Abstract
Heavy metal contamination was the main environmental problem around the Jinchang Ni–Cu mine area of Gansu, Northwest China. The concentration of heavy metals (Cr, Cu, Ni, Pb, and Zn) in various environmental mediums around the Jinchang Ni–Cu mine area were analyzed using atomic absorption spectrometry (AAS). The different chemical speciation of heavy metals was extracted using BCR (European Community Bureau of Reference) sequential extraction procedure, and the concentration of chemical speciation of each heavy metal was measured by inductively coupled plasma-atomic emission spectrometry. The results showed that Cu and Ni were the most important heavy metal pollutants in various mediums including cultivated soils, dust on slagheap surfaces, tailings, and sediments in waste water drains. In the tailings and sediments, the concentrations of Ni were obviously higher than those of Cu, whereas, in the soil and dust, the concentrations of Cu were higher than those of Ni. Analysis of chemical speciation indicated that Cr and Zn were mainly in residual fraction; Cu was mainly in oxidizable fraction; Ni was mainly in reducible fraction and acid soluble fraction; and Pb was mainly in reducible fraction and residual fraction. The extent of contamination of various environmental mediums was different because the heavy metals were derived from different sources. Furthermore, the mobility of various heavy metals was different because of the different distribution of chemical speciation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environment contamination caused by the mining and smelting activities has become a global environmental problem (Natarajan et al. 2006; Li et al. 2008; Rodríguez et al. 2009). Much research has been done in different metal mines and surroundings on the release and accumulation (Yang et al. 2006; Shuhaimi-Othman et al. 2006), transportation and transformation (Zhu et al. 2005), spatial distribution and bioavailability of the heavy metals (Zhang et al. 2004; Sun et al. 2008), as well as the potential environmental risks of heavy metals (Hu et al. 2006; Fan et al. 2007). Total heavy metal concentration is an important indicator of pollution risks. However, heavy metals associated with different fractions have different impacts on the environment (Tam and Wong 1996).
The toxicity and the mobility of heavy metals in soils depend not only on the total concentration, but also on their specific chemical speciation, their binding state, the metal properties, environmental factors and soil properties like pH, organic matter content and type, redox conditions and root exudates acting as chelates (Nyamangara 1998). The distribution of heavy metals in the various phases determines their behavior in the environment: their mobility, bioavailability and toxicity (Fuentes et al. 2008; Long et al. 2009). Therefore, fractionation is also necessary to evaluate the leaching behavior and environmental risks of heavy metal in soil and sediment around mine areas.
Jinchang, Gansu Province, northwestern China, known as the Nickel City, is the largest manufacture base of nickel (Ni) and cobalt (Co). The Jinchuan deposit, located in southwestern Jinchang City, is one of the super-large Ni–Cu–PGE (nickel–copper–platinum group elements) magmatic sulfide deposits containing the third largest amount of Ni in the world, and plentiful Cu, Co, and Pt elements (Tang and Li 1995; Tang and Barnes 1998). At present, the mining industry has already become a mainstay in Jinchang and it is a typical mining city. However, the heavy metal pollution is also one of the most prominent environmental problems in Jinchang. In the past half century, the mine exploitation has had huge impacts on the environment around Jinchang city (Liao et al. 2006). The present study is to understand the spatial distribution and chemical speciation of the heavy metals, especially, Ni and Cu in various environmental mediums around the Jinchang Ni–Cu mine area and discuss the behavior of heavy metals and their potential environmental risks.
Materials and methods
Materials
The studied area has the temperate continent-drought climate with an average annual precipitation of 200 mm and an annual evaporation of 2,100 mm. The local dominant wind direction is northwestern with a frequency of 30% and second strongest is southeastern with a frequency of 21% (Wu 2003). A large number of solid wastes are produced with the mineral processing and smelting, including mine tailings of mineral processing, water quenching slag, and electric furnace slag, which are over ground and stockpiled under outdoor conditions.
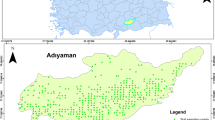
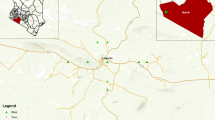
Forty-nine samples were taken in the surroundings of the Jinchang Ni–Cu mine area in the Gansu province, northwest China (Fig. 1). Samples collected in July and November of 2005, and July of 2006 were mainly from the different mediums including the cultivated soils, dust on slagheap surfaces, tailings, and sediments in waste water drains. The sampling sites in each medium were randomly selected. To ensure the quality of sampling, a mixed sample composed of 3–5 samples from the four vertices and center point of square cells (1 m length × 1 m height) are used making each mixed sample a good representation. One sample was made from the mixture using a quartation method, sealed in a plastic bag and then labeled. The method is suitable for the small area and inhomogeneous region of pollution, especially for tailing piles and slag heaps. All samples were air-dried in room temperature and passed through a 2-mm aperture plastic sieve to remove large stones and organic matter. Then a 100 g sample was taken using quartation, milled in a mortar, passed through 0.149-mm aperture plastic sieve, dried for 24 h at 105°C and finally placed in a dry clean container for the analysis.
Analysis
pH
The pH of each prepared sample was determined in the aqueous suspension in a soil-to-water ratio of 1:2.5 using pHs-3C pH meter.
Sample digestion for total metal determination
The prepared soil samples were analyzed for their heavy metal concentrations using the mixed acid (HNO3, HClO4 and HF) digestion method. Chromium (Cr), Cu, Ni, Plumbum (Pb), and Zinc (Zn) of the solutions were measured using an atomic absorption spectroscopy (AA240).
Sequential extraction of chemical speciation
The prepared samples were analyzed for the chemical speciation of Cr, Cu, Ni, Pb and Zn using a BCR (European Community Bureau of Reference) modified sequential extraction procedure (Ure et al. 1993; Rauret et al. 1999; Davidson et al. 2004; Wang et al. 2005). Selection of the most appropriate sequential extraction protocol for use in a particular study must always be carried out on the basis of “fitness for purpose” criterion. However, the revised BCR protocol, involving use of 0.5 mol/l NH2OH·HCl in the reducible step, appears to be more generally applicable than procedures involving acid ammonium oxalate (Davidson et al. 2004). A 1 g sample, taken from the prepared samples, was moved to a centrifuging tube, and each extraction reagent was added, respectively, in the following process:
-
1.
Acid-soluble fraction: 40 ml 0.1 mol/l HOAc was added into the 1 g sample, shaken for 16 h under 22 ± 5°C, and centrifuged for 20 min under 3,000g force;
-
2.
Oxidizable fraction: 40 ml 0.5 mol/l NH2OH·HCl was added into the remains of the above step, shaken for 16 h under 22 ± 5°C, and centrifuged for 20 min under a 3,000g force;
-
3.
Reducible fraction: 10 ml H2O2 (pH = 2~3) was added into the remains of step 2, kept under room temperature for 1 h, heated up to 85 ± 2°C for 1 h; and another 10 ml H2O2 was added and heated up to 85 ± 2°C for another hour; and 50 ml 1 mol/l NH4OAc (pH = 2) was added, shaken for 16 h under 22 ± 5°C, and centrifuged for 20 min under 3,000g force;
-
4.
Residual fraction: HCl,HNO3, HClO4, HF were added to the leftovers from all of the above steps. The concentration of each chemical speciation of heavy metals (Cr, Cu, Ni, Pb and Zn) was determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES) (type IRIS).
All glass- and plastic-wares were soaked in 10% nitric acid overnight and rinsed thoroughly with deionized water before use. For quality control, the national standard soil sample (GSS-1), reagent blanks and duplicate representing 10 and 20% of the total sample population, respectively, were incorporated in the analysis to detect contamination and assess precision and bias.
Results
Total heavy metal content in various environmental mediums
The concentration of heavy metals in the cultivated soils, dust on slagheap surface, tailings, and sediments in waste water drains around the Ni–Cu mine area is shown in Table 1.
The concentrations of Cu and Ni in the cultivated soils were 135.27 and 132.05 mg/kg, respectively. The Cu and Ni concentrations were 4–6 times of the soil background values in Gansu, and all the other elements were only slightly higher than the background values. In the dust on slagheap surfaces, which contained high contents of heavy metals, the maximum concentrations of Cu and Ni were 4,698.70 and 2,310.86 mg/kg, with averages of 1,744.87 and 1,172.14 mg/kg, which was 4–5 times of the third class values of the National Soil Quality Standard (State Environmental Protection Administration of China 1995). In the tailings, the concentrations of heavy metals were much higher than the background values in Gansu Province, and the maximum concentrations of Cu and Ni were 3,069.50 and 3,516.00 mg/kg. Their averages were 1,767.64 and 2,035.40 mg/kg, which was 4 and 10 times of the third class values of the National Soil Quality Standard (State Environmental Protection Administration of China 1995), respectively. Similarly, the heavy metals were highly concentrated in sediments in the waste water drains. The maximum concentrations of Cu and Ni were 8,377.73 and 13,112.42 mg/kg, and the averages were 5,583.86 and 7,652.12 mg/kg, which was much higher than the concentrations in other mediums (Fig. 2). The distribution pattern of Cu, Ni and Pb were very similar to the maximum concentration in sediments in the waste water drains nearby the old tailings pile. The highly concentrated heavy metals in sediments suggested that the heavy metals in the waste water were transported into the sediments. Moreover, since the drain is close to the tailings, it is expected that a great amount of heavy metals would be leached into the sediments in the waste water drains. The results showed that the concentrations of heavy metals were in descending order in sediments, tailings, dust, and soil.
Chemical speciation of heavy metals
As indicated by the analyzed results of chemical speciation of heavy metals (shown in Table 2), except for Cu, Ni and Pb, Cr and Zn have a similar distribution, mainly existing in residual fraction. The fractions of heavy metals in different mediums showed that the chemical speciation of Cu, Ni and Pb in different mediums were different (as Fig. 3).
Fractionation of elements from topsoil of wasteland (a), topsoil of abandoned greenhouse soil (b), tailings (c) and topsoil of cultivated soils (d). The result shows that Cr and Zn are mainly in residual fraction, Cu is mainly in oxidizable fraction, Ni is mainly in reducible fraction and acid-soluble fraction, and Pb is mainly in reducible fraction and residual fraction
In general, mine soils are mechanically, physically, chemically and biologically deficient (Vega et al. 2006) and characterized by instability and limited cohesion, with low contents of nutrients and organic matter and high levels of heavy metals (He et al. 2005). The results (Table 2; Fig. 3) indicate that Cr mainly existed in residual fraction, because through a series of reactions in soils Cr could be easily transformed into insoluble hydroxide precipitates, which stayed in the residue (Chen et al. 1993; Wang et al. 2000). The proportion of chemical speciation of Cu was relatively complex, and Cu mainly existed in oxidizable fraction (Zhang and Ma 1997; Lu et al. 2003). Oxidizable fraction is important because the lack of Cu in plants and accumulation of Cu on soil surface are all related to oxidizable fraction Cu (Yuan 1983). The largest portion of Cu was associated with sulfides/organic matter, because Cu has a high geochemical affinity for sulfides or organic matter (Kim et al. 2001). In DP samples Ni existed mainly in oxidizable fraction, and then in residual and reducible fraction, while in all other samples, most of the Ni was in reducible fraction. Overall, in most of the samples Ni was in active speciation (Xu and Yang 1995), including acid-soluble fraction, reducible fraction, and oxidizable fraction, which suggests that Ni in these mediums has strong activity and bioavailability. Pb was mainly in reducible fraction and then in residual fraction, and Zn was mainly in residual fraction, which is consistent with other research that showed reducible fraction of Pb was the most important compound form in soil, accounting for 40–50% (Rieuwerts et al. 2000) and Zn existed mainly as stable silicate minerals (Wang et al. 2000). Rodríguez et al.(2009) found that most Pb was associated with non-residual fractions, mainly in reducible form, in all the collected samples around a Pb–Zn mines in Spain. Zn appeared mainly associated with the acid-extractable form in mine tailing samples, while the residual form was the predominant one in samples belonging to surrounding areas. The studied area has the temperate continent-drought climate with an average annual precipitation of 200 mm and mean annual evaporation of 2,100 mm. It should be pointed out that oxide association does not guarantee the immobilization of contaminant elements in the subsurface, so metals associated with iron and manganese oxides are still labile and may be released upon decomposition of the oxides under reduced conditions, consequently having a significant impact on soil quality and biota (Chlopecka 1996).
Discussion
Origins of the heavy metal pollutants
As shown by the results of the spatial distribution of heavy metals (Table 1), the heavy metals, primarily Cu and Ni, the spatial area could be divided into two groups. The first region included the cultivated soils and dust on slagheap surface, where Cu concentrations were higher than Ni. The second region included the tailings and sediment in waste water drain, where Ni concentrations were obviously higher than Cu. The difference of distribution of Cu and Ni in the two regions were primarily related to the origins of Cu and Ni. The first group is in the urban areas of Jinchang, and the Cu and Ni concentrations in the atmospheric dust were 186.15 and 42.44 mg/kg, while in rural areas of Jinchang, 29.64 and 12.91 mg/kg (Zhao 2005a, b). This showed that Cu concentration was obviously higher than Ni in the atmospheric dust of the both areas, which contradicted the result that Ni concentration being higher than Cu of the soil background values in Gansu. The Cu content in the background soil is 24.10 and Ni is 35.50 mg/kg. Therefore, it is concluded that Cu and Ni in the urban and rural soils in Jinchang mainly came from the atmospheric dust, and the airborne particles emitted by smelting were the major cause of Cu and Ni in the atmosphere dust. As for the second group, the origin of Cu and Ni were related to ores from the Jinchang mine. Cu and Ni average concentrations of different types of ores normalized to 100% sulfide in Jinchuan deposits, were 9.38 and 13.17%, respectively (Tang and Li 1995), and Ni concentration was obviously higher than Cu in the ores. Of the tailings, which were the products of the floating filtration of the ores, Cu and Ni mainly came from the ores, so the content of Cu and Ni in the tailings was in agreement with those in the ores. The Cu and Ni concentrations in the sediments in waste water drains were influenced by the leaching of tailings, so they had the same distribution as those in tailings.
Interaction and proportions of chemical speciation of heavy metals
The proportions of chemical speciation were dramatically different in various mediums (shown in Table 2; Fig. 3). The proportions of chemical speciation of Cu and Ni in tailings were different from those in other mediums. Cu was mainly in residual fraction (50%) and acid-soluble fraction (30%) in the tailings, but mainly in oxidizable fraction in all the other mediums. Cu–organic matter complexes were generally considered relatively stable (Walter and Cuevas 1999). However, the percentage of Cu associated with organic matter fraction primarily depends on the quantity of Fe oxides in soil (Sliveira et al. 2006). The amounts of all the chemical speciation of Cu and Ni in abandoned greenhouse soils were higher than those in other mediums (Table 2), which may be due to the low pH in the greenhouse soils. Zn and Pb are often associated with sulphur, as sulfides. Under superficial environmental conditions, sulfides are quickly oxidized and these metals are released and separated from sulphur at an early stage of mineral weathering. During soil development, Zn associated with the step 2 BCR fraction tends to concentrate in Mn oxides, whereas Pb is more likely to be found enriched in Fe oxides and hydroxides (He et al. 2005). The various chemical speciation of heavy metals may transform among each other under suitable physical and chemical conditions. Reducible fraction and oxidizable fraction were potential sources of the acid-soluble fraction of heavy metals. Reducible fraction of heavy metals would transform to the acid-soluble fraction under the reductive condition or when pH decreases. Similarly, oxidizable fraction of heavy metals would transform to the acid-soluble fraction under the oxidative condition or when pH increases. Acid-soluble fraction is considered to be the most bioavailable among the different metal speciation, and it is also the most labile (Chlopecka 1996). Therefore, it is necessary to adjust the pH and Eh of the soils to avoid the transformation of reducible and oxidizable speciation to acid-soluble speciation which would increase environmental risks.
Conclusion
The perennial mining and smelting of ores have caused the obvious accumulation of Cu and Ni in various environmental mediums around the Jinchang Ni–Cu mine area. High levels of metal pollution were detected in the entire mine area. The concentration of Cu and Ni are in descending order in sediments in waste water drains, tailings, dust on slagheap surfaces, and cultivated soils. Moderately high concentrations of Cu and Ni were detected in many of the samples collected in the surrounding cropping and farming lands, showing a certain extent of dispersion of pollution from the smelter, mine spoil and tailings areas. The high correlation obtained between the different samples strongly suggests their common origin in mining activities during the operation of the Jinchang mine and, later, in the mine tailings dumped. The highly concentrated heavy metals in waste water drains and dust on slagheap surfaces, as well as similar distribution patterns with tailings, suggest that a lot of heavy metals have been released into the surrounding environment. Therefore, the tailings pile and slagheap are obviously the important pollution sources of the heavy metal pollution owing to their long-term existence and stockpiled over ground in outdoor conditions.
Sequential extraction showed that Pb was mainly associated with the reducible fraction in all the collected samples. Ni was mainly associated with the acid-soluble and reducible fractions in mine tailings, thus being more mobile and potentially more dangerous for the environment of the studied area. Cu was mainly associated with the oxidizable fraction, but residual fraction in tailings because of poor organic matters. Cr and Zn were mainly associated with the residual fraction, which indicates that the limited mobility of Cr and Zn in the soils from surrounding mining areas decreases the environmental risks. Therefore, it would be necessary to remove or stabilize the mine spoils and slagheap from the Jinchang mine, since still it is a source of soil pollution as the tailings continue to release heavy metals into the environment.
References
Chen YX, Zhu ZX, He ZY (1993) Mechanism of Cr (III) oxidation by Mn oxides. Acta Scientiae Circumstanitiae 13(1):45–50 (in Chinese)
Chlopecka A (1996) Assessment of form of Cd, Zn and Pb in contaminated calcareous and gleyed soils in southwest Poland. Sci Total Environ 188(2):253–262
Davidson CM, Hursthouse AS, Tognarelli DM, Urea AM, Urquhan G (2004) Should acid ammonium oxalate replace hydroxylammonium chloride in step 2 of the revised BCR sequential extraction protocol for soil and sediment. Anal Chim Acta 508(2):193–199
Fan QY, He J, Xue HX, Lu CW, Liang Y, Sun Y, Shen LL (2007) Competitive adsorption, release and speciation of heavy metals in the Yellow River sediments, China. Environ Geol 53(2):239–251
Fuentes A, Llorens M, Saez J, Aguilar MI, Ortuno JF, Meseguer VF (2008) Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol 99:517–525
He ZL, Yanga XE, Stoffellab PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Hu NJ, Li ZQ, Huang P, Cheng T (2006) Distribution and mobility of metals in agricultural soils near a copper smelter in South China. Environ Geochem Health 28(1–2):19–26
Kim KK, Kim KW, Kim JY, InS Kim, Cheong YW, Min JS (2001) Characteristics of tailings from the closed metal mines as potential contamination source in South Korea. Environ Geol 41:358–364
Li XH, Tang ZL, Chu FY (2008) Chemical forms of heavy metals in soil and sediment around Jinchuan and Baiyin Mines, Gansu. Geol Sci Technol Inf 27(4):95–100 (in Chinese)
Liao XY, Chen TB, Wu B, Yan XL, Nie CJ, Xie H, Zhai LM, Xiao XY (2006) Mining urban soil pollution: concentrations and patterns of heavy metals in the soils of Jinchang, China. Geogr Res 25(5):843–852 (in Chinese)
Long YY, Hu L, Fang CR, Wu YY, Shen DS (2009) An evaluation of the modified BCR sequential extraction procedure to assess the potential mobility of copper and zinc in MSW. Microchem J 91:1–5
Lu Y, Gong ZT, Zhang GL (2003) The chemical speciation of heavy metals of urban soils in Nanjing. Environ Chem 22(2):131–136 (in Chinese)
Natarajan KA, Subramanian S, Jean-Jacques B (2006) Environmental impact of metal mining biotechnological aspects of water pollution and remediation: an Indian experience. J Geochem Explor 88(1–3):45–48
Nyamangara J (1998) Use of sequential extraction to evaluate zinc and copper in a soil amended with sewage sludge and inorganic metal salts. Agric Ecosyst Environ 69(2):135–141
Rauret G, LóezSánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller Ph (1999) Improvement of the BCR three-step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Rieuwerts JS, Farago ME, Cikrt M, Bencko V (2000) Differences in lead bioavailability between a smelting and a mining area. Water Air Soil Pollut 122:203–229
Rodríguez L, Ruiz E, Alonso-Azcáratec J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manag 90:1106–1116
Shuhaimi-Othman M, Pascoe D, Borgmann U, Norwood WP (2006) Reduced metals concentrations of water, sediment and Hyalella azteca from lakes in the vicinity of the Sudbury metal smelters, Ontario, Canada. Environ Monit Assess 117(1–3):27–44
Sliveira ML, Alleoni LF, O’Connor GA, Chang AC (2006) Heavy metal sequential extraction methods—a modification for tropical soils. Chemosphere 64:1929–1938
State Environmental Protection Administration of China (1995) The national soil quality standard (15618). Chinese Standard Press, Beijing, China, pp 2–3 (in Chinese)
Station Environmental Monitoring of China (1990) The background values of soil chemical element of China. Chinese Environmental Science Press, Beijing, China, pp 87–90 (in Chinese)
Sun LN, Yang XB, Wang WQ, Ma L, Chen S (2008) Spatial distribution of Cd and Cu in soils in Shenyang Zhangshi Irrigation Area (SZIA) China. J Zhejiang Univ Sci B 9(3):271–278
Tam NFY, Wong YS (1996) Retention and distribution of heavy metals in mangrove soils receiving wastewater. Environ Pollut 94:283–291
Tang Zl, Barnes SJ (1998) Mineralization mechanism of magmatic sulphide deposits (in Chinese). Geological Publishing House, Beijing, pp 27–35
Tang ZL, Li WY (1995) Mineralization mechanism and deposit geology of Jinchuan Cu–Ni (PGE) sulphide deposits. Geological Publishing House, Beijing, China, pp 78–90 (in Chinese)
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments—an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission-of-the-European-Communities. Int J Environ Anal Chem 51(1–4):135–151
Vega FA, Covelo EF, Andrade ML (2006) Competitive sorption and desorption of heavy metals in mine soils: influence of mine soil characteristics. J Colloid Interf Sci 298:582–592
Walter I, Cuevas G (1999) Chemical fractionation of heavy metals in soil amended with repeated sewage sludge application. Sci Total Environ 226:113–119
Wang YP, Bao ZY, Hou SE (2000) Study on characteristics of heavy metal specie in the soils near the tailings. Rock Mineral Anal 19(1):7–13 (in Chinese)
Wang YP, Huang Y, Wang SM, Xu CX, Liu M (2005) Chemical speciation of elements in sediments and soils and their sequential extraction process. Geol Bull China 24(8):728–734 (in Chinese)
Wu YL (2003) Characteristic of pollution between total suspend grain and sulfur dioxide in atmosphere in Jinchang City. Environ Res Monit 16(4):326–328 (in Chinese)
Xu JL, Yang JR (1995) Heavy metal elements in terrestrial ecosystems. Chinese Environmental Science Press, Beijing, pp 110–118 (in Chinese)
Yang YG, Liu CQ, Wu P, Zhang GP, Zhu WH (2006) Heavy metal accumulation from zinc smelters in a carbonate rock region in He Zhang county, Guizhou province, China. Water Air Soil Pollut 174(1–4):321–339
Yuan KN (1983) The soil chemistry of nutritional elements in plant. Science Press, Beijing, pp 20–24 (in Chinese)
Zhang H, Ma DS (1997) Fractionation of heavy metals in soils from Nanjing. Acta Sci Circumst 17(3):346–352 (in Chinese)
Zhang X, Zhou TF, Yuan F, Shi XL, Fan Y, Liao XN (2004) Speciation and bioavailability of cadmium in Tongling mining soils. Ecol Environ 13(4):572–574 (in Chinese)
Zhao YF (2005a) Investigation and analysis on copper Sources in Jinchang City, Gansu province. J Environ Manag Coll 15(3):70–73, 87 (in Chinese)
Zhao YF (2005b) Investigation and analysis on nickel Sources in Jinchang City, Gansu Province. Environ Res Monit 18(2):10–12 (in Chinese)
Zhu JB, Chen FR, Lu L, Xie XH (2005) Heavy metal geochemistry behavior during the oxidation of the Fankou Pb–Zn mine tailings in Guangdong Province and the implications for environmental remediation of the mines. Acta Sci Circumst 25(3):414–422 (in Chinese)
Acknowledgments
This study was financially supported by the Chinese National Science Foundation (40534020) and Oceanic Science Young Foundation of State Oceanic Administration, China (2009310). Thanks to Professor Su for analytical services from Analytical Center, Lanzhou University. Appreciation should be directed to Dr. Edinger and an anonymous reviewer who offered valuable comments and detailed revisions for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XH., Tang, ZL., Chu, FY. et al. Characteristics of distribution and chemical speciation of heavy metals in environmental mediums around Jinchang mining city, Northwest China. Environ Earth Sci 64, 1667–1674 (2011). https://doi.org/10.1007/s12665-009-0438-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0438-1