Subbarao et al. 1996; Jayakumar and Siraz 1997; Jayaprakash et al. 2008; Abderamane et al. 2012; Hussein 2004; Yitbarek et al. 2012).
The software used to perform PCA is XLSTAT (version 7.5.2.), which is an add-on to Microsoft EXCEL to perform multivariate statistical analysis. The extraction method used in this study, implemented in XLSTAT, is known as ‘Principal Component method’ and looks for a solution that maximizes the explained variance with orthogonal components, i.e., independent of each other. The Varimax orthogonal rotation method (Kaiser 1958; Davis 2002) was used to maximize variance of loadings on each component. Each component is then explained by few variables.
The methods to help to choose the number of components are based on relations between the eigenvalues. According to the Kaiser criteria, eigenvalues larger than one (Harman 1960), explaining more variance than the average component, should be kept. An additional graphical method can also be used, the Scree diagram (or the elbow criterion). In this diagram, the eigen values are plotted vs. the number of the components. If the points on the graph tend to level out (show an “elbow”), these eigenvalues are usually close enough to zero that they can be ignored.
Saturation indice (SI)
The saturation indice (SI) was also evaluated to interpret groundwater hydrochemistry, using the software PHREEQC V2 (Parkhurst and Appelo 1999). PHREEQC can be used via the freeware DIAGRAMMES (Simler 2009). The thermodynamic database used to this purpose is Wateq.dat (Hounslow 1995). The saturation indices of minerals that were suspected to be responsible for the chemical composition of the Upper Cheliff groundwater were computed.
PHREEQC uses the specific ionic concentrations in the water and the mass balance approach to calculate all the stoichiometrically available reactions that are responsible for the observed chemical changes between end member waters (Plummer and Back 1980). The package calculates the saturation indices, SI, of minerals using the concentrations of the major ions in the system. The saturation index of a mineral is obtained from Eq. (1) (Appelo and Postma 1993; Yidana et al. 2008).
where IAP is the ion activity product of the chemical element in solution, K T is the equilibrium constant of the reaction considered at the temperature T(K). When the SI is below 0, the water is undersaturated with respect to the mineral in question. An SI of 0 means water is in equilibrium with the mineral, whereas an SI greater than 0 means a supersaturated solution with respect to the mineral in question.
Major ions chemistry
Table 2 shows the summary statistics for each water quality parameter for both high and low water periods. The average pH is 7.4 in high water and 8.2 in low water periods, which indicates that the Upper Cheliff groundwater is slightly alkaline. The average groundwater temperature is 21.0 °C in high water and 19.6 °C in low water. The TDS ranges from 723 to 4,064 mg/l with a mean of 1,870 mg/l in high water, and ranges from 740 to 3,230 mg/l with a mean of 1,953 mg/l in low water. The groundwater is slightly more mineralized in low water pointing out the effect of evaporation processes on water quality.
The variability of the ions contents, expressed using the coefficient of variation, is significantly higher during high waters. NO3 −, Cl−, Mg2+, and SO4 2− ions show the highest variability. Chloride concentrations range from 140 mg/l to 1,790 mg/l, sulfate from 61 to 568 mg/l, bicarbonate from 146 to 438 mg/l, calcium from 53 to 437 mg/l, magnesium from 19 to 222 mg/l, sodium from 66 to 582 mg/l and nitrate from 0 to 190 mg/l. The relative concentrations of the cations occur in the order of Na+, Ca2+, Mg2+ and of the anions in the order of Cl−, HCO3 −, SO4 2−, NO3 −.
Pearson’s correlation matrix (Swan and Sandilands 1995) was used to find relationships between two or more elements. The correlation matrix is shown in Table 3. TDS is strongly correlated with calcium (R = 0.78), magnesium (R = 0.84), sodium (R = 0.79), chloride (R = 0.93), and sulfate (R = 0.73). These relationships (Fig. 3) clearly identify the main elements contributing to the groundwater salinity and their tendency to follow a similar trend (e.g., due to concentration by evaporation).These element concentrations tend to increase as the salinity of the groundwater increases. The salinization of the groundwater would be expected to result from the increase in ionic concentrations as well as evaporation of recharge water and the effects of interactions between the groundwater and the geological formations.
The strongest correlations between elements of opposite sign combine Cl and Na+ (R = 0.82), Cl− and Mg2+ (R = 0.81), and Cl− and Ca2+ (R = 0.72). The Na+–Cl− relationship suggests dissolution of halite. The dissolution of halite in water releases equal concentrations of sodium and chloride into the solution:
The strong relationships Cl−–Mg2+ and Cl−–Ca2+ suggest that cation exchange can also significantly affect groundwater composition. The positive and significant correlations between sulfate and calcium (R = 0.53), sulfate and sodium (R = 0.66) and sulfate and magnesium (R = 0.59) indicate the contributions of evaporitic salts. An important evaporite present in the Upper Cheliff plain is gypsum (CaSO4·2H2O). The dissolution reaction for gypsum releases calcium and sulfate:
However, human activity related to agriculture may also contribute to these elements. In the study plain, farmers widely use such fertilizers based on potassium sulfate, ammonium sulfate and the sulfo-phosphate ammonium (Achour and Bouzelboudjen 1998; Gouaidia et al. 2011). Other associations between ions are further interpreted in terms of processes and origin of the mineralization of the groundwater and its evolution.
In recent times, multivariate methods have been widely used to study the sources of variation of groundwater chemistry. Factor analysis, as a multivariate statistical tool, reduces a large data set into a set of variables that represent the geochemistry without sacrificing much of the original information. In this study, a Principal Components Analysis (PCA) was performed for a better understanding of the groundwater hydrochemistry. The principles of this method have been presented earlier in a previous section. The data consist of 8 variables (Ca2+, Mg2+, Na+ + K+, Cl−, SO4 2−, HCO3 −, NO3 − and TDS) analyzed on all 49 samples collected during both campaigns of high and low waters in 2008.
Eigen values, percentage of the variance of each principal component (PC) and cumulative percentage of variance of the eight PCs are given in Table 4. The scree plot is shown in Fig. 4. This figure indicates, according to the elbow and Kaiser criteria, that the first two PCs should be kept for further consideration. They account for 74.1 % of the variance and are assumed to provide an adequate representation of the overall variance of the data set. Hence, in the factor matrix, only these two factors are considered.
To enhance the PC extraction, a Varimax rotation was performed. PC loading, communalities for each variable, percentage of the variance of each PC and cumulative percentage of variance of the two PCs are given in Table 5.
The 1st PC (Principal Component) accounts for 54.9 % of the variance in the data set. PC1 is interpreted as relating mainly to the mineralization of the groundwater as it is associated with Cl−, SO4 2−, Ca2+, Mg2+, Na+ + K+ and TDS. Loadings for these elements are high. PC1, therefore, opposes highly mineralized samples to weakly mineralized samples. PC2 accounts for 19.2 % of the data variance. It opposes HCO3 −–NO3 −. These two elements have the highest loadings on this factor. Possible pollution of the groundwater could be related to PC2, given its association with NO3. The plane associated with PC1 and PC2 accounts thus for 74.1 % of the total variance and is accordingly quite representative of the initial data variability. Figure 5 shows the plot of the samples on this plane. PC1-PC2 plane discriminates several groups of samples. It discriminates weakly mineralized waters lying North-West and West of the aquifer, on the borders of the Doui and Zaccar massives, highly mineralized waters located to the South of the groundwater on the left bank of wadi Cheliff, waters marked in bicarbonates located on the right bank of the wadi Cheliff, North of the groundwater near the borders of the Zaccar massive, and waters heavily loaded with nitrates located in the East and South of the groundwater close to the cities of Djendel and Ouled Khelifa.
Spatial distribution of the main elements and of TDS
Figure 6 shows the spatial distribution of the main elements in the groundwater. The spatial distribution of concentrations depends on several factors, such as lithology, the hydrodynamics of the water, the depth of the water table, climate conditions and urban and/or agricultural pollution sources. Figure 6 shows that Cl−, SO4 2−, Ca2+ and Na+ display relatively equivalent spatial pattern. The highest concentrations of these elements are located South of the plain, on the left bank of the wadi Cheliff, near the towns of Djelida and Ouled Khelifa.
Wastewater from the cities in the plain are not currently treated and are discharged into the environment as such. The total discharge is estimated at about 30,000 m3/d. The location of high concentrations may be associated with some extent with these spills. This is discussed in more detail later.
The distribution of nitrate is very different. The highest concentrations are located to the East near Djendel and to the South near Wadi Khelifa. These very high nitrate contents are due to the activity of orchards occupying large parts of the area. These lands receive overuse input of fertilizers, pesticides and manure (Bettahar et al. 2009).
The spatial distribution of TDS in high and low water is reported in Fig. 7. In the plain, TDS ranges from 723 to 4,064 mg/l. The high values are observed along the wadi Cheliff and to the South with a maximum recorded at Ouled Khelifa and Djelida. The low values are located in the North West Arib area during low water. The spatial distribution of TDS is quite similar to that of the chloride, sulfate, calcium and sodium. This corroborates the hypothesis that the main contributors to the groundwater salinity are chloride, sulfate, calcium and sodium.
Main hydrochemical facies
To properly identify the hydrochemical facies and to yield an indication of the qualitative aspects of groundwater, the graphical representation of the results of analysis proves an unavoidable tool. To achieve this goal, the hydrochemical data were processed using the Piper diagram (Piper 1944) and Stabler classification with the use of the software Diagrammes (Simler 2009). Figure 8 shows the Piper plot of samples taken during high water period. This diagram clearly shows that all samples have a dominance of chloride and nitrate ions for anions, while calcium is the cation which marks the majority of samples and is followed by the sodium. This reveals the dominance of chloride-calcium facies, and the secondary chloride-sodium facies. However, this representation has the disadvantage of involving chlorides with nitrates. This can lead to misinterpretation. In addition to the Piper diagram, the hydrochemical data have been processed using the method of classification of Stabler. Stabler classification compares reaction quantities of cations and anions expressed as percentages (%), and separately classifies the anions and cations in descending order to determine the chemical facies.
Figure 9 shows that the most common chemical facies are the chloride-calcium type (14 samples out of 28 or 50 % in period of high water and 14 samples out of 22 or 64 % in period of low water), followed by chloride-sodium facies (10 samples out of 28 or 36 % in high water and 6 samples of 22 or 27 % in low water). The bicarbonate-calcium facies represents 11 % (3 samples) and 9 % (2 samples), respectively, in high and low water. Finally, the presence of a chloride-magnesium facies is noted for a single sample in high water.
The chloride-calcium facies is the most dominant and spreads in the North East and West of the plain. It can be bound to the presence of Mio-Plio-Quaternary alluvial formations and gypsiferous marls associated with a process of inverse cation exchange. The chloride-sodium facies develops South of the plain; this is probably due to the presence of recent alluvium of fine texture, while the bicarbonate-calcium facies is localized in the North West and has its origin in carbonate formations bordering the groundwater, following the dissociation reaction:
The Stabler classification, based on the reaction quantities, proved useful as a complementary method to the Piper diagram.
Binary diagrams and mineralization process
Dissolved species and their relationship with each other can reveal the origin of solutes and the processes that generated observed composition of the groundwater (Hussein 2004; Gupta et al. 2008; Sujatha and Reddy 2003; Aboubaker et al. 2013; Moussa et al. 2008; Kuldip et al. 2011; Yuce 2007; Jalali 2009; Nandimandalam 2011; Diaw et al. 2012). The relationships between concentrations of major dissolved elements are shown in Fig. 10. The Cl− vs. Na+ relationship has been often used to identify the mechanism for acquiring salinity. The Na+ and Cl− show a good correlation (R = 0.82) indicating that Na+ and for the most part Cl− are derived from the dissolution of disseminated halite in fine-grained sediments. A noticeable feature of the groundwater in the Upper Cheliff is, however, the enrichment in Cl− relative to Na+ (Fig. 10d). The excess of Cl− can be explained by the combined effect of another source for this ion than the dissolution of halite and the Na+ losses due to the phenomenon of base exchange, as clays bedrock can release Ca2+ after setting the Na+. The excess of Cl− may also have an anthropogenic origin. Indeed, as stated above, urban wastewater are discharged untreated in the environment and can reach groundwater by infiltration. On the other hand, as the region is agricultural, fertilizers can also contribute to Cl−.
HCO3 − is poorly correlated with Ca2+ (R = 0.10; Fig. 10b) and Mg2+ (R = 0.13; Fig. 10f) indicating that dissolution of carbonate rocks (calcite, dolomite) is not the only source for these elements. Correlation of SO4 2− with these two elements is significant (respectively, R = 0.53 and R = 0.59; Fig. 10a, e) and shows that these elements (Ca2+, Mg2+, SO42−) are partly derived by the dissolution of gypsum and a Mg-sulfate mineral.
The plots of Ca2+ vs. SO42− (Fig. 10a), Ca2+ vs. HCO3 − (Fig. 10b) and Ca2+ vs. Mg2+ (Fig. 10c) display, however, a substantial excess of Ca2+, showing that the origin of Ca2+ is not the only dissolution of calcite and gypsum. This is consistent with the hypothesis of a contribution of Ca2+ by ion exchange reaction via a basic reaction, such as:
Na+ can exchange Ca2+ and Mg2+ sorbed on the exchangeable sites of the clay minerals, resulting in the increase of Ca2+ and Mg2+ and the decrease of Na+ in groundwater. During this process, the host rocks are the primary sources of dissolved solids in the water. Groundwater in which the alkaline earths (Ca2+, Mg2+) have been exchanged for the Na+ ions is referred to as base exchange-hardened water (Gupta et al. 2008).
Knowledge of the changes undergone by the chemical composition of the groundwater during its travel in the aquifer is essential. The ion exchange between the groundwater and its host aquifer during travel can be understood analyzing the plot of Ca2+ + Mg2+ versus SO4 2− + HCO3 −. In a Ca2+ + Mg2+ versus SO4 2− + HCO3 − scatter diagram, the points falling along the equiline (Ca2+ + Mg2+ = SO4 2− + HCO3 −) suggest that these ions have resulted from the dissolutions of calcite, dolomite and gypsum (Datta and Tyagi 1996; Rajmohan and Elango 2004; Cerling et al. 1989; Fisher and Mulican 1997). If reverse ion exchange is the dominant process, it will shift the points to the left due to a large excess of Ca2+ + Mg2+ over SO4 2− + HCO3 −. Most of the points in this study fall in the Ca2+ + Mg2+ (Fig. 11) side, suggesting that reverse ion exchange is the major hydrogeochemical process operating in this aquifer. Ion exchange process is further discussed using the chloroalkaline indices (CAI) (Schoeller 1977):
All values are expressed in meq.l−1. When there is an exchange between adsorbed Na+ or K+ with Mg2+ or Ca2+ in the groundwater, the CAI will be negative and if there is a reverse ion exchange prevalent (exchange between adsorbed Mg2+ or Ca2+ with Na+ or K+ in the groundwater) then this indice will be positive. Most samples of the plain have a positive indice (See Table 1). This dominance of positive values reflects the substitution of sodium and potassium in groundwater with calcium and magnesium in the underground environment. This corroborates well the relationship previously identified between various elements in solution in the groundwater.
Mineral saturation indices
Saturation indices of minerals are very useful for evaluating the extent to which water chemistry is controlled by equilibrium with solid phases (Appelo and Postma 1993). PHREEQC was used to calculate the saturation indices of the following minerals in both water periods: calcite, dolomite, anhydrite, gypsum and halite.
Calculations showed that carbonate minerals have different degrees of saturation. The dolomite SI ranges from −0.56 to +0.87, that of calcite ranges from −0.25 to +0.44 (Table 6). Assuming that equilibrium is in the range of −0.5 to +0.5, the results show that calcite has reached equilibrium and dolomite is in a state of supersaturation. The evaporitic minerals show degrees of saturation lower than the carbonate minerals. Gypsum SI ranges from −1.82 to −0.68 (100 % of water points analyzed are under-saturated), and halite SI ranges from −6.6 to −4.68, indicating that the groundwater is very under-saturated regarding this mineral.
Role of evaporation
To characterize the effect of evaporation on the hydrochemistry of the Upper Cheliff groundwater, the ratio (Na+/Cl−) was plotted vs. TDS (Fig. 12). This figure shows that the points fall along a horizontal line which means that the (Na+/Cl−) remains almost constant despite the increase in TDS in both periods of high and low water, reflecting the effect of the phenomenon of evaporation in the study area.
Groundwater potability assessment
The Upper Cheliff groundwater potability was assessed according to international standards (World Health Organization 2008) and to the water hardness. The hardness is expressed in French degrees (°F). A French degree (°F) corresponds to the hardness of a solution containing 10 mg/l of CaCO3. 1 °F is equivalent to 4 mg of calcium per liter and 2.4 mg of magnesium per liter. 1 meq of calcium ion is equivalent to 5 °F.
The contents of the main elements of samples in high water and low water periods were compared with WHO standards and are reported in Table 7. This table shows that only 5 % of the samples have calcium and magnesium contents below WHO standards in low water period. All samples have higher calcium compared to the standards during high water. 14 and 18 % of the samples have Cl concentrations below standards in October and April. For sulfate, 50 % of the samples have lower contents vs. standards for both periods, whereas for bicarbonates 36 % of the samples are substandard in April and 23 % in October. Nitrate and dissolved solids are in excess of the standards in the majority of samples in both periods.
The samples were classified according to their hardness (De Fulvio and Olori 1976). Table 8 shows that almost all the analyzed samples are very hard in both periods, with total hardness greater than 54 °F (French degrees). The contents of magnesium and calcium that exceed the threshold set by WHO cause such high hardness.
These results show that the groundwater in the study area is of poor to bad quality for drinking purpose.
Summary and conclusions
Understanding the groundwater hydrochemistry and quality is vital to preserve this resource so that it can meet the present and future water needs in many countries. In North West Algeria, groundwater resources in the Upper Cheliff plain play a vital role in supplying water for drinking and agricultural purposes. However, there is an increase in degradation of this valuable resource reflecting a lack of knowledge of the groundwater mineralization processes and a lack of rational management.
This study allowed first for a geological and hydrogeological synthesis of the aquifer of the Upper Cheliff plain. This system is mainly composed of Mio-Pliocene-Quaternary formations. Quaternary formations are represented by alluvium. The Miocene and Pliocene consist mainly of sandstone. All these formations have a hydraulic continuity and form a single aquifer system. The carbonate formations bordering the plain are of Secondary age (Zaccar and Doui massives). Groundwater flow is generally in a East–West direction, with a drainage axis which coincides with the course of the wadi Cheliff.
The hydrogeochemical study of the groundwater was conducted using several methods (Piper and Stabler diagrams, multivariate statistics, ions exchanges, saturation indices of various minerals). This study showed the presence of several hydrochemical facies: chloride-calcium, chloride-sodium and bicarbonate-calcium. Mineralization of groundwater is due to the process of dissolution of carbonate and evaporite formations. The exchange of ions significantly affects the chemical composition of groundwater. Human activities (urban waste, fertilizers) also contribute to the mineralization of the water. Nitrate mapping in the plain of Upper Cheliff showed that the area close to Djendel and Ouled Khelifa cities is much more exposed to pollution. Nitrate contents over there exceed 50 mg/l. These high nitrate contents can be explained by the presence of various sources of pollution mainly related to agriculture, livestock and urban practices (domestic and industrial waste). The role of evaporation due to high temperatures in the plains was also highlighted.
In the plain of Upper Cheliff, the majority of chemical elements analyzed exceed the standards set by WHO. The groundwater is accordingly unfit for human consumption.
The results of this study helped to significantly improve the understanding of the aquifer which is an important resource for the development of this region. Urgent action must be taken promptly by the authorities to address the serious deterioration of the resource.
References
Abderamane H, Razack M, Vassolo S (2012) Hydrogeochemical and isotopic characterization of the groundwater in the Chari-Baguirmi depression Republic of Tchad. Environ Earth Sci 69(7):2337–2350
Aboubaker M, Jalludin M, Razack M (2013) Hydrochemistry study of a volcano-sedimentary aquifer using major ion and environmental isotope data. Dalha basalts aquifer, southwest of Republic of Djibouti. Environ Earth Sci 70(7):3335–3349
Achour F, Bouzelboudjen M (1998) Variabilité spatio-temporelle des ressources en eau en région semi-aride: application au bassin du Cheliff, Algérie. Spatio-temporal variability of water resources in semi-arid region: application to the Cheliff basin, Algeria. Water resources variability in Africa during XXth Century. Proceedings Abidjan’98 Conference, Abidjan, Cote d’Ivoire, IAHS Publ. 252, (In French)
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2000) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
ANRH Agence Nationale des Ressources Hydrauliques (2004) Annuaire Hydrogéologique de la nappe alluviale du Haut et Moyen Cheliff. Hydrogeological Yearbook of the alluvial aquifer of the Upper and Middle Cheliff. Unpublished report, Alger, (In French)
Appelo CAJ, Postma D (1993) Geochemistry. Groundwater and Pollution, Balkema
Ashley RP, Lloyd JW (1978) An example of the use of factor analysis and cluster analysis in ground water chemistry interpretation. J Hydrol 39:355–364
Bettahar N, Ali Benamara A, Kettab A, Douaoui A (2009) Risque de pollution nitratée des zones semi-arides : cas de la vallée du moyen Cheliff occidental (Nord Algérien). Risk of nitrate pollution in semi-arid areas: case of the valley of the Middle Western Cheliff (North Algerian). Revue Sciences Eau 22(1):69–78 (In French)
Boulaine J (1957) Étude des sols des plaines du Cheliff. Study of the soils of the Cheliff plains. Unpublished report. University of Alger, (In French)
Cerling TE, Pederson BL, Damm KLV (1989) Sodium-Calcium ion exchange in the weathering of shales: implications for global weathering budgets. Geology 17:552–554
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater regime. J Geol Soc India 47:179–188
Davis JC (2002) Statistics and data analysis in geology. Wiley (ASIA) Ltd, Singapore, New York, pp 526–540
Dawdy DR, Feth JH (1967) Application of factor analysis in study of chemistry of groundwater quality, Mojaveriver Valley California. Water Resour Res 3(2):505–510
De Fulvio S, Olori L (1976) Definitions and classification of naturally soft and naturally hard waters. In: Proc. Hardness of drinking water and public health. European Scientific Colloquium, Luxembourg 1975, Pergamon Press, New York, p 95
Diaw M, Faye S, Stichler W, Maloszewski P (2012) Isotopic and geochemical characteristics of groundwater in the Senegal River delta aquifer: implication of recharge and flow regime. Environ Earth Sci 66(4):1011–1020
Domenico PA, Schwartz FW (1990) Physical and chemical hydrology. Wiley, New York
EPA (1983) Methods for the chemical analysis of water and wastes. EPA/600/4-79/020, USA, p 491
Esteller MV, Andreu JM (2004) Anthropic effects on hydrochemical characteristics of the Valle de Toluca aquifer (central Mexico). Hydrogeol J 13:378–390
Fisher RS, Mulican WF III (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Chihuahuan desert, Trans-Pecos, Rexas, USA. Hydrogeol J 10:455–474
Gouaidia L, Boudoukha A, Djabri L, Guefaifia O (2011) Évaluation de la vulnérabilité d’une nappe en milieu semi-aride et comparaison des méthodes appliquées : cas de la nappe de Meskiana (Est Algérien). Vulnerability assessment of groundwater in semi-arid and comparison of methods: Meskiana groundwater (Eastern Algeria). Revue Sécheresse 22(1):35–42
Gupta S, Mahato A, Roy P, Datta JK, Saha RN (2008) Geochemistry of groundwater, Burdwan District, West Bengal, India. Environ Geol 53:1271–1282
Harman HH (1960) Modern factor analysis. University of Chicago Press, Chicago
Hitchon B, Billings GK, Klovan JE (1971) Geochemistry and origin of formation waters in the western Canada sedimentary basin-III factors controlling chemical composition. Geochim Cosmochim 35:567–598
Hounslow A (1995) Water quality data: analysis and interpretation. CRC Press, Boca Raton
Hussein MT (2004) Hydrochemical evaluation of groundwater in the Blue Nile Basin, eastern Sudan, using conventional and multivariate techniques. Hydrogeol J 12:144–158
Jalali M (2009) Geochemistry characterization of groundwater in an agricultural area of Razan, Hamadan, Iran. Environ Geol 56:1479–1488
Jayakumar R, Siraz L (1997) Factor analysis in hydrogeochemistry of coastal aquifers—a preliminary study. Environ Geol 31:174–177
Jayaprakash M, Giridharan L, Venugopal T, Krishna Kumar SP, Periakali P (2008) Characterization and evaluation of the factors affecting the geochemistry of groundwater in Neyveli, Tamil Nadu, India. Environ Geol 54:855–867
Kaiser HF (1958) The Varimax criteria for analytical rotation in factor analysis. Psychometrika 23:187–200
Karanth KR (1997) Groundwater assessment, development and management. Tata McGraw-Hill, New Delhi
Kuldip S, Hundal H, Dhanwinder S (2011) Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 64:1823–1833
Lawrence FW, Upchurch SB (1976) Identification of geochemical patterns in ground water by numerical analysis. In: Zaleem EA (ed) Advances in Groundwater Hydrology. America Water Resources Association, pp 199–214
Lawrence FW, Upchurch SB (1983) Identification of recharge areas using factor analysis. Ground Water 20:680–687
Llamas MR, Martínez-Santos P (2005) Intensive groundwater use: a silent revolution that cannot be ignored. Water Sci Technol Ser 51(8):167–174
Mania J, Djeda F (1990) Hydrogéologie de la plaine alluviale du Haut Cheliff de la région de Khemis–Miliana (Algérie). Hydrogeology of the alluvial plain of the High Cheliff. Region of -Khemis Miliana (Algeria). Bull Soc Géol France 8-VI(3): 505–513, (In French)
Mattauer M (1958) Etude géologique de l’Ouarsenis oriental (Algérie). Geological study of the eastern Ouarsenis (Algeria). Publ Serv Carte Géol Algérie, Alger, Bull 17, (In French)
Meghraoui M, Cisternas A, Philip H (1986) Seismotectonics of the lower Cheliff basin: structural background of the El Asnam (Algeria) earthquake. Tectonics 5:809–836
Moussa A, Zouari K, Oueslati N (2008) Geochemical study of groundwater mineralization in the Grombalia shallow aquifer, north-eastern Tunisia: implication of irrigation and industrial waste water accounting. Environ Geol. doi:10.1007/s00254-008-1530-7
Nandimandalam JR (2011) Evaluation of hydrogeochemical processes in the Pleistocene aquifers of Middle Ganga Plain, Uttar Pradesh, India. Environ Earth Sci 65(4):1291–1308
Narasimhan TN (2005) Hydrogeology in North America: past and future. Hydrogeol J 13:7–24
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)—A Computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. United States Geological Survey, Water Resources Investigations Report 99-4259, Washington, p 326
Perrodon A (1957) Etude géologique des bassins néogènes sublittoraux de l’Algérie Nord Occidentale. Geological survey of sublittoral Neogene basins of Western North Algeria. Publ Serv Carte Géol Algérie, Alger, Bull 12, (In French)
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Plummer L, Back W (1980) The mass balance approach: application to interpreting the chemical evolution of hydrologic systems. Amer J of Sci 280:130–142
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
Razack M, Dazy J (1990) Hydrochemical characterization of groundwater mixing in sedimentary and metamorphic reservoirs with combined use of Piper’s principle and factor analysis. J Hydrol 114:371–393
Rodier J, Legube B, Merlet M, Brunet R (2009) L’analyse de l’eau. Ed. Dunod, Paris, p 1600
Scanlon BR, Jolly I, Sophocleous M, Zhang L (2007) Global impacts of conversions from natural to agricultural ecosystems on water resources: quantity versus quality. Water Resour Res 43(3):W3437
Schoeller H (1977) Geochemistry of groundwater. In: Brown RH et al (eds) Groundwater studies—an international guide for research and practice. UNESCO, Paris, pp 1–18
Seyhan EV, Van de Caried AA, Engelen GB (1985) Multivariate analysis and interpretation of the hydrochemistry of a dolomite reef aquifer, Northern Italy. Water Resour Res 21:1010–1024
Simler R (2009). Diagrammes software. Downloadable at http://www.lha.univ-avignon.fr/LHA-Logiciels.htm
Sujatha D, Reddy RB (2003) Quality characterization of groundwater in the south-eastern part of the Ranja Reddy district, Andhra Pradesh, India. Environ Geol 44(5):579–586
Swan ARH, Sandilands M (1995) Introduction to geological data analysis. Blackwell, Oxford
UNEP (United Nations Environment Programme) (2010) Clearing the Waters. A Focus in Water Quality Solutions. Nairobi, UNEP. http://www.unep.org/PDF/Clearing_the_Waters.pdf
Usunoff EJ, Guzman AG (1989) Multivariate analysis in hydrochemistry. An example of the use of factor and correspondence analysis. Ground Water 17:27–34
WHO World Health Organization (2008) Guidelines for Drinking-Water Quality, 2nd edn. Geneva. http://www.who.int/water_sanitation_health/dwq/2edvol1i.pdf
WWAP (World Water Assessment Programme) (2009) United Nations World Water development report 3: water in a changing world. UNESCO, Paris
WWAP (World Water Assessment Programme) (2012) The United Nations World water development report 4: managing water under uncertainty and risk. UNESCO, Paris
Yidana S, Ophori D, Yakubo B (2008) Hydrochemical evaluation of the Voltaian system.The Afram Plains area, Ghana. J Environ Manag 88:697–707
Yitbarek A, Razack M, Ayenew T, Zemedagegnehu E, Azagegn T (2012) Hydrogeological and hydrochemical framework of Upper Awash River basin, Ethiopia: with special emphasis on interbasins groundwater transfer between Blue Nile and Awash Rivers. J Afr Earth Sc 65:46–60
Yuce G (2007) A Geochemical study of the groundwater in the Misli basin and environmental implications. Environ Geol 51:857–868
Zaporozec A (1972) Graphical interpretation of water quality data. Groundwater 10(2):32–43
Acknowledgments
The authors gratefully acknowledge three anonymous reviewers, for their critical evaluation and suggestions, which greatly helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Touhari, F., Meddi, M., Mehaiguene, M. et al. Hydrogeochemical assessment of the Upper Cheliff groundwater (North West Algeria). Environ Earth Sci 73, 3043–3061 (2015). https://doi.org/10.1007/s12665-014-3598-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3598-6