Abstract
Salt weathering is a crucial process that brings about a change in stone, from the scale of landscapes to stone outcrops and natural building stone façades. It is acknowledged that salt weathering is controlled by fluctuations in temperature and moisture, where repeated oscillations in these parameters can cause re-crystallisation, hydration/de-hydration of salts, bringing about stone surface loss in the form of, for example, granular disaggregation, scaling, and multiple flaking. However, this ‘traditional’ view of how salt weathering proceeds may need to be re-evaluated in the light of current and future climatic trends. Indeed, there is considerable scope for the investigation of consequences of climate change on geomorphological processes in general. Building on contemporary research on the ‘deep wetting’ of natural building stones, it is proposed that (as stone may be wetter for longer), ion diffusion may become a more prominent mechanism for the mixing of molecular constituents, and a shift in focus from physical damage to chemical change is suggested. Data from ion diffusion cell experiments are presented for three different sandstone types, demonstrating that salts may diffuse through porous stone relatively rapidly (in comparison to, for example, dense concrete). Pore water from stones undergoing diffusion experiments was extracted and analysed. Factors controlling ion diffusion relating to ‘time of wetness’ within stones are discussed, (continued saturation, connectivity of pores, mineralogy, behaviour of salts, sedimentary structure), and potential changes in system dynamics as a result of climate change are addressed. System inputs may change in terms of increased moisture input, translating into a greater depth of wetting front. Salts are likely to be ‘stored’ differently in stones, with salt being in solution for longer periods (during prolonged winter wetness). This has myriad implications in terms of the movement of ions by diffusion and the potential for chemical change in the stone (especially in more mobile constituents), leading to a weakening of the stone matrix/grain boundary cementing. The ‘output’ may be mobilisation and precipitation of elements leading to, for example, uneven cementing in the stone. This reduced strength of the stone, or compromised ability of the stone to absorb stress, is likely to make crystallisation a more efficacious mechanism of decay when it does occur. Thus, a delay in the onset of crystallisation while stonework is wet does not preclude exaggerated or accelerated material loss when it finally happens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“…questions associated with prospective geomorphological consequences of contemporary climate change have received far less attention than they deserve” (Church 2010, 281).
This paper seeks to explore the ways in which climate change is likely to impact on salt weathering (a key process in stone decay, weathering and geomorphological activity as a whole) in temperate climates where seasonality of precipitation is projected to increase throughout the twenty-first century (Smith et al. 2011a). There is a growing recognition among environmental geomorphologists, perhaps especially those involved in the heritage sector (Viles 2002; Hall 2009) that there is a need to understand change “not just in a dynamic world, but in a world where the nature of the dynamics is constantly changing” (Smith et al. 2008, 455). Weathering processes are an appropriate place to tackle this issue, as weathering is the ‘initiator’ of change in stone (both on buildings and in natural landscapes). In the context of stone decay in the built environment, salt weathering is perhaps the most ubiquitous decay process. In light of recent research into climate change impacts on decay (see for example, Viles 2002; Smith et al. 2004, 2011a; Brimblecombe and Grossi 2007), consideration of how this process in particular may change will be key to understanding stone (particularly sandstone) response to shifting climatic trends.
Geomorphological studies have long considered environmental processes in the context of systems (Chorley and Kennedy 1971; Schumm 1979; White et al. 1992). In any environmental system, there are controls that play a major role in determining system behaviour (Gregory 2010). In the case of porous stone in the presence of soluble salts, temperature and moisture have been highlighted as crucial controls/drivers of decay (Turkington et al. 2002). Any change in these controls is likely to result in a change in inputs to the stone decay system (in terms of moisture and salts), a change in storage (how long and how deeply moisture and salt may move into stone), and a change in outputs (alteration of the material over time). Of course, many natural systems are non-linear in nature, where small changes in inputs may result in a disproportionate change in output and overall system behaviour (Viles 2005).
“Weathering studies continue to be characterised… by uncertainties and gaps in knowledge” (Smith 2009). When it comes to projected climate change impacts on weathering systems, uncertainty abounds at each stage—not only in terms of how the climatic data is dealt with (choice of GCM, emission scenario, downscaling protocol, see for example, Crawford et al. 2007), but also in terms of ‘downscaling’ in an attempt to understand what a specific change in climatic controls may actually mean at process and mechanistic levels (McCabe et al. 2011a). Making the ‘leap’ from climate data to stone decay processes is another area of inherent difficulty in terms of uncertainties, gaps in present knowledge and method. Thus, in his classic volume on microclimate, ‘the Climate Near the Ground’, Rudolph Geiger discusses how ambient climate data is an abstraction, designed to tell the user what a specific parameter will be at about the height of a human being walking down a street—“the meteorological station is thus ‘representative’ of a wider surrounding area, and the climate thus measured is that experienced by a man walking upright” (Geiger 1965, 1). Consequently, it is with caution that such data is applied to, for example, stone surfaces and/or interiors. In fact, there is a wealth of data demonstrating that natural rock surfaces and building stone surfaces do not necessarily follow the same patterns as the ambient temperatures to which they are exposed (see Jenkins and Smith 1990; Smith and Warke 1997). Ambient air temperatures, whilst providing information on the climate history of a given area, cannot necessarily be used to illustrate or characterise the weathering regime of a given environment—“such undertakings are meaningless for they take no account of rock warming by the sun [or] rock to rock variations resulting from albedo affects” (Hall and Andre 2001, 24). Thus, ambient temperatures and stone surface/subsurface temperatures are not the same thing, and should not be treated as such. The same principle may be applied to moisture—the relationship between rainfall and moisture flux within the stone is not straightforward (depending on, for example, aspect, wind direction and speed, and the connected porosity/water absorption capacity characteristics of the stone, surface modification of the stone). Though the relationship is complex, internal stone moisture data has shown that prolonged periods of rainfall over successive days can bring about ‘deep wetting’ in stone to a depth of >25 cm from a building stone surface (see McAllister et al. 2011) in wet conditions in the west of Northern Ireland. Of course, dampness is already a major issue in natural stone buildings—a complex and widespread problem with a huge economic impact (Oliver 1988), which may only become more pressing in the coming decades.
Given the difficulty of extrapolating wider climatic data to stone microenvironments and interiors, when it comes to understanding how future climate change regimes may impact decay processes such as salt weathering, a thoughtful approach is needed—essentially, to ‘get inside’ the stone; conceptually and experimentally. “In exploring the impacts of climate change on stone-built structures, there is a need to ‘downscale’, not only in terms of climatic projections to the site scale, but experimentally to investigate impact at process and mechanistic levels” (McCabe et al. 2011b).
Conceptualising the process change
The ‘traditional’ view of how salt weathering proceeds (Smith et al. 2002, 2008) is based on the frequent wetting and drying of stone in the near-surface zone, where repeated crystallisation and expansion/contraction of salts breaches strength thresholds—for example, at grain boundaries, causing granular disaggregation and salt efflorescence (see Fig. 1). There are some variations on this where salts may produce scaling or multiple flaking, depending on the speed at which the stone surface is dried (Smith and McGreevy 1988; Huinink et al. 2004; Rodriguez-Navarro and Doehne 1999; Smith et al. 2011b). These views of salt weathering and associated decay forms have led to models of sandstone decay based on episodic retreat (especially in a building stone context), where a stone surface may appear stable and quiescent for a long period of time, until the stresses caused by accumulated salts breach the stone strength threshold and material loss is triggered (Smith et al. 2002).
However, as climate change scenarios are being incorporated into models of deterioration, this picture of episodic decay may warrant change or modification (see for example, Smith et al. 2011a). Increased winter rainfall means that stone may remain damp for long periods during winter months and beyond: “periods of quiescence may be longer than before. The outer zone of the block may experience regular wetting/drying cycles, but the block interior may only experience wetting/drying in response to seasonal cycling. Thus, while the onset of surface decay events may be delayed, when they do occur their severity may be increased as a result of deeper penetrating salts and enhanced surface/substrate heterogeneity” (Smith et al. 2011a, 1698).
If blocks are to remain damp for longer, as evidence from the NW UK increasingly suggests, it may be possible that blocks could experience saturation over the course of a winter (Smith et al. 2011a; McAllister et al. 2011). In this context, ion diffusion may be particularly important as a mechanism for salt transport (when there is no or little bulk water movement). Ion diffusion describes the mixing of molecular constituents in response to a concentration gradient. In porous media, ions will diffuse from areas of high concentration to areas of low concentration until equilibrium is reached (whereupon diffusion ceases).
The implications of such a salt transport mechanism for weathering have been outlined by Smith (2009): “this process could lead to complex, three dimensional distribution of salts throughout a rock mass and also facilitate chemical reactions with rock constituents”. Potential implications of ion diffusion for decay are related to the consequences of sandstone blocks being saturated by a saline solution for extended periods. “Weakening of rock structure may… be accomplished in quartz sandstone by selective silica dissolution under saline conditions, revealed as solutional etching on grain surfaces” (Smith 2009). The increase in solubility of quartz grains and amorphous silica cements with increasing pH is well established (Goudie 1997)—common evidence is provided in, for example, the formation of silicate deposits within the evaporate sequences of alkaline lakes (see Eugster 1967, 1969). Thus, the solubility of silica may increase dramatically above pH 9 (Young and Young 1992), and less developed crystalline structure may be more susceptible even at a lower pH. It is true that salt weathering is yet “incompletely understood” (Doehne 2002, 51), and a better understanding, or even a raising of awareness, of the potential chemical impacts of salt in solution is an important way forward.
This paper, then, seeks to make links between climatic inputs into the stone decay system, and how changes in those inputs may alter how the process of salt weathering proceeds, both in terms of the transportation of salts to depth in blocks (by ion diffusion), and also in regard to the potential for chemical alteration in the stone matrix (by analysis of pore water after stone exposure to an electrolyte solution). It is crucial to understand the factors by which diffusion is controlled or encouraged within these stones so that potential changes in salt weathering patterns accompanying increased time of wetness may be identified and understood.
Methods
Materials
The sandstones used in experiments were Peakmoor Sandstone, Locharbriggs Sandstone and Scrabo Sandstone. These stone types commonly used across the NW of the UK, one of the areas where winter rainfall is projected to increase, and time of wetness in stone may increase as a response. Petrographic analysis was carried out on thin sections with a polarising optical microscope (see Fig. 2).
Locharbriggs is a Permian red very fine to fine grained, moderately well sorted immature litharenite with sub-angular to angular grains, well-developed iron oxide coatings and syntaxial and silica cement. Rock fragments correspond to highly weathered metamorphic and volcanic rock. Metamorphic rock fragments form a muscovite pseudomatrix locally when deformed by diagenetical processes. This rock is quarried from Dumfries and Galloway (Scotland) and has 15.8 % porosity accessible to water and 4.57 % (by wt) water absorption capacity.
Peakmoor is a Carboniferous, buff, well-cemented, medium grained, moderately to well sorted sub-litharenite with subrounded to rounded grains. Rock fragments correspond to igneous rocks formed by quartz, potassium feldspar and biotite, in the main. This rock is quarried from Derbyshire (England) and has 16.5 % porosity accessible to water and 4.45 % (by wt) water absorption capacity.
Scrabo is laminated up to thin section scale. It is Triassic buff to pink, very fine to fine grained, well sorted, visibly very porous sub-arkose to arkose with lenses of smectite. It has a high content of opaque minerals (iron oxides and hydroxides mainly) and presents zircon, tourmaline and apatite as accessory minerals. This rock is quarried from Down (Northern Ireland) and has 22.4 % porosity accessible to water and 10.12 % (by wt) water absorption capacity.
Mercury porosimetry
Connected porosity was characterised by means of mercury intrusion porosimetry (MIP) with an Autopore IV 9500 Micrometics mercury porosimeter. The pore size characterisation ranged from 0.001 to 1,000 μm, which corresponds, respectively, to pressures from 15 to 60,000 psi. MIP was performed on small irregular rock samples of approximately 6.5 cm3. In addition to overall connected porosity and pore size distributions, other parameters related to solution movement were calculated with the results from this technique, such as specific surface and tortuosity. For the tortuosity, values obtained directly from the data reduction software of the Micrometrics MIP Autopore IV were used. These values are calculated through Hager’s (1998) modification of Katz and Thompson (1986) expression as described by Webb (2001).
Ion diffusion cell experiment
Salt concentrations in this diffusion cell experiment were determined by ascertaining pore water salt concentration from a ‘real-world’ building stone, exposed in Belfast (a polluted, maritime environment) (described by McCabe et al. 2011a). A 0.15 M solution was used for NaCl, while a 0.02 M solution was used for CaSO4 (combined for a mixed solution experiment).
To achieve sample saturation (necessary for the diffusion experiment to work), sandstone cores of the stones described above (50 mm thick, 100 mm in diameter) were placed in a vacuum desiccator with both ends of the core exposed. The pressure was reduced to 10–50 mbar and held steady for 3 h. Deaerated deionised water was added until the core was completely immersed. This vacuum was maintained for a further hour before allowing air to re-enter the desiccator after which the core was left for 16 h.
The diffusion cell experiment is based on studies carried out by Poupeleer et al. (2003). It operates by setting up a concentration gradient in the stone core that salts diffuse along until equilibrium is reached. The experimental set-up is illustrated in Fig. 3. Salt solution was placed in cell A and deionized water in cell B. A sandstone core saturated in deionized water was placed between the two cells. This core remains saturated for the duration of the experimental run, as salts diffuse from cell A, through the stone core, to cell B. Sampling from cell B at intervals, the concentration of chloride (and/or sulphate) can be determined in that cell using an ion chromatograph (IC). In this way, the diffusion of salts through the saturated stone can be monitored and quantified.
The experiment set-up requires the following assumptions: (1) the solution in the cell outside the stone sample is well mixed (this can be achieved with magnetic stirrers within the cells—see Fig. 3. (2) The water within the matrix of the core is immobile (i.e. the stone remains saturated). (3) The temperature of the solutions in the cells is constant (achieved by carrying out the experiment within a climate cabinet). (4) Sorption is negligible (not necessarily true with mineralogically complex sandstones).
Pore water analysis
Following the diffusion cell experiment, samples (stone cores) were crushed to remove the pore water. To achieve this, the sample was loaded to a maximum of 300 tonnes (at 5 tonnes per hour). Once this was completed, the load was removed and the pore water collected in a sealed container to avoid loss and contamination.
Pore water from this sample was tested for pH, anion analysis (using IC) and cation analysis (using AAS), allowing, to some extent, an ‘inside the stone’ perspective on the process (thought future work will explore this further using sequential extraction techniques—see for example, McAlister et al. 2003). A colorimetric technique was used for the detection of silica in pore water. This spectrophotometric method for analysing dissolved silica is based on the reaction between silicic acid and ammonium molybdate to form a yellow complex. The molar absorptivity of the complex is low and this is overcome by forming a reduced blue complex with oxalic acid and metol-sulphite solutions. This reaction prevents the reduction of excess molybdate and interference from phosphate. Absorbance is read at 810 nm.
Results
Mercury porosimetry
As observable in Table 1 and Fig. 4, Scrabo presents the highest overall connected porosity, with a marked bias towards larger pore sizes and very low specific surface (which means fewer surfaces to “attract” ions in solution if the surface presents free charge). This matches with the thin section observations of the open and relatively direct porosity (Fig. 2). Locharbriggs, on the other hand, presents the lowest values of overall connected porosity, the highest values of tortuosity and specific surface and the smallest average pore sizes of the three sandstones. This is caused partly by the diagenetical processes that compressed and disaggregated the mica-based metamorphic rock fragments, generating a pseudomatrix that both clogged the pores and made the pore system more convoluted. These parameters might be expected to exert significant control on the diffusion process.
Diffusion cell experiments
Figure 5 shows the diffusion of salt solutions (0.15 M NaCl alone, and 0.15 M NaCl mixed with 0.02 M CaSO4) through different sandstones. Results show that diffusion happens more rapidly in the Scrabo Sandstone, while the process seems suppressed in the case of Locharbriggs. Consistently, the presence of the CaSO4 in solution appears to slow down the diffusion of NaCl, which is relatively rapid in the single-solution experiment over the period of the experimental run.
Ion diffusion (0.15 M NaCl alone, and 0.15 M NaCl mixed with 0.02 M CaSO4) through different sandstone cores demonstrated in the increased ion concentrations in cell B: a NaCl through Peakmoor Sandstone, b NaCl + CaSO4 through Peakmoor Sandstone, c NaCl through Scrabo Sandstone, d NaCl + CaSO4 through Scrabo Sandstone, e NaCl through Locharbriggs Sandstone, f NaCl + CaSO4 through Locharbriggs Sandstone
Pore water chemistry
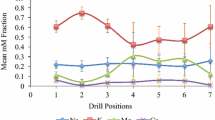
Table 2 shows the pH, Si, cation and anion results from analysis of the extracted pore water from each of the diffusion cell experiments.
Though at a low concentration, the presence of Si in the pore water is significant in demonstrating that it is being brought into solution (and experiencing limited re-distribution—Si was detected at 0.66 ppm in cell B in the Peakmoor Sandstone NaCl experiment) in the presence of electrolytes (see for example, Dove 1999). Background Si in deionized water after preparation of the cores was not greater than 0.2 ppm, demonstrating that the Si has originated from the stone (rather than the input salt solution). It suggests that the dissolution of silica (possibly amorphous) from the sandstone matrix may be taking place over a period of just 3 months with realistic-strength salt solutions (although simple leaching may also play a role). Pore water results from all cores (following the diffusion experiment) indicate that the highest concentrations of salts were held in the cores themselves (i.e. in the pore system). The pH of this pore water was recorded and shown in Table 2—often pH is quite strongly alkaline (over 8 in most cases), confirming that silica dissolution is likely to occur. Mg and K show variable levels of dissolution and mobility (perhaps related to weathering of clays and feldspar in the different stone types).
Discussion
The experiment carried out is the traditional approach of engineers seeking to investigate how long it may take for chlorides to reach rebars in concrete structures. As stone decay scientists, however, we want to ‘see inside’ the stone—for example, what elements within the stone does the salt solution mobilize? IC analysis of the pore water has shown that, by the end of the experiment, the highest concentrations of salts were held in solution within the stone core. The experimental run took place over a 2-month period. Clearly it is difficult to extrapolate this to stones with a long and complex exposure history. However, diffusion through blocks is likely due to long periods of block saturation (or near-saturation) (see for example, McAllister et al. 2011), and alteration of the material may be much more significant that what is implied in this short-term experiment (also bearing in mind declining stone strength over a long exposure period).
Controls on diffusion
What is controlling diffusion in the different sandstones, in terms of the transportation of salts to depth?
1. The continued saturation of the cores drives the process and ensures that it continues (although diffusion can occur in any wet area to mix molecular constituents). This is essentially an external driver of change, but related to and mediated by internal stone characteristics, such as the connectivity of pores. Thus, saturation is likely to depend on the stone being subjected to enough consecutive wet days to maintain wetness. However, even if the surface/near-surface zone is allowed to dry out, there can still be a significant reservoir of moisture at depth in the stone (see McAllister et al. 2011). While it is likely that evaporative flux will exist in the near-surface zone (i.e. absolute saturation may be rare), deep internal wetness may remain and drive chemical action in the stone interior (Srinivasan et al. 2009; Smith et al. 2011a). Where a moisture gradient is present in a stone block, it will encourage the ingress of moisture to depth, toward achieving equilibrium across the block (provided that moisture supply is maintained).
2. Pore connectivity/tortuosity—the connectivity of pores is the physical control that allows ions to move through the stone along the concentration gradient. However, pore connectivity can change as weathering proceeds (McCabe et al. 2011b)—especially where salt solutions are near saturation and may crystallize out, blocking pore throats.
“The length of the tortuous path through the pores to get from A to B (in particular from one side of a block of material to another) may be much longer than the direct distance AB… tortuosity has nothing to do with the size of the pores but entirely depends on the connectivity of the pore system” (Hall and Hoff 2012, 24–25). The tortuosity value (Table 1) brings to light the relationship between porosity and permeability (knowing the connectedness of pores does not give us an understanding of the tortuous route that moisture/ions actually make though the pore system—the tortuosity describes how the pore system and its connectedness is configured) and therefore, the higher the tortuosity, the higher the interaction between grain surface and salt solution. Ionic diffusion will be slowed if ions in solution tend to adsorb to grain surfaces. So, in general, the less interactions between grain surfaces and salt solutions the quicker ion diffusion may be (this is the likely scenario for Scrabo Sandstone, which shows a higher rate of diffusion over the period of the experimental run). Conversely, pore systems with high surface areas will be more prone to have slower ion diffusion rates, as there is a higher chance of surfaces reacting with salt solutions. For the same reasons, smaller pores will also favour a slower movement of ions (this is the likely scenario for the tortuous route through Locharbriggs Sandstone).
3. Mineralogy—as one would expect with sedimentary stones, there will be variability in mineralogy depending on provenance and diagenesis. The importance of micas and clays should be noted in terms of their potential to ‘fix’ ions in the interlamellar spaces of their structure. There might be evidence of this in both Locharbriggs and Scrabo. As mentioned elsewhere, diagenetical processes in Locharbriggs disaggregated the mica-rich metamorphic rock liberating a pseudomatrix that in addition to contributing to decreased porosity and increase tortuosity may also contribute to adsorption of hydrated ions and therefore decrease even further the overall diffusion process. This might also be the case of smectite lenses in Scrabo, thus making sense of the apparent disparate higher pore water concentrations of Na, Cl, Ca and SO4 in the case of the Scrabo stone core. Although micas are also observed in Peakmoor, these are “trapped” within the granitic rock fragments, and do not exert the same influence.
The presence of iron oxides and hydroxides and the way they are distributed may also play a role in controlling diffusion. Iron oxides and hydroxides are commonplace in sedimentary building stones and are often an aesthetic cause for their selection due to the deep red and yellow colours that iron oxides and hydroxides can generate at the surface. Iron minerals may be present as grains dispersed through the rock or as grain coatings. Depending on this, diffusion will be modified. Grain coatings modify both the free charges on the pore surfaces and the contact angle (wettability) between the salt solutions and the silicate grains in comparison to sandstones without grain coatings. Grain coatings are formed by iron oxides and hydroxides and they show intense variability in their contact angle with water (e.g. Iveson et al. 2004). The adsorption capacity of silicates is also modified by the existence of grain coatings. The studied samples (Fig. 2) show three different scenarios in relation to grain coatings and the presence of iron oxides. Peakmoor does not show abundant iron minerals, Locharbriggs shows well-developed iron oxide and hydroxide coatings and Scrabo do not show developed coatings, but a lot of iron oxides and hydroxides dispersed throughout the stone.
4. The behaviour of the different salts (anions and cations) in solution under pore conditions. This is a complex area of work, and can be difficult to investigate. Rodriguez-Navarro and Doehne (1999) have stated that the physical characteristics of a salt solution within porous media will influence how weathering proceeds. In each of the sandstones, the presence of CaSO4 appears to slow down the diffusion of NaCl. The authors suggest that this may be a physical effect, if the near-saturated CaSO4 is coming out of solution under pore conditions—i.e. it is likely that the CaSO4 is crystallizing out in pores even though the stone core is saturated (due to the very low solubility of CaSO4). This would then block pore throats, slowing down the diffusion of the NaCl. The available data suggests that this is the case (Fig. 5).
5. Sedimentary structural controls—Locharbriggs Sandstone has a higher water absorption capacity than Peakmoor Sandstone (connected porosity very similar in the MIP data), implying greater connectivity in the pore system. Yet the rate of diffusion is lower in this sandstone core. This may be due to high levels of tortuosity and abundance of micropores, but is also likely to be related to structural controls—Locharbriggs sandstone is heavily bedded, and salts needed to diffuse through several bedding layers perpendicular to the concentration gradient. A future test would run bedding parallel to the diffusion gradient, perhaps allowing faster diffusion.
Changes to the system and future questions
The introduction to this paper described salt weathering in terms of environmental systems. We return to that theme now to briefly discuss how the factors considered in the paper (in terms of block saturation and ion diffusion) may change the dynamics of the stone/salt system.
-
Inputs—increased moisture input means a greater depth of wetting front (so long as the moisture supply is kept up), and greater facilitation of salt ingress into block interiors.
-
Storage—salts may be held in storage differently, in terms of being in solution for longer. This has myriad implications in terms of the movement of ions by diffusion (along 3-dimensional gradients, rather than the simplified ‘uni-directional’ gradient encouraged in this study) and the potential for chemical change in the stone (especially in more mobile constituents), leading to a weakening of the stone matrix/grain boundary cementing. High alkalinity is observed in the pore water chemistry—in some cases, above pH 10. There are obvious implications for weathering—even in this relatively short experimental period, it is clear that the material is undergoing alteration in the presence of moisture and soluble salts. The potential mobilisation of elements that are traditional viewed as stable is noted—the bringing of Si into the pore solution, even in small amounts, is significant for long-term weathering and decline in the ability of the stone matrix to resolve stresses with material loss.
-
Output—the ‘output’ may be mobilisation and precipitation of elements leading to, for example, uneven cementing in the stone. As already outlined by Smith et al. (2011a), this reduced strength of the stone, or compromised ability of the stone to absorb stress, is likely to make crystallisation a more efficacious mechanism of decay when it does occur. Thus, a delay in the onset of crystallisation while stonework is wet does not preclude exaggerated or accelerated material loss when it finally happens.
In any environmental system, there is the potential for the establishment of feedback, where one variable in the system impacts on others, which in turn changes the original variable—changes in a system will be echoed by changes in morphological structure, which, in turn, can alter how the system operates, changing the inputs (Chorley and Kennedy 1971). Surface moisture will encourage colonisation by algae and other biological agents (Cutler and Viles 2010)—which may aid in moisture retention at and below the surface for several reasons. First, the organisms can retain moisture at the organism/stone interface (Gorbushina 2007), and second, the surface growth will reduce the permeability of the stone surface, reducing the stone’s ability to ‘breathe’ properly. This can interfere with the natural drying process, trapping moisture and soluble salts in the stone interior (see for example, McCabe et al. 2006).
Many historic sandstones have, of course, already undergone surface modification, so there remain a number of research questions regarding understanding how stone with a long and complex exposure history (and hence surface modification) may respond to climate change and ‘deep wetting’ (in terms of subsurface response) in relation to the process of salt weathering. There is thus a need for further investigation into the following areas:
-
How does surface alteration caused by climate change (for example, algal growth, weakening and surface loss) impact subsurface patterns of moisture and salt distribution?
-
How does climate-driven surface change alter albedo and sub-surface temperature patterns?
-
How do surface treatments designed to minimise the impact of climate change alter subsurface patterns of moisture and salt distribution?
-
How deeply may salts diffuse through stone over a 6-month period? What is the chemical impact of such a period of saturation?
-
How does rapid summer drying impact the crystallisation and distribution of salts within historic stone?
Research to answer these questions is underway, and seems crucial to understanding the changing dynamics of stone systems and the management of stone heritage under present and future conditions in much of the UK and NW Europe.
Conclusions
Those studying geomorphological and stone decay processes should have an awareness of the dynamism of the systems they deal with, in response to environmental and climatic change. The way in which processes act to bring about change will inevitably shift as a result of/response to control-shifts. This paper has looked at one process that is likely to change in particular regions—with implications for building stones (and, by extension, stone in the natural environment).
Greater moisture input into stones is likely to place emphasis on the diffusion of ions through stone, potentially to depth. Although in saturated media it is easier to ‘force’ rates of ionic transport through ‘unidirectional’ models (as in the present study, setting up a strong gradient from cell A to B), it perhaps ignores more complex factors such as gravitational effects. From the results, it seems the movement of ions in natural building sandstones is more complex than simply moving continuously through an inert ‘pipe’ of connected pores—the concentration/accumulation of ions inside the pore systems suggests that there are extra factors that an unidirectional model can not predict/detect (these experiments are using this 1D kind of assumption). Stone saturated with a saline/electrolyte solution may be subject to incremental chemical changes that, when placed in the context of complex stress histories, are significant in contributing to decay. The potential for silica mobilisation (and other constituents) under alkaline pore water conditions has been highlighted, with implications for long-term stone durability associated with uneven silica cementation (see for example, Nespereira et al. 2010).
In an attempt to address some complex questions on the potential impact of climatic change on the process of salt weathering, the authors are aware that this may have asked and raised further questions. We are hopeful that this will open up a potentially fruitful avenue of enquiry in the future.
References
Brimblecombe P, Grossi CM (2007) Damage to Buildings from Future Climate and Pollution. American Preservation Technology Bulletin 38:13–19
Chorley RJ, Kennedy BA (1971) Physical Geography: A systems approach. Prentice Hall International Inc., London
Church M (2010) The trajectory of geomorphology. Prog Phys Geogr 34:265–286
Crawford T, Betts NL, Favis-Mortlock D (2007) GCM grid-box choice and predictor selection associated with statistical downscaling of daily precipitation over Northern Ireland. Climate Research 34:145–160
Cutler N, Viles HA (2010) Eukaryotic microorganisms and stone biodeterioration. Geomicrobiol J 27(6):630–646
Doehne E (2002) Salt weathering: a selective review. In Seigesmund S, Weiss T, Vollbrecht A (eds) Natural Stone, Weathering Phenomenon, Conservation Strategies and Case Studies. Geological Society, London, Special Publications 205, pp 51-64
Dove PM (1999) The dissolution kinetics of quartz in aqueous mixed cation solutions. Geochim Cosmochim Acta 63:3715–3727
Eugster HP (1967) Hydrous sodium silicates from Lake Magadi, Kenya: precursors to bedded chert. Science 157:1177–1180
Eugster HP (1969) Inorganic bedded cherts from the Magadi area, Kenya. Contrib Miner Petrol 22:1–31
Geiger R (1965) The Climate Near the Ground. Harvard University Press, Massachusetts, 4th Edition
Gorbushina A (2007) Life on the rocks. Environ Microbiol 9(7):1613–1631
Goudie AS (1997) Weathering processes. In: Thomas DSG (ed) Arid Zone Geomorphology, 2nd edn. Wiley, Chichester, pp 25–39
Gregory KJ (2010) The Earth’s Land Surface. SAGE Publications Ltd., London
Grossi CM, Brimblecombe P (2007) Effect of long-term changes in air pollution and climate on the decay and blackening of European stone buildings. In: Prikryl R, Smith BJ (eds) Building Stone Decay: From Diagnosis to Conservation. Geological Society, London, Special Publications 271, pp 117–130
Hager J (1998) Steam Drying of Porous Media, Ph.D. Thesis, Department of Chemical Engineering, Lund University, Sweden
Hall K (2009) Rock art vs cultural stone: some geomorphological perspectives on weathering and conservation under a changing climate. S Afr Geogr J 91:58–62
Hall K, Andre M-F (2001) New insights into rock weathering from high–frequency rock temperature data: an Antarctic study of weathering by thermal stress. Geomorphology 41:23–35
Hall C, Hoff WD (2012) Water transport in brick, stone and concrete. Spon Press, Oxon
Huinink HP, Pel L, Kopinga K (2004) Simulating the growth of tafoni. Earth Surf Proc Land 29:1225–1233
Iveson SM, Holt S, Biggs S (2004) Advancing contact angle of iron ores as a function of their hematite and goethite content: implications for pelletising and sintering. Int J Miner Process 74:281–287
Jenkins KA, Smith BJ (1990) Daytime rock surface temperature variability and its implications for mechanical rock weathering: tenerife, Canary Islands. Catena 17:449–459
Katz AJ, Thompson AH (1986) A quantitative prediction of permeability in porous rock. Phys. Rev. B. 34:8179–8181
McAlister JJ, Smith BJ, Curran JM (2003) The use of sequential extraction to examine iron and trace metal mobilisation and the case-hardening of building sandstone: a preliminary investigation. Microchem J 74:5–18
McAllister D, McCabe S, Srinivasan S, Smith BJ, Warke PA (2011) Moisture dynamics in building sandstone: monitoring strategies and implications for transport and accumulation of salts. In: Ioannou I., Theodoridou M (eds) Salt Weathering on Buildings and Stone Sculptures 2011, University of Cyprus, pp 39-46
McCabe S, Smith BJ, Warke PA (2006) Calcium loading of building sandstones by lime rendering: implications for decay. In: Fort R, Alvarez de Buergo M, Gomez-Heras M, Vazquez-Calvo C (eds.) Heritage, Weathering and Conservation. Taylor & Francis Group, London, pp 177–182
McCabe S, McKinley JM, Gomez-Heras M, Smith BJ (2011a) Dynamical instability in surface permeability characteristics of building sandstone in response to salt accumulation over time. Geomorphology 130:65–75
McCabe S, Smith BJ, McAlister JJ, McAllister D, Srinivasan S, Basheer PAM, Curran JM (2011) Linking climate change, moisture dynamics and salt movement within natural building sandstones: implications for salt transport by diffusion. In: Ioannou I., Theodoridou M (eds) Salt Weathering on Buildings and Stone Sculptures 2011, University of Cyprus, pp 63–70
Nespereira J, Blanco JA, Yenes M, Pereira D (2010) Irregular silica cementation in sandstones and its implication on the usability as building stone. Eng Geol 115:167–174
Oliver A (1988) Dampness in Buildings. BSP Professional Books, Oxford
Poupeleer AS, Carmeliet J, Roels S, Van Gemert D (2003) Validation of the salt diffusion coefficient in porous materials. International Journal for Restoration of Buildings and Monuments 9:663–682
Rodriguez-Navarro C, Doehne E (1999) Salt weathering: influence of evaporation rate, supersaturation and crystallization pattern. Earth Surf Proc Land 24:191–209
Schumm SA (1979) Geomorphic thresholds: the concept and its applications. Transactions of the Institute of British Geographers, New Series 4(4):485–515
Smith BJ (2009) Weathering processes and forms. In: Parsons AJ, Abrahams AD (eds) Geomorphology of Desert Environments (2nd ed), Springer Science + Business Media B. V., pp 69–100
Smith BJ, McGreevy JP (1988) Contour scaling of a sandstone by salt weathering under hot desert conditions. Earth Surf Proc Land 13:697–705
Smith BJ, Warke PA (1997) Controls and uncertainties in the weathering environment. In: Thomas DSG (ed) Arid Zone Geomorphology, 2nd edn. Wiley, Chichester, pp 41–54
Smith BJ, Turkington AV, Warke PA, Basheer PAM, McAlister JJ, Meneely J, Curran JM (2002) Modelling the rapid retreat of building sandstones: a case study from a polluted maritime environment. In: Seigesmund S, Weiss T, Vollbrecht A (eds) Natural Stone, Weathering Phenomenon, Conservation Strategies and Case Studies. Geological Society, London, Special Publications 205, pp 347–362
Smith BJ, Warke PA, Curran JM (2004) Implications of climate change and increased ‘time-of-wetness’ for the soiling and decay of sandstone structures in Belfast, Northern Ireland. In: Prikryl R (ed) Dimension Stone. Taylor & Francis Group, London, pp 9–14
Smith BJ, Gomez-Heras M, McCabe S (2008) Understanding the decay of stone-built cultural heritage. Prog Phys Geogr 32:439–461
Smith BJ, McCabe S, McAllister D, Adamson C, Viles HA, Curran JM (2011a) A commentary on climate change, stone decay dynamics and the ‘greening’ of natural stone buildings: new perspectives on ‘deep wetting’. Environmental Earth Sciences 63:1691–1700
Smith BJ, Srinivasan S, Gomez-Heras M, Basheer PAM, Viles HA (2011b) Near-surface temperature cycling of stone and its implications for scales of surface deterioration. Geomorpohology 130:76–82
Srinivasan S, Basheer PAM, Smith BJ, Gomez-Heras M, Grattan KTV, Sun T (2009) Use of fiber optic and electrical resistance sensors for monitoring moisture mocement in building stone subjected to simulated climatic conditions. Journal of ASTM International 7(1):1–11
Turkington AV, Smith BJ, Basheer PAM (2002) The effect of block retreat on subsurface temperature and moisture conditions in sandstone. In: Prikryl R, Viles HA (eds) Understanding and managing stone decay. The Karolinum Press, Prague, pp 113–126
Viles HA (2002) Implications of future climate change for stone deterioration. In: Seigesmund S, Weiss T, Volbrecht A (eds) Natural Stone, Weathering Phenomenon, Conservation Strategies and Case Studies. Geological Society, London, Special Publications, 205, pp 407–418
Viles HA (2005) Can stone decay be chaotic? In: Turkington AV (ed) Stone Decay in the Architectural Environment. Geological Society of America Special Paper 390, pp 11–16
Webb PA (2001) An Introduction To The Physical Characteriazation of Materials by Mercury Intrusion Porosimetry with Emphasis On Reduction And Presentation of Experimental Data. Micromeritics Instrument Corp, Norcross, Georgia
White ID, Mottershead DN, Harrison SJ (1992) Environmental systems: an introductory text. Chapman & Hall, London. Second edition
Young R, Young A (1992) Sandstone Landforms. Springer, Heidelberg
Acknowledgments
This research was funded by a Historic Scotland Research Fellowship and by EPSRC grant EP/G01051X/1. Miguel Gomez-Heras was supported by Regional Government of Madrid Geomateriales S2009/MAT-1629 and by a PICATA postdoctoral fellowship of the Moncloa Campus of International Excellence (UCM-UPM, CSIC). We thank anonymous reviewers for their comments, which helped improve this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCabe, S., Smith, B.J., McAlister, J.J. et al. Changing climate, changing process: implications for salt transportation and weathering within building sandstones in the UK. Environ Earth Sci 69, 1225–1235 (2013). https://doi.org/10.1007/s12665-013-2278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2278-2