Abstract
The aim of this work was to investigate the effects of sorbent (natural and modified zeolite and bentonite, iron filings and ferric sulfate) on the speciation and bioavailability of arsenic in contaminated soil. The soil used in this experiment was collected from Zarshuran area (Western Azerbaijan province, NW Iran). The sorbents were added to the soil in various rates separately. After a month of incubation, sunflower was planted in pots. After harvest, soil and plant samples of each pot were analyzed. Then various species of arsenic were estimated in soil solutions by MINTEQ software program. Water-soluble arsenate, arsenite and exchangeable arsenic from each pot measured. The results showed that the sorbents had no effect on the speciation of arsenic. Mobility of arsenite in the soil solutions has not changed. Soils treated with natural bentonite and zeolite increased soluble arsenate concentration and decreased exchangeable arsenic concentration. Although Fe-zeolite increased soluble arsenate concentration, Fe-bentonite, iron filings and ferric sulfate decreased soluble arsenate concentration and exchangeable arsenic concentration. Finally, iron filings (containing 354 mmol Fe+3) vigorously increases in the plants biomass and decreases in the arsenic concentration in plants tissue, is suggested as the best sorbent for arsenic stabilization in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is a known metalloid since the ancient times and accounted a toxic element for humans (Bech et al. 1997; Tseng 1977). High levels of arsenic in drinking water reported from India, Bangladesh and Taiwan (Chatterjee et al. 1995). The Zarshuran mine in Iran are well known for gold mining that its history dates back a hundred years ago (Modabberi and Moore 2004). In during time, high levels of arsenic accumulated in downstream mine (Mehrabi et al. 1999; Asadi and Hale 2001). Due to the high toxicity of arsenic in the Zarshuran soil, people’s health is compromised (Karimi et al. 2009).
The adverse function of arsenic in soil is not only dependent to total concentration, but it is dependent to fractionation and speciation of arsenic (Ho et al. 2013). Different fractions are including soluble and exchangeable arsenic, bound to carbonates, iron and manganese oxides, organic matter, sulfides and residual (in the crystal lattices of silicate minerals) (Kim et al. 2003). The bioavailability, toxicity and mobility of As in soil are largely determined by the distribution and fractionation between the solution and soil matrix (Wenzel et al. 2001).
Behavior of arsenic in soil solution depends on the pH and the abundance of different arsenic species (Ho et al. 2013). Due to the high cost and time-consuming measurement of different species of arsenate and arsenite in soil solution, their species are estimated via geochemical speciation models (e.g., MINTEQ software) (US EPA 2007). However, those models are based on chemical reactions of solution and solid phase (Shum and Lavkulich 1999; Van Herreweghe et al. 2002). Although arsenate and arsenite are the main two forms of arsenic in soil solution, the toxicity of arsenite is 25–60 times more than arsenate (Kim et al. 2003).
Several methods are used to stabilize arsenic in the soil. The stabilize process can be achieved by adding sorbents such as zeolites, bentonite (Hamidpour et al. 2010) and iron (Kim et al. 2003). Due to low cost and high availability of zeolites and bentonites, these materials are used for stabilizing heavy metals in contaminated soils (Inglezakis et al. 2007; Garcıa-Sánchez et al. 1999). Clay minerals with negative surface charges are not efficient for the remediation of metalloids such as arsenic. Therefore, it is necessary that surface properties of the natural clay minerals modified with materials such as iron for binding anionic pollutants (Krishna et al. 2001; Malekian et al. 2011; Sarkar et al. 2010a, b, 2011a, b, 2012a ). A recent study, Malekian et al. (2011) reported a successful stabilization of nitrate in soil by surfactant-modified clinoptilolite. Other studies have also reported that adding organo-clay to As-contaminated soils will reduce the mobility of As species (Sarkar et al. 2012b). Iron compounds such as iron filings and ferric sulfate because of low price and high adsorption capacity have been widely used for arsenic removal from environmental (Nicolaose et al. 2003; Kim et al. 2003). The effects of natural zeolite and bentonite enriched with iron on the arsenic distribution among solid phase (especially exchangeable phase) and soil solution are not well understood. Although Zarshuran is greatest gold mine in Iran, it has produced high amount of arsenopyrite. There are no data about immobilization of As in contaminated soil of Zarshuran area. Therefore, the aims of this study were to investigate the effect of natural zeolite and bentonite, Fe-bentonite, Fe-zeolite, iron filings and ferric sulfate on the: (1) arsenic distribution among exchangeable and solution phase, (2) speciation of arsenate and arsenite in soil solution and (3) arsenic bioavailability in soil around the Zarshuran gold mine site.
Materials and methods
Soil
Sample collection and preparation
Soil was collected from agricultural site in Zarshuran area, about 50 km2 located at 36°43′21′′N and 47°8′25′′E, 42 km north of the Takab town in West Azerbaijan province, NW Iran. Soil samples (0–30 cm depth) were collected, air-dried, mixed, homogenized and sieved through 2- and 8-mm grid for physicochemical analysis and culture, respectively.
Soil characterization
Soil properties were measured using standard methods. pH in a soil paste saturated with water; organic matter by Tiurin method (Jackson 1960); cation-exchange capacity by ammonium acetate, and texture were measured using a pipette method (Salt et al. 1972). Total elemental composition of the soil was determined by hot digestion with HNO3 + HClO4 + HF (Van Herreweghe et al. 2003). Solution obtained from digestion was analyzed by graphite furnace atomic absorption spectrometry (GFAAS) for As, Cu, Cd, Ni, Pb and Zn (Fig. 1).
Preparation and characterization of the sorbents
The bentonite and zeolite were, respectively, obtained from Anarak and Firouzkoh mines in central and northern Iran. Natural bentonite and zeolite samples for 12 h were put in contact with 0.3 M NaCl solution (1:6.5 solid–liquid ratios) under turbulent flow. This process was repeated several times. The solid and liquid phases were separated by centrifugation, and the solid phases were washed. To ensure the removal of chlorine used the AgNO3 test (Macedo-Miranda and Olguín 2007). Then, the bentonite and zeolite samples were dried at 60 °C for 24 h. After modification with Na, clays were treated with (0.024 M FeCl3 for zeolite and 0.019 M for bentonite) under agitation for 24 h to prepare Fe-bentonite and Fe-zeolite (equivalent to 50% of their CEC).
The solid phases were separated by centrifugation, washed with distilled water and dried at 50 °C for 17 h. Then, each sample was thermally treated at 100 °C for 24 h, and then, the Fe-bentonite and Fe-zeolite were prepared (Macedo-Miranda and Olguín 2007). The X-ray diffraction patterns of natural and modified clays were obtained by X-ray diffraction (Fig. 2). The total elemental analysis of natural clays was performed by fluorescence spectroscopy using a Spectro X-Lab 2000 X-Ray instrument (Table 3). The cation-exchange capacity of natural clays was measured with sodium acetate (Salt et al. 1972), and pH of natural clays was measured in the extract 2:1 (Table 3).
The iron filings were obtained from Mobarakeh Steel complex (Isfahan, Iran). Iron filings were heated at 550 °C for 1 h and were passed by sieve (Mesh at 140). The total elemental analysis of iron filings was performed by emission spectrometry (Table 4).
Pot experiments
Plastic pots were filled with soil (1 kg) mixed with the designated amounts of the sorbents (Table 1). After 4 weeks of equilibrium in moisture at 80% of field capacity, two healthy sunflowers (Helianthus annuus L.) planted into each pot. At the end experiment (10 weeks), the plants were harvested, and shoots and roots separated, washed by distilled water and oven-dried at 65 °C to a constant mass for determination of the As concentration (GFAAS following HNO3–HClO4 digestion) (Harborne 1998).
Chemical analysis of soil after the culture
Soil samples were collected from each pot and water-soluble arsenate (soil and Milli-Q water were mixed in 1:10 w/w proportion), exchangeable arsenicFootnote 1 (soil and Na2HPO4 0.1M and pH 8 were mixed in 1:10 w/w proportion) and arsenite measured (Kim et al. 2003). The arsenate and arsenite concentration were determined by HG–AAS (hydride generation–AAS).
Processing data
The pot experiment was set up in a randomized complete block design with three replications. The ANOVA (SAS version 9.2) (SAS Institute 1999) was employed for statistical analysis of the data. Moreover, the data from soil solution samples were used as the input in a Visual MINTEQ version 3.0 in order to predict As speciation in soil solution. Input consisted of the measured soil solution concentrations of K, Ca, Mg, Fe, Na, As(III), As(V), Cu, Mn, Ni, Pb, Zn, and anions such as Cl−, PO −34 , NO3 −, SO −24 , dissolved inorganic carbon (DIC), dissolved organic carbon (DOC), electrical conductivity (ionic strength) and pH of soil solution in each pot. The specified redox couples were Fe+2/Fe+3, H3AsO3/AsO −34 , Cu+1/Cu+2, HS−/SO4−2, Mn+2/Mn+3 and NO 2−/NO 3−.
Results and discussion
Characteristics of the soil
Some physical and chemical characteristics of soil are presented in Table 2. The pH value of the Zarshuran soil (7.8) was close to neutral. Total concentrations of As and some heavy metals in the soil, comparing with the standards (Kabata-Pendias and Pendias 1984), indicated that total As, Pb and Cd concentrations were extremely high. The main reason of high As in Zarshuran area is the most abundant sulfide and orpiment (As2S3) in this soil (Mehrabi et al. 1999).
Characteristics of the sorbents
The cation-exchange capacity of zeolite and bentonite were, respectively, 94 and 76 meq/100 gr (Table 3). The natural zeolite and bentonite are alkaline properties (pH about 8.3 and 8, respectively). The main reason of alkaline properties for the natural bentonite and zeolite is the alkaline parent material bedrock. Also, the elemental analysis of natural zeolite and bentonite (Table 3) indicated the existence of alkali and alkaline earth metals on the clay surfaces, though, after the modification of clays with iron, their properties were acidic. The amount of iron in the iron filings was equivalent to 98.08 wt%, and other elements such as Cr, Mo and Ni were in low amount (Table 4).
The XRD patterns (Fig. 2) show that the bentonite is contained of 85.5% montmorillonite, 12% quartz and 2.5% illite, the zeolite of 80.6% clinoptilolite, 9.6% quartz, 5.8% illite and 4% feldspar. A strong peak at 13.1 Å in the natural bentonite is the indication of high amount of montmorillonite in the sample (Fig. 2). The XRD pattern for the Fe-bentonite was similar to the natural bentonite. But a strong peak at 14.6 Å indicated that trivalent iron was caused to increasing interlayer of space in Fe-bentonite. The strong peaks at 2.96, 3.94 and 9 Å in the natural zeolite were the indication of high amount of clinoptilolite in the natural zeolite. The XRD pattern Fe-zeolite was similar to the natural zeolite.
Speciation of arsenate and arsenite in soil solutions
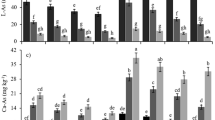
Arsenic in soil solution is considered as an important part of plant uptake. Understanding the distribution of species arsenic in solution is essential. Plants for arsenic uptake prefer to H2AsO −14 species.
According to the result of MINTEQ (Fig. 3), in soil solution without sorbent (control) the abundance of H2AsO −14 species was 12%. But adding different levels of natural zeolite and bentonite to the soil caused to the H2AsO −14 species 2–6% lower than the control. Application of Fe-zeolite, Fe-bentonite, iron filings and ferric sulfate in the soil by decreasing of soil pH increased abundance of H2AsO −14 species and decreased of HAsO −24 species. In soil solution without sorbent (control), abundance of H3AsO3 species was 95% but no difference between other sorbents and control (Fig. 4).
Mobility of arsenite and arsenate in soils
Arsenite concentration in the soil solutions was 0.43–0.5 mg/kg. It has no significant differences between the treatments and control (Table 5) which might be due to the small changes of soil pH (7.1–8.2) (Sparks 2003). H3AsO3 is the dominant species of arsenite at pH of about 7.1–8.2. Sparks (2003) reported that at pH less than 9, H3AsO3 is the most stable species of arsenite. The reason for low concentrations and lack of stabilization of H3AsO3 by different sorbents is related to soil ventilation and non-react ability, respectively. Liu et al. (2005) reported that soils with adequate ventilation had low concentration of arsenite, while the arsenate concentration was high.
Soluble arsenate concentration in soil without sorbent (control) compared to the other treatments was significantly different (Table 5). Soluble arsenate concentration in soil solution of the control was 9.25 mg/kg. With applying natural bentonite and zeolite (1, 6 and 12% w/w), soluble arsenate concentration in soil was significantly increased. Alkaline properties of natural zeolite and bentonite caused to increases in soil pH and the amount of OH− and releasing arsenate from exchangeable site. Increasing soil pH is due to repelling anionic phenomenon and released arsenate into the solution. Bohn et al. (2002) stated that with increasing soil pH, net negative charges increased and repelling anionic phenomenon in the soil would occur. EA-Na2HPO4 in soils treated with natural bentonite and zeolite decreased significantly compared to the control. Reducing of EA-Na2HPO4 indicates that the natural bentonite and zeolite released arsenate at exchangeable phase to the solution phase.
Increasing the levels of Fe-zeolite raised the amount of arsenate solution in soil subsequently. Probably, Fe-zeolite with decreasing soil pH increased solubility of magnesium and calcium arsenate, and arsenate is increased in the solution phase (Robins 1981). Again due to low external cation-exchange capacity and non-expandable structural of zeolite, low amount of arsenate could enter to the Fe-zeolite pit and stabilized by iron. Therefore, arsenic released from the dissolution of magnesium and calcium arsenate is more than the arsenic stabilized on the Fe-zeolite surface. Lack of significant difference in the amounts of EA-Na2HPO4 in control compared to the soil enriched with Fe-zeolite indicates that arsenic has been adsorbed by monodentate electrostatic bond on the Fe-zeolite sites and thus easily moved back into solution. Treating soil with 6 and 12 wt% Fe-bentonite caused to the amount of soluble arsenate relative to the control, be decreased about 53 and 73 percent, respectively. Possibly adding Fe-bentonite to the soil reduced soil pH and increased the solubility of magnesium and calcium arsenate and soluble arsenate. On the other side of laminate structure, expandable layer and high interlayer space of bentonite allowed that the arsenic easily bonded with iron (loaded on the clay). It seems that the amount of arsenate stabilized by Fe-bentonite is more than the arsenic released from magnesium and calcium arsenate. The comparing XRD pattern of Fe-bentonite and natural bentonite indicated that Fe3+ increases the interlayer space of bentonite (Fig. 2). Probably arsenate by electrostatic adsorption mechanism (monodentate and bidentate) and ligand exchange (monodentate and bidentate) immobilized onto Fe-bentonite adsorption sites. Part of arsenate that immobilized by mechanism of electrostatic adsorption is reversible to the solution phase, and the other part of arsenate that adsorbed by ligand exchange is nonreturnable to the solution phase. Sample et al. (1980) stated that when the phosphate adsorbed on the iron hydroxides, if with one hydroxyl bonded, its monodentate and phosphate is returnable, but phosphate with two hydroxyl bonded, its bidentate and phosphate is nonreturnable to the solution. Also Sparks 2003 stated that ligand exchange is a kind of specific absorption and its nonreturnable. Due to the similar characteristics of arsenate and phosphate, adsorption behavior of arsenic onto iron hydroxides likes phosphorus. Significant reducing of EA-Na2HPO4 in soil enriched with Fe-bentonite compared to the control indicates that great part of arsenic has been stabilized by ligand exchange and nonreturnable.
Applying iron filings and ferric sulfate into the soil reduced soluble arsenate and EA-Na2HPO4 compared to the control. Arsenic stabilization mechanism of adsorption (ligand exchange and electrostatic adsorption) and precipitation (FeAsO4) processes was done by iron filings and ferric sulfate, respectively. However, both sorbents by decreasing of soil pH increased the solubility of magnesium and calcium arsenate, but the amount of arsenic immobilizes is higher than in arsenic releases from magnesium and calcium arsenate. Decreasing of EA-Na2HPO4 in soil treated with iron filings indicated that arsenic immobilized by ligand exchange processes, and in soils enriched with ferric sulfate, extractable arsenic entered to the solution and then precipitated with iron.
Reactions of the absorption of As by sorbent (Macedo-Miranda and Olguín 2007) might be summarized as:
-
1.
Bidentate inner sphere complex:
$$\begin{aligned} & \left( {{\text{Columbic}}\;{\text{and}}\;{\text{Lewis}}\;{\text{acid}} - {\text{base}}\;{\text{interactions}}} \right) \\ & 2{\text{Clay}} - {\text{FeOH}}_{2}^{ + } \leftrightarrow {\text{HAsO}}_{4}^{2 - } \\ \end{aligned}$$ -
2.
Monodentate inner sphere complex:
$$\begin{aligned} & \left( {{\text{Columbic}}\;{\text{and}}\;{\text{Lewis}}\;{\text{acid}} - {\text{base interactions}}} \right) \\ & {\text{Clay}} - {\text{FeOH}}_{2}^{ + } \leftrightarrow {\text{H}}_{2} {\text{AsO}}_{4}^{ - } \\ \end{aligned}$$ -
3.
Ligand exchange
$$\begin{aligned} & {\text{Clay}-\text{Fe}-\text{OH}} + {\text{H}}_{2} {\text{AsO}}_{4}^{ - } \leftrightarrow {\text{Clay}-\text{Fe}-\text{H}}_{2} {\text{AsO}}_{4} + {\text{OH}} \\ & 2{\text{Clay}-\text{Fe} -\text{OH }} + {\text{ HAsO}}_{4}^{ - 2} \leftrightarrow 2{\text{Clay}-\text{ Fe}-\text{HAsO}}_{4} + 2 {\text{OH}} \\ \end{aligned}$$
Reaction precipitates of As by ferric sulfate (Sparks 2003):
Reactions of the solubility of magnesium and calcium arsenate (Robins 1981) as follows:
Biomass and as uptake by sunflower
Arsenic concentrations in roots and shoots of sunflower grown in the soil without sorbent (control) rather than other treatments were significantly different (Table 5). The amount of arsenic concentration in the roots had a significantly correlation with soil pH and soluble arsenate (Table 6). So soil pH is directly and indirectly affected the arsenic concentrations in roots. The indirect influence of pH on arsenic concentrations in root related to changes of arsenic speciation (especially in the soil enriched with bentonite 12% w/w) and lowering abundance of H2AsO4 − species. Correlation about 0.74 soil pH and soluble arsenate in soil indicates directly the effect of soil pH on the bioavailability of arsenic. Iron filings, Fe-bentonite and ferric sulfate by decreasing of soluble arsenate reduced arsenic concentration in roots and shoots. Significant correlation of arsenic concentration in root and shoot with dry weight of shoot stated that iron filings, Fe-bentonite and ferric sulfate by lowering of arsenic concentration in shoot enhance dry weight of shoots.
Conclusion
Adding sorbents (zeolite or bentonite) to the contaminated soil caused: changing of soil pH (about 0.2–0.7), high enhancing mobility of arsenate, lowering the mobility of arsenite and affecting the arsenic speciation in soil solution. Natural zeolite and bentonite and Fe-zeolite could increase the solubility of arsenate and thus result in increasing of arsenic contents in sunflower. In contrast, Fe-bentonite, iron filings and ferric sulfate could stabilize arsenic in soil. Iron filings with low cost rather than Fe-bentonite and ferric sulfate can be to use in barriers for isolating contaminated soil of Zarshuran to prevent groundwater pollution and As into food chain.
Notes
EA Na2HPO4.
References
Asadi HH, Hale M (2001) A predictive GIS model for mapping potential gold and base metal mineralization in Takab area, Iran. Comput Geosci 27:901–912
Bech J, Poschenrieder C, Llugany M, Barceló J, Tume P, Tobias F, Barranzuela J, Vásquez E (1997) Arsenic and heavy metal contamination of soil and vegetation around a copper mine in Northern Peru. Sci Total Environ 203:83–91
Bohn HL, Myer RA, O’Connor GA (2002) Soil chemistry. Wiley, London
Chatterjee A, Das D, Mandal BK, Chowdhury TR, Samanta G, Chakraborti D (1995) Arsenic in groundwater in six districts of West Bengal, India: The biggest arsenic calamity in the world— Part 1. Arsenic species in drinking water and urine of the affected people. Analyst 120:643–650
Garcıa-Sánchez A, Alastuey A, Querol X (1999) Heavy metal adsorption by different minerals: application to the remediation of polluted soils. Sci Total Environ 242:179–188
Hamidpour M, Afyuni M, Kalbasi M, Khoshgoftarmanes AH, Inglezakis VJ (2010) Mobility and plant-availability of Cd (II) and Pb(II) adsorbed on zeolite and bentonite. Appl Clay Sci 48:342–348
Harborne JB (1998) Phytochemical methods a guide to modern techniques of plant analysis. Springer, Berlin
Ho HH, Swennen R, Cappuyns V, Vassilieva E, Van Gerven T, Van Tran T (2013) Speciation and mobility of selected trace metals (As, Cu, Mn, Pb and Zn) in sediment with depth in Cam River-Mouth, Haiphong, Vietnam. Aquat Geochem 19:57–75
Inglezakis VJ, Stylianou MA, Gkantzou D, Loizidou MD (2007) Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 210:248–256
Institute SAS (1999) SAS user’s guide: statistics, version 8. SAS Institute Inc., Cary
Jackson ML (1960) Soil chemical analysis. Prentice-Hall Inc, Englewood Cliffs, p 489
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and plants. CRC Press, Boca Raton, pp 143–157
Karimi N, Ghaderian S, Maroofi MH, Schat H (2009) Analysis of arsenic in soil and vegetation of a contaminated area in Zarshuran, Iran. J Phytoremediat 12:159–173
Kim JY, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ Sci Technol 37:189–195
Krishna B, Murty D, Jai Prakash B (2001) Surfactant-modified clay as adsorbent for chromate. Appl Clay Sci 20:65–71
Liu WJ, Zhu YG, Smith FA (2005) Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 277:127–138
Macedo-Miranda M, Olguín M (2007) Arsenic sorption by modified clinoptilolite–heulandite rich tuffs. J Incl Phenom Macrocycl Chem 59:131–142
Malekian R, Abedi-Koupai J, Eslamian SS (2011) Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J Hazard Mater 185:970–976
Mehrabi B, Yardley BWD, Cann JR (1999) Sediment-hosted disseminated gold mineralization at Zarshuran, NW Iran. Miner Deposita 34:673–696
Modabberi S, Moore F (2004) Environmental geochemistry of Zarshuran Au-As deposit, NW Iran. Environ Geol 46:796–807
Nicolaose NP, Dobbs GM, Lackovic JA (2003) Arsenic removal by zero-valent iron: field, laboratory and modeling studies. Water Res 37:1417–1425
Robins RG (1981) The solubility of metal arsenates. Metall Trans 12:103–109
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, SCS (Soil Conservation Service) (1972) Soil survey laboratory methods and procedures for collecting soil samples. USDA, Washington
Sample E, Soper R, Racz G (1980) Reactions of phosphate fertilizers in soils. The role of phosphorus in agriculture pp 263–310
Sarkar B, Xi Y, Megharaj M, Krishnamurti GS, Naidu R (2010a) Synthesis and characterisation of novel organopalygorskites for removal of p-nitrophenol from aqueous solution: isothermal studies. J Colloid Interface Sci 350:295–304
Sarkar B, Xi Y, Megharaj M, Krishnamurti GS, Rajarathnam D, Naidu R (2010b) Remediation of hexavalent chromium through adsorption by bentonite based Arquad® 2HT-75 organoclays. J Hazard Mater 183:87–97
Sarkar B, Megharaj M, Xi Y, Naidu R (2011a) Structural characterisation of Arquad 2HT-75 organobentonites: surface charge characteristics and environmental application. J Hazard Mater 195:155–161
Sarkar B, Megharaj M, Xi Y, Naidu R (2011b) Orange II adsorption on palygorskites modified with alkyl trimethylammonium and dialkyl dimethylammonium bromidean isothermal and kinetic study. Appl Clay Sci 51:370–374
Sarkar B, Megharaj M, Xi Y, Naidu R (2012a) Surface charge characteristics of organo-palygorskites and adsorption of p-nitrophenol in flow-through reactor system. Chem Eng J 185:35–43
Sarkar B, Naidu R, Rahman MM, Megharaj M, Xi Y (2012b) Organoclays reduce arsenic bioavailability and bioaccessibility in contaminated soils. J Soils Sediments 12:704–712
Shum M, Lavkulich L (1999) Speciation and solubility relationships of Al, Cu and Fe in solutions associated with sulfuric acid leached mine waste rock. Environ Geol 38:59–68
Sparks DL (2003) Environmental soil chemistry. Access Online via Elsevier
Tseng W (1977) Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect 19:109–119
US EPA (2007) Framework for metals risk assessment. US Environmental Protection Agency, Washington, DC 20460. http://www.epa.gov/raf/metalsframework/pdfs/metals-risk-assessment-final.pdf. Accessed 20 Jan 2012
Van Herreweghe S, Swennen R, Cappuyns V, Vandecasteele C (2002) Chemical associations of heavy metals and metalloids in contaminated soils near former ore treatment plants: a differentiated approach with emphasis on pH stat-leaching. J Geochem Explor 76:113–138
Van Herreweghe S, Swennen R, Cappuyns V, Vandecasteele C (2003) Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ Pollut 122:323–342
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, LombiZ Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Acknowledgements
This work was supported by Soil Science Department and Soilless Cultivation Research Center at Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Hari Pant.
Rights and permissions
About this article
Cite this article
Shahmoradi, S., Afyuni, M. & Hajabbasi, M.A. Speciation, fractionation and plant availability of arsenic as induced by sorbents mixed with soil of Zarshuran (Iran). Int. J. Environ. Sci. Technol. 14, 767–776 (2017). https://doi.org/10.1007/s13762-016-1195-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1195-9