Abstract
Previous studies showed that 85 % of total organic matter (TOM) in digested sewage sludge (biosolids) used as a sealing layer material over sulfide tailings at the Kristineberg Mine, northern Sweden, had been degraded 8 years after application, resulting in a TOM reduction from 78 to 14 %. To achieve a better understanding of the field observations, laboratory studies were performed to evaluate biodegradation rates of the TOM under anaerobic conditions. Results reveal that the original biosolid consisted of ca. 60 % TOM (48.0 % lignin and 11.8 % carbohydrates) that had not been fully degraded. The incubation experiments proved that 27.8 % TOM in the biosolid was further degraded anaerobically at 20–22 °C during the 230 days’ incubation period, and that a plateau to the biodegradation rate was approached. Based on model results, the degradation constant was found to be 0.0125 (day−1). The calculated theoretical gas formation potential was ca. 50 % higher than the modeled results based on the average degradation rate. Cumulated H2S equated to 0.65 μmoL g−1 of biosolid at 230 days. However, the large sulfurous compounds reservoir (1.76 g SO4 2− kg−1 biosolid) together with anaerobic conditions can generate high concentrations of this gas over a long-term perspective. Due to the rate of biodegradability identified via anaerobic processes, the function of the biosolid to serve as an effective barrier to inhibit oxygen migration to underlying tailings, may decrease over time. However, a lack of readily degradable organic fractions in the biosolid and a large fraction of organic matter that was recalcitrant to degradation suggest a longer degradation duration, which would prolong the biosolid material’s function and integrity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental hazards caused by mining activities are one of the most serious problems worldwide. Large quantities of sulfide-tailings are discarded into the environment each year and are subject to atmospheric weathering, oxidation and acid rock drainage (ARD) formation. The inherent effect of ARD on peripheral surface and groundwater systems is of great environmental concern, posing an adverse impact to humans and ecosystems (Gerhardt et al. 2004; Johnson and Hallberg 2005; Lottermoser 2010).
Current policies for mine tailings remediation techniques are directed in Europe by the “Management of Waste from Extractive Industries Directive (2006/21/EC)” (European Parliament Council 2006). Engineered dry covers are commonly used as a remediation technique to cap the tailings material to limit oxygen and meteoric precipitation. The efficiency of sulfidic mine waste remediation and ARD mitigation is not only associated with geochemical and hydrological systems, but is also connected to microbial activities within the mine tailings (Schippers et al. 1998).
Traditionally, clayey till is utilized as a sealing layer material, but availability, large volumes and excavations for its use are required. This adds to problems already associated with mining activities. The implementation of new European legislation has led to stricter land-filling permission of organic residues in Sweden (van Praagh and Persson 2006). Waste residues such as fly ash from the paper mill industry and treated sewage sludge from waste water treatment plants have thus been evaluated to replace till (Lindgren 2005; Neuschütz and Greger 2010; Nason et al. 2013). The purpose of using industrial waste residues for tailings remediation is to solve both environmental disposal problems simultaneously. However, a systematic evaluation with respect to the geochemical, microbiological and hydrogeological effects of sewage sludge is required. Furthermore, an interaction between the biosolid and the mine waste is also of importance.
Digested sewage sludge (biosolid) has been tested for use as an organic substrate, and as a source of nutrients to promote vegetation establishment on agricultural land, landfill covers, and onto waste rock and tailing repositories (Sopper 1993; Tordoff et al. 2000; Wang et al. 2010). On the other hand, repeated long-term applications of biosolids above agronomic rates should be avoided (Schroder et al. 2008). Biosolids have been used as a material for sealing and vegetation layers to cover mine tailings (Neuschütz and Greger 2010; Li et al. 2013). Peppas et al. (2000) states that biosolids may have advantages over other types of covers, as it possesses properties such as a high alkalinity, high cation exchange capacity, and a low hydraulic permeability.
On the other hand, the long-term integrity, function, and efficiency of biosolids when used for mitigating ARD is a concern due to increased degradation of the high organic matter content of the material. An array of factors such as the nutrient availability, moisture, temperature, and pH conditions can affect the materials behavior in the natural environment (Peres et al. 1992; Gallert and Winter 1997; Lü et al. 2007). In addition, special care has to be taken regarding potential health and environmental risks in connection to the presence of elevated concentrations of metals and nitrate (Li et al. 2011a; Zanuzzi et al. 2013), and toxic levels of pathogenic microorganisms (Dean and Suess 1985). Due to degradation, the physical properties of the biosolids may be altered over time, which may affect the ability of the material to function appropriately to its applicability as an engineered dry cover to treat ARD abatement.
Increased research activities have been conducted in respect to risk assessment for biosolids when applied as a sub-surface sealing layer cover material for tailings (Peppas et al. 2000). Previous field investigations at the Kristineberg Mine, northern Sweden showed that ca. 85 % of organic matter in the biosolid, when applied as a sub-surface sealing layer barrier material over sulfide-tailings, was degraded within 8 years due to combined aerobic and anaerobic degradation (Nason et al. 2013). This reduced the original high organic content of the sludge (78 %) to a minor amount of 14 %. The reduction in mass may be a limiting factor to effective long-term remediation function efficiency.
For the production of biosolid waste residues, anaerobic digestion techniques are employed in waste-water treatment reactors. The process generates CH4 and CO2 due to the degeneration of organic matter. The complete microbial catalyzed reaction is simplified and expressed as follows:
However, obtaining complete anaerobic digestion can be difficult, due to intermediates that exist such as formic acid, acetic acid, as well as less degradable organic matter fractions such as hemicellulose and cellulose, and persistent organics such as lipids (represented by fats, oils or greases) and lignin. These often remain in the biosolid due to incomplete digestion during waste treatment (Peres et al. 1992). The ratio of CH4 to CO2 in reality is often considered to be >1 due to the amount of intermediates that are formed in the anaerobic conditions (Asgari et al. 2011; Kavuma 2013). By evaluating the gas released from the biosolid, a calculation of the mass balance can be achieved by using the total mass prior to, and at the termination of the laboratory experiments. This may be used to provide evidence for long-term predictions of biosolid use in sealing layers, as a consequence of the mass loss identified from the experimental data.
The production of volatile sulphur compounds such as hydrogen sulfide (H2S) may emit an unpleasant odor. Moreover, H2S in biogas is undesirable, since it is bio-toxic and can cause corrosion (APHA 1998; Dhar et al. 2011). In biogas, H2S may be present in concentrations from several hundred to a couple of thousand parts per million (ppm) (Zhao et al. 2010). Long-term H2S gas control is an issue to consider in relation.
Optimal anaerobic digestion of sewage sludge in bio-processing is normally performed in mesophilic (30–37 °C) and thermophilic (45–60 °C) conditions (Verstraete et al. 1996; Kim et al. 2002). Under ambient temperature ranges (20–22 °C), the degradation is expected to be much slower. Nevertheless, it can be deemed to be an accelerated process when compared to sub-Arctic field conditions within a Nordic climate.
The feasibility to use biosolids to cover mine waste tailings has been geochemically evaluated in a few studies (Hallberg et al. 2005; Nason et al. 2013). The present study is intended to quantitatively evaluate the proportion of the organic matter fraction of the biosolid that can be degraded anaerobically. Simultaneous aerobic laboratory experiments are currently in progress for the purpose of providing evidence on how the biosolid will behave if air infiltration is not a limiting factor. The comprehensive anaerobic and aerobic investigations are intended to place an insight into two extreme cases in comparison with field experimental data.
Laboratory studies were performed and compared to the field data derived from Nason et al. (2013) to achieve a better understanding of the observations. The objective of the present study was to determine the degradability of biosolids under anaerobic conditions to predict the long-term biological stabilization of using it as a sealing layer cover material. Particular emphasis was directed at the rate of biodegradation. It was the aim of the present study to predict and model the life-time of the organic content in the biosolid, and the implications this may have for a biosolid to function appropriately as a sealing layer for the remediation of sulfide-mine tailings.
Materials and methods
Source of field-scale biosolids and experimental set-up
The Kristineberg Mine site (Latitude 62.788582, Longitude 16.742800; Findthedata 2014) is located in the western part of the Skellefte ore district, approximately 175 km south-west of the city of Luleå in northern Sweden (Fig. 1). The volcanic massive sulfide deposit is hosted in 1.9 Ga ore-bearing volcanic rocks which are overlain by sedimentary rocks. Pyrite rich sulfide ores have been mined extensively since 1940 to the present. New Boliden AB originally processed the ore onsite until 1990, and deposited the tailings in the nearby vicinity of the mine within four major impoundments (1–4). The mine site has been the focus of an extensive Swedish mining scientific research study (MiMi) into ARD formation and remediation approaches (Höglund and Herbert Jr 2004).
The climate of the area is classified as sub-Arctic. Temperatures remain at sub-zero between October and April, with a mean annual temperature and precipitation amount at 0.7 °C and 600 mm year−1, respectively (Axelsson et al. 1991). The dominant vegetation and soil type in the area is coniferous forest and podzol-weathered till respectively (Carlsson 2002).
The pilot-scale test cell located at the Georange Environmental Test site was constructed in 2002 (Fig. 1). Each concrete cell measured 5 × 5 m2 by 3 m deep, was lined with an inert high density polyethylene (HDPE) liner that resisted acid attack from the materials contained therein, and was covered by a protective layer of locally derived glacial till (1.2 m), opened at the top to atmospheric conditions. The cell contained a 1.0 m thick fresh unoxidized high-sulfidic tailings layer (48 % pyrite and 4.8 % pyrrhotite). Above this layer, a 0.25 m thick sealing layer barrier consisting of biosolids was applied. This was sourced from a nearby waste-water treatment plant in Lycksele (Fig. 1). The processing of the biosolid involved sedimentation of the raw sludge, aluminum-sulfate thickeners added, and afterwards anaerobic digestion. Above this layer, an inert glacial till was applied as a protective layer to a thickness of 1.2 m. Drainage layers facilitated artificial runoff.
Two field biosolid samples were collected for analysis: a fresh biosolid sample applied at the beginning of the experiment (BFF); and an aged biosolid sample (BFA) collected after 8 years since application. Triplicate spot samples of the BFF were collected upon application in the pilot-scale cells. Duplicate samples of the BFA after 8 years of in situ field conditions were taken using a sediment core from an Atlas Copco Cobra TT percussion hammer drill. The samples were collected in a 0.05 m diameter core at 0.05 m intervals and extracted into a non-diffusive plastic bag within an argon filled chamber and were stored in a cool, dark environment until sample analysis. All sampling took place in an anoxic environment.
Source of laboratory-scale biosolids
Due to the experimental time differences between the field (0–8 years) and laboratory experiments (0–1 year), and the availability of the field samples upon collection, the biosolid sources between the two experiments were different, but directly comparable in this study. Two laboratory biosolid samples were used in the laboratory study: a fresh laboratory biosolid (BLF); and an aged laboratory biosolid sample (BLA). The BLF sample originated from the Skellefteå Biogas plant situated in northern Sweden. It was a mixture of aerobic and anaerobically digested biosolid waste residues generated from slaughterhouse and domestic wastes that would have been used to produce biogas. Spot samples were collected from the Boliden mine Gillervattnet tailings impoundment in northern Sweden immediately after application. The BLA spot samples (also derived from the Skellefteå Biogas plant) were collected from the same tailings impoundment after it was applied as a vegetation layer onto fresh tailings 1 year earlier. Samples were 20–30 kg each and were thoroughly homogenized with a shovel and stored at 4 °C before use in the experiments.
Chemical analysis
Physicochemical characteristics
For the lab samples (BLF vs. BLA), paste pH was determined with a Metrohm 704 portable pH meter. It corresponded to 20 g total solid (TS) of biosolid in Milli-Q™ water (ratio 1:10 solid to liquid) after being mixed in a rotating table mixer for 24 h. Measurement of the redox potential was conducted using a pH/Ion meter with an Ag/AgCl electrode. The measured value was corrected to the standard hydrogen electrode value according to the instruction manual by adding a value of 207 mV. Measurement of electrical conductivity (EC) was performed using a HI 8733 multi-range conductivity meter. Determination of total solids (TS) was performed routinely. Measurement was repeated in triplicate.
Organic contents
The BLF was analyzed for complex organic composition analysis. The BFF, BFA and BLA samples were analyzed for total organic matter (TOM) only. The differences in organic compound analysis between the samples were due to discrepancies in the original time-frame of the field experiment and the aims therein. Also, limited sample volumes were collected from the field sites, and the laboratory samples could be more extensively analyzed.
Note that hereafter only the BLF samples were used for complex organic content analysis and the subsequent incubation tests. The BFF and BFA samples were solely analyzed for their loss of ignition (LOI) contents, for use as an indication of TOM. The samples were dried at 105 °C for 24 h for the dry weight determination, and LOI was performed by taking an aliquot of the sample and heating it to 1,000 °C. The results are an extension of those obtained by Nason et al. (2013).
The BLF sample was sent to an accredited commercial laboratory, ALS Scandinavia AB in Luleå, Sweden. Analysis of Tot-P in the biosolid followed DIN EN ISO 11885 (E22), aqua-regia digestion (DIN EN 13346). Analysis of Tot-N, NH4-N, NO3-N and NO2-N after leaching was carried out following ISO 11261, EN ISO 11732, DIN EN ISO 10304, and DIN EN ISO 13395, respectively. Analysis of sulfate and chloride was conducted using liquid chromatography of ions following DIN EN ISO 10304-1/-2 (D19/20). Total organic carbon (TOC) was analyzed using a LECO CR-412 Carbon Analyzer following CSN EN 13137. Analysis of lignin followed the Tappi T222 standard. Volatile fatty acids (C1–C4 including formic acid and acetic acid) and neutral lipids (triglycerides, sterols, steryl esters) were measured following more research of the intern methods, KA 80.309 and KA 10.213, respectively. The acids derived from the leachate of the material were analyzed by high-performance liquid chromatography (HPLC). Analysis of hemicellulose and cellulose followed the standard SCAN CM-71. The hemicelluloses and cellulose were determined from these results and calculated as the percentage of total carbohydrate in the sample. Carbohydrates were analyzed by ion chromatography after acidic hydrolysis with sulfuric acid. Lignin was analyzed as Klason lignin after acidic hydrolysis of the carbohydrates; the acid-insoluble lignin was filtered off, dried and weighed out.
GC-Åbo was used for analyzing extractives where unions are categorized into three groups; fatty acids and resin acids, steryl esters, and triglycerides. The final determination was performed using gas chromatograph (GC). An estimate of the substances that were soluble in the extractant was determined. Fatty acids with carbon chains longer than C14 were determined with the GC-Åbo method, while the others were not detected.
Laboratory microcosms
Prior to the experiment, glass infusion flasks together with 32 mm airtight rubber stoppers and aluminum rings were rinsed with Milli-Q™ water. They were sterilized in an autoclave at 120 °C for 4 h.
Anaerobic batch incubation
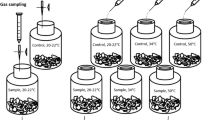
Triplicates of 20 g dry mass portions of fresh biosolids (83.40 ± 0.05 g with moisture) were put into a 500 mL sterilized transparent glass infusion flask. The flasks were closed with rubber stoppers and tightened using aluminum rings. The needle, connected with a three-way stopcock (in a closed position unless sampling), was placed on the septum. To create anaerobic conditions, the sealed flasks containing the biosolids were purged with helium gas by a needle penetrating through the rubber cap during which another needle was used to ventilate the flasks for 5 min to allow helium to exist ubiquitously in the headspace of the testing flask. The flasks were then incubated at room temperature (20–22 °C) in the dark.
Control
One control was included in each batch. In the control microcosm, the biosolid (corresponding to a 20 g dry mass) was put into a 500 mL glass infusion flask and was sterilized at 120 °C for 4 h. The rubber stopper and aluminum ring was sterilized simultaneously.
Gas sampling and measurements
Field pilot-scale experiment
Gas measurements were carried out 586–1,076 days after the BFF application and the experimental setup was initiated. The time deficiency between the application of the biosolids, and the gas sampling initiation was to allow stabilization of the material, settling, and to allow the geochemistry to plateau. The measurements, therefore, represent a period of time between after the BFF sample application and are hereafter referred to as post-fresh field biosolid sample (BPFF). Gas concentrations (%) of O2, CH4, and CO2 were collected from a 1 m3 quartzite filled geotextile ball installed 0.5 m below the biosolid sealing layer in the anoxic tailings (Fig. 1). Using a Maihak S710 gas analyzer following procedures described by Hallberg et al. (2005), the gas samples were analyzed biweekly during spring, summer, and autumn in 2004 and once a month during 2005. Samples could not be retrieved between October and April each year due to sub-zero temperatures and hence a lack of sample retrieval. CH4 and CO2 were calibrated with specific gas concentrations prior to the sampling. The precision of the instrument was better than 2 % of the analyzed value according to the manufacturer.
The gas concentrations (%) were converted into mmoL gas. Atmospheric pressure was assumed and the temperature (Kelvin) was measured biweekly using a thermistor located at a 0.05 m interval next to the geotextile balls. The gas concentrations were converted per unit gram of biosolid, as in the laboratory experiments. A known gas volume was calculated using the porosity of the biosolid and the underlying tailings, which equated to 15,392 L assuming that the biosolids were 95 % water-saturated. The total mass of the biosolid could be calculated as the volume (0.25 m × 5 m × 5 m) and arbitrary sludge density (721 kg m−3) were known. This conversion enabled a direct comparison between the field- and laboratory-experimental gas data per unit mass of biosolid.
Laboratory experiments
Before sampling, the 22 mL vials to be used for the gas analysis in the GC were prepared by clamping them with rubber septa, followed by them being purged with 2.5 bar helium for 2 min. The overpressure was checked and released when necessary to maintain atmospheric pressure in the vials.
The scheduled sampling was conducted on day 5, 10, 20, 40, 90, 135, 180, and 230 for all the samples. In each scheduled gas sampling, sufficient gas was withdrawn from the experimental flasks. Then the gas amount was adjusted and 11 mL gas was transferred to the vial. The total amount of gas that represented the overpressure, was withdrawn with a 100 mL Auswechselbar—interchangeable glass syringe. Thereafter the overpressure was released until atmospheric pressure was obtained in the gas collecting vials.
The gas (CO2, CH4, and H2S) concentrations were measured by injecting the sample from the vial into a stream of helium flowing through a gas chromatograph (GC, Clarius 580, PerkinElmer) with a built-in syringe auto-sampler. The collected data were compiled as instantaneous and cumulated gas production, as well as mass balance curves based on the volume and calculated amount of organic matter in the BLF samples.
Calculation of free ammonia
Free ammonia (NH3-N) in the laboratory microcosm was calculated according to Anthonisen et al. (1976):
where N concentrations are in mg L−1 and T is in °C. K a is the dissociation constant of ammonia in water. K b is a measure of constant of protolysis. K w is the dissociation constant for water. Taking into consideration the water content in the biosolid, the NH4 + concentration was converted into the unit of mg L−1 from mg kg−1 TS.
Calculation of organic nitrogen
The organic nitrogen content was calculated based on the difference between the contents of the total nitrogen and inorganic nitrogen contents (summary of ammonia, nitrate and nitrite) in the biosolid.
Modeling based on laboratory experimental results
Theoretical gas formation potential, G p (mL g−1) can be calculated by the following equation:
where C 0 is the content of degradable carbon in the waste (mg g−1); T is temperature (°C).
Modeling was performed based on a relationship originally developed for the anaerobic digestion of sewage sludge by Tabasaran (1976):
where G t is accumulated gas generation until time t (mL g−1); G p is gas formation potential (mL g−1); k is degradation constant (day−1), k = ln(2)/t 1/2; t is time (day). Mean residence time (MRT, day) can be described by MRT = 1/k.
The G t and k values were allowed to change until the best fit of experimental data with the modeling results was achieved. t 1/2 is half-life of the biosolid degradation (day). Sensitivity of the key controlling factors in the model was examined by doubling or halving each parameter. The parameters checked were G p and k.
Results and discussion
Characterization of biosolids
Table 1 presents the physicochemical characterization for the four types of biosolid. The pH of the BLF and BLA samples was 7.3 and 4.3. The difference in pH may be a consequence attributed to the readable oxidation of reduced ammonium (NH4 +) fractions by oxygen that have been found to be prevalent in surface applications of biosolids to treat mine waste (Neuschütz 2009; Nason et al. 2014). The redox potential was higher in the BLA than in the BLF, implying a decrease of reactivity in the aged biosolid. The pH was higher in the BFA than in the BFF. TS was low in all the four types of biosolids.
The results of the BLF for anions and organic compounds such as carbohydrate and volatile fatty acids, and for fat and neutral lipids, are summaried in Table 2. The main organic group in the BLF was lignin (48.0 % in TS) and carbohydrates (11.8 % in TS), giving a sum of ca. 60 % of organic matter. The carbohydrates were identified as arabinose, galactose, glucose, xylose, and mannose. These sugar types analyzed are the main carbohydrates in wood.
The volatile fatty acid concentration represented by C1-C4 (e.g. acetic acid), was 0.11 g kg−1 TS. Long chain fatty acids can inhibit the growth of anaerobic microorganisms including acetogenic bacteria and methanogens (Palatsi et al. 2010). However, this was not an issue concerning the use of biosolid as a cover material since the content of fatty acid was so low. The total amount of lipids in the BLF was as low as 1.8 g kg−1 TS, which meets the criterion for an ideal cover material over tailings. The organic nitrogen was calculated to be 40.41 g kg−1 TS. The nitrate and nitrite content were negligible (Table 2), and the free ammonia (NH3) was calculated to be 6.4 × 10−7 mg L−1 at room temperature using Eqs. 2 and 3.
The lipids and fatty acids found in the BLF indicate that it underwent anaerobic processes at the biogas plant but that the digestion was not 100 % complete. This is shown from the ca. 11.8 % (TS) carbohydrate in the BLF, of which consisted of 6.6 % cellulose and 5.2 % hemicellulose. Cellulose and hemicelluloses are present in paper and wood products and are the dominant biodegradable polymers in municipal waste (Barlaz 2006). The BLF contained a low content of lipids such as fat, grease, and oil (Table 2). This may be a result of both the aerobic and anaerobic digestion processes in the biogas plant. Alternatively, low amounts of these organics might be from the original slaughterhouse- and domestic-wastes.
The TOC content of the BLF was 44 % (in TS) (Table 2). The C/N ratio was calculated to be 11:1, indicating that the biosolid was not readily degradable. The ratio of stabilized compost is typically from 15:1 to 20:1 (Golueke 1981; Goyal et al. 2005). To enhance microbial growth in an anaerobic digestion system, the recommended operating C/N ratio ranges from 20:1 to 30:1 with an optimal ratio of 25:1 (Li et al. 2011b). An improper C/N ratio can lead to a high NH4-N release-potential and/or high volatile fatty acid accumulation in the digester, which might inhibit biosolid degradation. The BLF results show that although it has already been degraded in the biogas plant, due to its low C/N ratio it was not fully complete.
The high content of organic matter (60 % TS) in the biosolid assures it as a potential organic barrier to cover tailings, and in addition, the low C/N indicates that there is a lack of readily degradable organic fractions, suggesting long degradation duration, which would prolong the biosolid material’s function and integrity.
Biodegradation
Pilot-scale experiment for BPFF
The calculated cumulative concentrations of CO2 and CH4 per gram of BPFF as a function of time are shown in Fig. 2. In the pilot-scale experiments, it is clear that the system exhibited a release of CO2 preferentially over CH4. Nason et al. (2013) indicated that the combination of CO2 released from aerobic degradation of organic matter in the biosolid from atmospheric oxygen exposure, together with CO2 released from anaerobic degradation, may create a larger combined CO2 release. Atmospheric oxygen may have also oxidized CH4 to form CO2 which may have resulted in the elevated CO2 concentration.
The pilot-scale experiment gas data were initiated 586 days after application. Hence, it is likely that peak CH4 release had already occurred. This indicates that the methane released due to the anaerobic degradation of organic matter decreased with time. Though detectable, H2S concentrations within the geotextile balls in the pilot-scale cell were below the detection limit.
LOI in the original BFF samples was 78.0 % (Table 1). The total organic matter content in the BFA after 8 years of field conditions was reduced to 14 %, displaying a mass reduction of 85 % (Nason et al. 2013). The depletion of organic fractions may be explained by the detailed organic matter analysis performed in the microcosms (Table 2).
Laboratory microcosm for BLF
Overpressure of the overall released gas concentrations for BLF in response to incubation time against measured gas generation are presented in Fig. 3. According to OECD/OCDE (2006), the biodegradation of readily degradable organic matter is crucial in the first 10 days. In this period in the present study, degradation was low (Fig. 3) suggesting that the organic fraction in the biosolid is recalcitrant towards biodegradation. On the other hand, the degradation rate of organic matter depends on the initial number of microorganisms in the biosolid, as well as the solubility and sorption of the organic matter into the sludge matrix (Maier et al. 2000).
Biodegradation rates from the laboratory experiments were modeled, and a plateau for the cumulated gas was reached (Fig. 3) suggesting the further production of methane is of minor importance, which is in agreement with the data in the pilot-scale experiment. The plateau likely indicates an exhaustion of readily degradable organic matter fractions, which in turn, decreases the amount of gas released. This is used as a direct indicator to enable the prediction of the life-time of a biosolid sealing layer when used to remediate sulfide-mine tailings. It may also be a guide to the applicability to use it as a landfill cover. However, this is in regard to its use as an organic reactive barrier (Peppas et al. 2000). Nevertheless, biosolids may act successfully as a physical barrier extrusion when water-saturated (Nason et al. 2013).
The optimal G p and K values to fit the measured data within the modeled results were found to be 67 mL g−1 and 0.0125 (day−1), respectively. The half-life of the biosolid degradation (t 1/2) was calculated to be 55.5 days under anaerobic conditions at 20–22 °C. The MRT was calculated to be 80 days.
The calculated cumulative concentrations of CO2 and CH4 per gram of BLF as a function of time are shown in Fig. 4. The results suggest that the biosolid was biodegradable in the microcosm system. This was confirmed by the generation of CO2 and CH4. The laboratory experiment was initiated directly from fresh biosolids and the peak in CH4 concentrations occurred after only 50 days. The magnitude of CH4 concentrations in the microcosm was higher than in the pilot-scale experiment because of the experimental time difference and possibly also the sealed closed system in the laboratory microcosm compared to the open system field conditions.
LOI in the original BLF was 72.3 % (TS; Table 1). As explained, carbohydrate (11.8 % in TS) in the BLF consisted of 6.6 % cellulose and 5.2 % hemicellulose. A number of steps and a wide variety of microbial groups are involved in the degradation of cellulose under anaerobic conditions (Barlaz 1997). Methane associated with the incubation is principally due to the decomposition of cellulose and hemicelluloses, as was the case for lignin and some intermediate products (Table 2).
However, lignin is recalcitrant to degradation under methanogenic conditions due to its molecular structure (Ruiz-Dueñas and Martínez 2009; Wong 2009). Although many types of bacteria and fungi have the ability to degrade lignin (Bugg et al. 2011), they can mineralize lignin only to an extent (Buswell and Odier 1987). Moreover, the correct enzyme has to be present in a narrow range of temperatures (Wong 2009; Gasser et al. 2012). Lipids, characterized by fat and resin acid, steryl esters, triglycerides, and sterols, which are present in high quantities in slaughterhouse waste, are potential inhibitors of methane production (Palatsi 2010; Lalman and Bagley 2002) and are responsible for microorganism toxicity, causing a reduction in the degradation process. However, these were present in relatively low concentrations in the biosolid (Table 2).
Nitrogen present in inorganic forms in the BLF was dominantly in the form of ammonium (NH4 +) (Table 2), and the oxidation of this fraction to nitrate may explain the decrease in pH from 7.3 (BLF) to 4.3 (BLA). However, ammonium only contributed <8 % of the total nitrogen as it was mainly in the form of organic-N. Nevertheless, the anaerobic digestion of waste can be inhibited by high ammonium concentrations. In the BLF, ammonium (NH4 +) occurred at a concentration of 3.7 g kg−1 (equivalent to 1.1 g L−1 NH4 + in biosolid liquid phase). This is similar to that of the ammonium released (1 g L−1 NH4 +) due to the mesophilic process obtained in Gallert and Winter (1997), who showed that thermophilic microorganisms can tolerate at least twice as much free ammonium than the mesophilic microorganisms. Therefore, the ammonium concentration in the BLF is likely not toxic to the methanogenesis process.
Though detectable, H2S concentrations in the microcosm were as low as 16 ppm g−1 biosolid, and the cumulated amount was 0.65 µmoL g−1 biosolid after 230 days (Fig. 4b). Nevertheless, the high level of sulfate composition (1.76 g kg−1 SO4 2−; Table 2) in the BLF can potentially release more H2S under anaerobic conditions over time. Since the H2S gas is toxic and may cause erosion of the sealing layer (APHA 1998; Dhar et al. 2011), the release of H2S from the biosolid has to be monitored over a long-term perspective.
Mass balance calculation of organic matter conversion in the BLF can be carried out by three approaches: (i) theoretical gas-forming potential using the G p equation (Eq. 5); (ii) theoretical organic matter needed based on modeling results; and (iii) the gas production related to the theoretical organic matter in the biosolid using Eq. 1 in connection with the laboratory incubation.
Assuming 1 mol. gas at 20 °C is 22 L, and referring to Eq. 1, the degradation of 1 mol. of glucose leads to 6 mol. of gas corresponding to 3 mol methane. The cumulated gas in overpressure in the microcosm was 1.2 L after 230 days which required 3.3 g organic matter as glucose. In 20 g TS BLF ca. 60 % (i.e. 12 g) was organic matter. Thus it can be calculated that 27.8 % of the organic matter was degraded under the anaerobic conditions in the microcosm experiment, leaving a total of 43.3 % organic matter.
Based on the results from the chemical analysis, if one assumes that 11.8 % (mainly cellulose and hemicellulose) of the total biosolid is degradable, the G p is calculated to be 98.4 mg g−1 biosolid by applying Eq. 4. This value is ca. 50 % higher in comparison to the value obtained from the modeling results. This probably reflects that a fraction of cellulose and hemicellulose cannot be degraded (at least during the incubation period), or that intermediate products are formed during the course of the experiment.
Though the results of the mass balance from the three approaches differ largely, it highlights the extent for the proportion of biosolid degradation.
Link between pilot-scale investigation and laboratory microcosm evaluation
There was a difficulty to maintain similar conditions between the laboratory microcosm and the field conditions. The closed microcosm provided a quantitative estimate of anaerobic degradation rates, whereas the field experiment may represent a combination of aerobic and anaerobic degradation processes due to open field conditions. Nevertheless, it is notable that the gas diffusion pathways in these two systems can be different and the microbial communities can also be different in comparison to the laboratory microcosm with the field samples. It is recommended to perform more work related to these topics. Nevertheless, the laboratory experiment was intended to provide the worst case scenario when biosolid biodegradation is entirely represented by anaerobic degradation, and in regards to being accelerated under the ambient incubation temperature (20–22 °C), when compared to the lower field temperatures experienced.
The cumulated biogas production (Fig. 2 vs. 4), for the microcosm and pilot-scale experiments, shows a difference in the magnitude of CO2 and CH4 gas release when compared to a fixed amount of biosolid. Biogas release was higher in the microcosm than in the pilot-scale experiment (Fig. 2 vs. 4). The difference in biogas release may be a function of the amount of readily degradable organic matter that remained due to continuing degradation over time, as the two experiments differed on collected gas time frames. Nason et al. (2013) indicated that organic matter degradation in the biosolid sealing layer was 85 % after 8 years, a reduction from 78 % organic matter in the BFF to 14 % in the BFA. Also, cumulated CO2 and CH4 differ between the two experiments. In the first 200 days of both experiments, the BLF released 7× as much cumulated CO2, and 20× CH4 than the BPFF, possibly due to an abundance of fresh, readily degradable organic matter in the BLF. The data indicates that the BPFF had undergone peak degradation prior to derived gas data collection (<586 days) and that the degradation rate had significantly declined by the time the study commenced. Ideally, the field experiment gas data should have been conducted on a similar time-scale as with the laboratory microcosm experiments. Nevertheless, importantly, the data identifies that CO2 and CH4 formation, as an indication of organic matter degradation rates in biosolid material, declines over time, due to the exhaustion of readily degradable organic fractions.
A number of environmental factors in the pilot-scale experiment may have affected field degradation rates. Temperatures in the biosolid sealing layer were below freezing between December and March during 2004–2005 (Shcherbakova 2006), and the sealing layer was found to be highly water saturated in the spring-melt and autumn storm events. Because of the environmental limitations in the sub-Arctic environment where the cells were situated in northern Sweden, the 6-month incubation at room temperature in the microcosm can be roughly estimated to an equivalent 2-year period under the in situ conditions in the pilot-scale experiment.
Prospects to use biosolids as a sealing layer material for sulfide mine tailings remediation
The present study demonstrates that a large proportion of the organic matter fraction of a biosolid, if applied as a sub-surface sealing layer material, may be anaerobically degraded. Based on the chemical components analysis of the present study, the long-term degradability of the biosolid is of concern, and is identified as a major limiting factor on the materials integrity and function to serve as an effective barrier to oxygen mitigation for long-term applications. Moreover, after a fraction of organic matter is depleted in the biosolid, the reduction in mass may change the composition, form and shape of the sealing layer, which can lead to a variation of gas diffusion patterns, such as oxygen.
In Sweden, frost penetration depth typically varies between 1.0 and 2.5 m in common glacial till material, and conventional protective layers constructed from this material for sulfide mine tailings remediation ranges from 1 to 1.5 m deep (Höglund and Herbert Jr 2004). A load of a soil column about 1 m deep generates a pressure of 20 kPa. A decrease of mass due to the anaerobic biodegradation rates identified may result in the remaining biosolid undergoing a compaction process. As a consequence, the pore volume in the biosolid may be reduced due to the pressure (20–30 kPa) of the uppermost protective layer. Thus oxygen infiltration is theoretically reduced in response to the biodegradation of the biosolid, despite the reduction in mass. Oppositely however, there is a potential risk of the decreased biosolid mass and layer thickness to effect the function of the barrier. Melting snow or heavy rain events may increase the chance for the water infiltration via cracking in the thinner biosolid layer. Preferential flow paths may increase the possibility of oxygen diffusion to the underlying tailings. Consequently, the chance of uncertainly increases with respect to the stabilization of the sealing layer if a reduction in thickness occurred, attributed to the decrease in mass from organic matter biodegradation.
In the present study, the geotechnical and hydrological properties with respect to the biosolid applied in the field has not been evaluated, especially over the long-term. Factors such as the shear strength (for the placement on a slope such as hillside and tailings stack), permeability [the guideline value is 10−9 m s−1 for barrier layer in Sweden; (Herrmann et al. 2009)], water retention capacity, saturation degree, particle size distribution, and freeze–thaw processes (especially in cold climates) play an important role in tailings remediation, especially if this material is to be used as a long-term (100 year) amendment.
Overall, the design, operation and the efficiency of an organic cover to prevent oxygen diffusion and mine waste contaminants from being released may depend on the type of underlying mine wastes [causing ARD, or neutral rock drainage (if it contains high levels of carbonates)]. The local and regional climate, the characteristics of the biosolid composition (organic and inorganic), and the interaction between the organic cover and the underlying tailings may all govern the potential environmental impact if the biosolid sealing layer were to fail.
Komnitsas et al. (2000) developed a model in predicting the life expectancy of biosolid covers when established over reactive sulfide wastes in various climatic conditions based on a laboratory test over a 9 month period. The established method to prevent influx of oxygen into the underlying mine waste had been carried out using a model by recording the O2 concentration in pore air (directly related to the moisture of the organic matter). The moisture depended on the rainfall rate and upon the organic matter layer depth. By correlating the two parameters, together with the known initial moisture, the moisture content within the biosolid, as well as its monthly variations, can be accurately predicted over the long-term.
Santibáñez et al. (2007) evaluated nitrate leaching for 21 weeks from mine tailings amended with biosolids. They found that by establishing a permanent plant cover, the potential of biosolid-derived nitrate leaching can be reduced under Mediterranean semi-arid climate conditions. In contrast, without vegetation application, a separate biosolid cover which had been applied over Zn–Pb tailings at Garpenberg, in central Sweden was found to add large volume of nutritious elements to the underlying tailings, though it decreased the oxidation and weathering of the tailings and the leaching of elements from the tailings (Lu et al. 2013).
An evaluation of a 6-year-old sequence of paper sludge amended Au mine tailings in Ontario showed that the C/N ratio of the paper sludge increased, possibly due to N depletion from leaching or plant uptake (Cousins et al. 2009). The results from the 13C labeled analyses show that the organic matter in the paper sludge was recalcitrant to decomposition, indicating that the paper sludge amendment may be appropriate for long-term mining reclamation strategies.
Kabas et al. (2012) studied the impact of marble waste and pig slurry on the growth of native vegetative and metal mobility in an abandoned Pb–Zn–Cd tailing pond in southeast Spain. The application of organic matter for 1 year was shown to be effective with respect to the growth of native vegetation, increment of organic cover, richness and biodiversity, and in the reduction of metal mobility in the tailings pond.
The effect of goethite by a biosolid application on the mobilization of arsenic from mine tailings at a former gold mine in Ontario, Canada, had been assessed by Paktunc (2013) using a leaching test. The study showed that reductive dissolution of goethite influenced by the biosolid cover resulted in the mobilization of Arsenic, as As3+ forms. The long-term storage or disposal of tailings is a challenging task with respect to the leaching of arsenical minerals from biosolids.
Though the present study has proved that the emission of H2S is of minor importance under laboratory-scale experimental conditions, sulfurous compounds in tailings mixed with anaerobic conditions can create high concentrations of this dangerous gas. Therefore, the generation of H2S should be taken into consideration in remediation of sulfidic mine waste, in large-scale amendments.
If the prerequisites such as geochemical, microbiological, hydrological and geotechnical requirements are all met, the goal to use biosolids as a long-term cover material over tailings can be achieved. Thus comprehensive action is required to achieve these goals to mitigate ARD released into the environment.
Conclusions
In the present study, biosolids have been evaluated as a sealing layer cover material over mine waste tailings. Fresh biosolids derived from a biogas plant contained ca. 60 % of organic residue indicating that it had not been fully degraded in the digestion phases at the plant before application. After 230 days of anaerobic incubation under 20–22 °C, ca. 27.8 % organic matter in the biosolid had degraded, with lignin and carbohydrates being the main remaining organic components. These un-degraded fractions were biochemically stable. Model results indicate that peak degradation would be reached within 2 years if biosolids were used as a subsurface sealing layer. Thereafter, degradation would slow down due to the readily degradable fraction being exhausted, leaving a less degradable organic fraction which may act as a physical barrier against oxygen transport. The cumulated H2S was 0.65 µmoL g−1 biosolid at 230 days. However, the large sulfurous compounds reservoir (1.76 g SO4 2− kg−1 biosolid) together with anaerobic conditions can lead to high concentrations of this gas being released over a long-term perspective. Field applications of biosolid sealing layers, if water-saturated and highly compacted may act as a physical barrier for further mitigating oxygen diffusion, even though the organic reactivity (biodegradation rate) is reduced. Alternatively, the presence of sub-zero temperatures for part of the year may subdue microbial activity leading to a reduction in biodegradation rates. More work should be focused towards how the biosolid behaves after the relative easily degradable organic fraction is degraded. This would provide more evidence of the life-time of the biosolid used as a potential cover material.
References
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. J Water Pollut Control Fed 48:835–849
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Asgari MJ, Safavi K, Mortazaeinezahad F (2011) Landfill biogas production process.In: 2011 International conference on Food Engineering and Biotechnology, IPCBEE, vol 9. IACSIT Press, Singapore, pp 208–212
Axelsson C-L, Ekstav A, Holmén J, Jansson T (1991) Efterbehandling av sandmagasin i Kristineberg. Hydrogeologiska förutsättningar för åtgärdsplan, Lakvattenbalanser och vittringsbegränsande åtgärder (Report in Swedish), Golder Geosystem AB Report
Barlaz MA (1997) Microbial Studies of Landfills and Anaerobic Refuse Decomposition. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology American society for microbiology. ASM Press, Washington DC, pp 541–557
Barlaz MA (2006) Forest products decomposition in municipal solid waste landfills. Waste Manag 26:321–333
Bugg TDH, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400
Buswell JA, Odier E (1987) Lignin biodegradation. CRC. Crit Rev Biotechnol 6:1–60
Carlsson E (2002) Sulphide-rich tailings remediated by soil cover—evaluation of cover efficiency and tailings geochemistry: Doctoral Dissertation, Luleå University of Technology
Cousins C, Penner GH, Liu B, Beckett P, Spiers G (2009) Organic matter degradation in paper sludge amendments over gold mine tailings. Appl Geochem 24:2294–2300
Dean RB, Suess MJ (1985) The risk to health of chemicals in sewage sludge applied to land. Waste Manag Res 3:251–278
Dhar BR, Youssef E, Nakhla G, Ray MB (2011) Pretreatment of municipal waste activated sludge for volatile sulfur compounds control in anaerobic digestion. Bioresour Technol 102:3776–3782
European Parliament Council (2006) Directive 2006/21/EC of the European Parliament and of the Council of 15 March 2006 on the management of waste from extractive industries and amending Directive 2004/35/EC—Statement by the European Parliament, the Council and the Commission. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32006L0021:EN:NOT
Findthedata (2014) Kristineberg mine site. Mineral mines, deposites and resources. http://mineral-resources.findthedata.org/l/155944/Kristineberg
Gallert C, Winter J (1997) Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: effect of ammonia on glucose degradation and methane production. Appl Microbiol Biotechnol 48:405–410
Gasser CA, Hommes G, Schäffer A, Corvini PFX (2012) Multi-catalysis reactions: new prospects and challenges of biotechnology to valorize lignin. Appl Microbiol Biotechnol 95:1115–1134
Gerhardt A, Janssens de Bisthoven L, AMVM Soares (2004) Macroinvertebrate response to acid mine drainage; community metrics and on-line behavioural toxicity bioassay. Environ Pollut 130:263–274
Golueke CG (1981) Principles of biological resource recovery. Biocycle 22:36–40
Goyal S, Dhull SK, Kapoor KK (2005) Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bioresour Technol 96:1584–1591
Hallberg RO, Granhagen JR, Liljemark A (2005) A fly ash/biosludge dry cover for the mitigation of AMD at the falun mine. Chem Erde Geochem 65:43–65
Herrmann I, Svensson M, Ecke H, Kumpiene J, Maurice C, Andreas L, Lagerkvist A (2009) Hydraulic conductivity of fly ash–sewage sludge mixes for use in landfill cover liners. Water Res 43:3541–3547
Höglund LO, Herbert R Jr (eds) (2004) MiMi—performance assessment main report. MiMi 2003:3. The MISTRA-programme MiMi, Mitigation of the environmental impact from mining waste. MiMi Print, Luleå
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Kabas S, Faz A, Acosta JA, Zornoza R, Martínez-Martínez S, Carmona DM, Bach J (2012) J Geochem Explor 123:69–76
Kavuma C (2013) Variation of methane and carbon dioxide yield in a biogas plant. MSc. Thesis. KTH Industrial Engineering and Management. http://www.diva-portal.org/smash/get/diva2:604559/FULLTEXT02. Accessed 15 Nov 2013
Kim M, Ahn Y-H, Speece RE (2002) Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res 36:4369–4385
Komnitsas K, Peppas A, Halikia I (2000) Prediction of the life expectancy of organic covers. Miner Eng 13:1589–1601
Lalman JA, Bagley DM (2002) Effects of C18 long chain fatty acids on glucose, butyrate and hydrogen degradation. Water Res 36:3307–3313
Li XH, Tang ZL, Chu FY, Yang LY (2011a) Characteristics of distribution and chemical speciation of heavy metals in environmental mediums around Jinchang mining city, Northwest China. Environ Earth Sci 64:1667–1674
Li Y, Park SY, Zhu J (2011b) Solid-state anaerobic digestion for methane production from organic waste. Renew Sustain Energ Rev 15:821–826
Li SQ, Di XY, Wu DM, Zhang JT (2013) Effects of sewage sludge and nitrogen fertilizer on herbage growth and soil fertility improvement in restoration of the abandoned opencast mining areas in Shanxi, China. Environ Earth Sci 70:3323–3333
Lindgren M (2005) Efterbehandling av anrikningssanden på Rönnskär, plan för provytya. Boliden Mineral AB (In Swedish)
Lottermoser BG (2010) Mine wastes: characterization, treatment, and environmental impacts, 3rd edn. Springer, Berlin
Lu J, Alakangas L, Jia Y, Gotthardsson J (2013) Evaluation of the application of dry covers over carbonate-rich sulphide tailings. J Hazard Mater 244–245:180–194
Lü F, He PJ, Shao LM, Lee DJ (2007) Effects of ammonia on hydrolysis of proteins and lipids from fish residues. Appl Microbiol Biotechnol 75:1201–1208
Maier RM, Pepper IL, Gerba CP (2000) Environmental Microbiology. Academic Press, San Diego
Nason P (2013) Novel advances using sewage sludge in engineered dry covers for sulphide mine tailings remediation. Doctoral Dissertation, Luleå University of Technology
Nason P, Alakangas L, Öhlander B (2013) Using sewage sludge as a sealing layer to remediate sulphidic mine tailings. a pilot-scale experiment, northern Sweden. Environ Earth Sci 70:3093–3105
Nason P, Alakangas L, Öhlander B (2014) Impact of sewage sludge on groundwater quality at a formerly remediated tailings impoundment. Mine Waste Environ 33:66–78
Neuschütz C (2009) Phytostabilization of mine tailings covered with fly ash and sewage sludge. Doctoral Thesis, Stockholm University
Neuschütz C, Greger M (2010) Stabilization of mine tailings using fly ash and sewage sludge planted with Phalaris arundinacea L. Water Air Soil Pollut 207:357–367
OECD/OCDE (2006) OECD guidelines for the testing of chemicals, ready biodegradability—CO2 in sealed vessels (headspace test), pp 1–18
Paktunc D (2013) Mobilization of arsenic from mine tailings through reductive dissolution of goethite influenced by organic cover. Appl Geochem 36:49–56
Palatsi J, Illa J, Prenafeta-Boldú FX, Laureni M, Fernandez B, Angelidaki I, Flotats X (2010) Long-chain fatty acids inhibition and adaptation process in anaerobic thermophilic digestion: batch tests, microbial community structure and mathematical modeling. Bioresour Technol 201:2243–2251
Peppas A, Komnitsas K, Halikia I (2000) Use of organic covers for acid mine drainage control. Miner Eng 13:563–574
Peres CS, Sanchez CR, Matumoto C, Schmidell W (1992) Anaerobic biodegradability of the organic components of municipal solid wastes (OFMSW). Water Sci Technol 25:285–293
Ruiz-Dueñas FJ, Martínez AT (2009) Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol 2(2):164–177
Santibáñez C, Ginocchio R, Varnero MT (2007) Evaluation of nitrate leaching from mine tailings amended with biosolids under Mediterranean type climate conditions. Soil Biol Biochem 39:1333–1340
Schippers A, Jozsa PG, Sand W (1998) Evaluation of the efficiency of measures for sulphidic mine waste mitigation. Appl Microbiol Biotechnol 49:698–701
Schroder JL, Zhang H, Zhou D, Basta N, Raun WR, Payton ME, Zazulak A (2008) The effect of long-term annual application of biosolids on soil properties, phosphorus, and metals. Soil Sci Soc Am J 72:73–82
Shcherbakova E (2006) Geochemical and hydrological aspects of interactions between water and mine waste. Licentiate Dissertation, Luleå University of Technology
Sopper WE (1993) Municipal sludge use in land reclamation. Lewis Publishers, London
Tabasaran O (1976) Uberlegungen zum Problem Deponiegas. Müll und Abfall, vol no 7. Heft 7 S
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the vegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
van Praagh M, Persson KM (2006) National Translation of the EU landfill directives: will Swedish Landfills become sustainable? Int J Sustain Dev Planing 1:46–60
Verstraete W, de Beer D, Pena M, Lettinga G, Lens P (1996) Anaerobic bioprocessing of organic wastes. World J Microbiol Biotechnol 12:221–238
Wang B, Zhang HY, Fan ZM, Ju YY (2010) Compacted sewage sludge as a barrier for tailings impoundment. Environ Earth Sci 61:931–937
Wong D (2009) Structure and action mechanisms of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209
Zanuzzi A, Faz A, Acosta JA (2013) Chemical stabilization of metals in the environment: a feasible alternative for remediation in mine soils. Environ Earth Sci 70:2623–2632
Zhao Q, Leonhardt E, MacConnell C, Frear C, Chen S (2010) Purification Technologies for Biogas Generated by Anaerobic Digestion. CSANR Research Report 2010-001. Climate Friendly Farming. Chapter 9. Compressed Biomethane. pp 1–24. http://csanr.wsu.edu/publications/researchreports/CFF%20Report/CSANR2010-001.Ch09.pdf. Accessed 21 Nov 2013
Acknowledgments
The authors wish to thank the GEORANGE program and the Center of Advanced Mining and Metallurgy (CAMM) for financial support. Anton Lundkvist from Boliden Mineral AS, is thanked for field assistance during biosolid sample collection. Désirée Nordmark and Tommy Wikström are thanked for their laboratory assistance and to Jacqueline Vigilanti for her English proof-reading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, Y., Nason, P., Alakangas, L. et al. Degradation of digested sewage sludge residue under anaerobic conditions for mine tailings remediation. Environ Earth Sci 72, 3643–3654 (2014). https://doi.org/10.1007/s12665-014-3275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3275-9