Abstract
Salt weathering represents a major cause of damage on stone monuments worldwide. However, processes of salt weathering still cannot be explained satisfactorily. Further systematic investigation of stone monuments is required for the improvement of knowledge on active salt weathering processes and controlling factors. Assessment of the dynamics of salt crystallization–dissolution processes is a focus of modern salt weathering research. The overall aim of the ‘petraSalt’ research project are real-time/real-scale weathering models that depict characteristic interdependencies between stone properties, monument exposure regimes, environmental influences, salt loading, salt crystallization–dissolution behaviour and salt weathering damage. The rock-cut monuments of ancient Petra in Jordan were selected for studies. A main part of the project is the joint evaluation of salt load and environmental conditions acting on it, allowing a differentiated, depth-dependent quantification of the complex salt crystallization–dissolution processes, considering diurnal and seasonal variation. The approach is exemplified.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Stone monuments
- Salt weathering
- Wireless sensor network
- Environmental monitoring
- Salt crystallization models
1 The City of Petra and Salt Weathering Damage
The ancient city of Petra is located in a mountainous region of Southwest Jordan. In Petra many hundred monuments like tombs, sanctuaries and places of worship were carved by the Nabataeans from sedimentary bedrocks (mainly sandstones of Cambrian to Ordovician age) about 2,000 years ago. In 1985 UNESCO inscribed Petra on the list of World Heritage. All rock-cut monuments have suffered damage from weathering, partly of alarming extent. Results of a previous research project (1996–1999) had revealed salt weathering as major cause of damage (e.g., Heinrichs 2008). This was the reason for focusing the ‘petraSalt’ project on this significant weathering process. Considering the huge masses of rock removed for monument creation, unweathered condition of the monuments can be assumed for their initial phase of exposure. Thus, active salt weathering processes can be limited to a period of about 2,000 years (Heinrichs and Nguyen 2011).
2 Environmental Monitoring
An autonomously operating wireless sensor network was applied in Petra as an innovative technology for high-resolution monitoring of environmental conditions affecting the monuments and acting as driving forces for salt weathering processes (Heinrichs et al. 2012). The system was developed in cooperation with TTI GmbH—TGU Smartmote (Stuttgart, Germany). The system consists of sensor nodes and gateways. The sensor nodes comprise processor board, radio module, long-life batteries, sensors for the measurement of temperature, relative humidity and electrical impedance in various depths (1, 3, 6, 9, 13 and 18 cm) inside the stone and sensors for the measurement of temperature, relative humidity, stone surface temperature, wind, rain and light at the stone surface. The sensor nodes were programmed to collect data every 5 min. They sent the collected data to gateways in Petra, equipped with solar energy supply units. The gateways relayed the measurement data to a long-distance network (GSM) for remote access per internet. The sensor network collected data from September 2012 to June 2013. In this way, the sensor system provided an extraordinary information output regarding environmental conditions at the Petra monuments considering diurnal, seasonal and depth-dependent variation. The data were exported from the database on daily basis for further evaluation.
3 Case Study
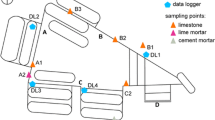
The presented case study was chosen for reasons of rather high salt load and salt load to great depth. The investigation area was located at the right side of the ‘Silk Tomb’ (Monument No. 770). The case study refers to sensor node 770-10. It was installed in a height of 3.36 m above ground level. The stone surface steeply inclines to the north (direction of inclination: 10°, angle of inclination: 88°). The stone surface is mainly shadowed (effect of insolation rather low). The sensor node was positioned at the margin of a water run-off path, showing granular disintegration as weathering form. The case study concerns a grey-red variety of the multicoloured, matrix-rich, fine-grained sandstone of the Cambrian Umm Ishrin Sandstone Formation (middle part). Considering certain gaps in the series of climate data, mainly due to disturbances of the GSM network in Petra, complete sets of climate data were available for 208 days comprising 306,000 temperature and humidity data in total.
4 Salt Analysis and Salt Crystallization Models
Drill cores obtained from dry drilling in the course of the sensor network installation were taken for ionic analysis of soluble salts. The quantitative analysis of salt crystallization–dissolution processes requires knowledge about the salt crystallization behaviour under changing temperature and humidity conditions. The crystallization behaviour of mixed salt systems is rather complex and, thus, prediction not easy. However, there has been considerable progress in the use of salt crystallization models such as ECOS-RUNSALT—used here—which allows prediction of the crystallization pathways and phase transitions even for complex salt mixtures (Steiger and Heritage 2012). RUNSALT serves as user interface to the ECOS program “Environmental Control of Salt Damage—thermodynamic model for the prediction of the crystallization behaviour of salt mixtures under changing climate conditions” (Bionda 2002–2005; Price 2000). Following ions are included in the ECOS-RUNSALT model parameterization: Na+, K+, Mg2+, Ca2+, Cl−, NO3 − and SO4 2−. These ions represent the typical constituents of salt mixtures found at the Petra monuments. The drill cores were analyzed segment-wise (0–0.5 cm, 0.5–1.5 cm, 1.5–2.5 cm,…17.5–18.5 cm). Application of the ECOS-RUNSALT program requires that the sum of the cation and anion charges is equal (Steiger and Heritage 2012). Thus, a charge balance has to be made first. In the samples of the case study a systematic cation excess was found. Besides salt minerals, calcite characteristically occurs in the Petra rocks as further secondary mineral. From experience it is known, that a certain fraction of calcite is mobilized by the eluting procedure as dissolved Ca2+ and HCO3 − ions despite the low solubility of calcite. Therefore, the charge balance can be equalized by corresponding reduction of the Ca2+ content. The real content of calcite was determined by testing with hydrochloric acid. The corrected weight fractions of the ions were related to one kilogram salt-free and calcite-free stone material. These weight fractions represented the input data for the ECOS-RUNSALT program. Soluble salt was found in the drill core segments from 0 to 15.5 cm. With respect to weight fractions of ions, highest concentration of soluble salt occurs in the outermost part of the drill core (0–3.5 cm). Na+ is the prevailing cation in the outermost 4.5 cm, followed by Ca2+. Further to depth, Ca2+ and Mg2+ become prevalent. Cl− is the predominant anion all over the profile, followed by NO3 − and SO4 2−. The ECOS-RUNSALT cannot predict the crystallization behaviour in case of very complex salt solutions containing Ca2+ and SO4 2− at the same time, like found in all segments of drill core 770-10. In this case the program asks for removal/reduction of SO4 2− and Ca2+ fractions that may form gypsum. Although gypsum is a major salt in many stone monuments, also in the Petra rock-cut monuments, its removal from the system does not mean a great problem, because gypsum is considered as very inactive with respect to climate fluctuations due to its very low solubility (Steiger and Heritage 2012). Nevertheless, the concentration of gypsum was calculated separately from those portions of Ca2+ and SO4 2− removed from the input data.
The ECOS-RUNSALT program offers the calculation of the salt crystallization behavior for range of temperature between −30 and 50 °C and relative humidity between 15 and 98 %. Volume of crystallized salt was selected as mode of evaluation. Regarding the case study, the program predicted halite (NaCl), sylvite (KCl), calcium nitrate (Ca(NO3)2), niter (KNO3), carnallite (KClMgCl3 · 6 H2O) and nitrocalcite (Ca(NO3)2 · 4 H2O) as potential salt phases. Due to loo low salt concentration, only halite was confirmed by X-ray diffraction analysis. However, previous X-ray diffraction analysis of surface samples had identified sylvite, calcium nitrate, carnallite, nitrocalcite, niter and gypsum as potential salt phases. Based on export and evaluation of data obtained from systematic query, salt crystallization models were derived individually for all drill core segments displaying salt phase formation for the overall ranges of temperature and relative humidity. It is well known that the deliquescence relative humidity of a single salt can be considerably reduced by the influence of other salt phases. This was also found here. In case of complete salt crystallization, halite, carnallite, calcium nitrate and gypsum occur in all segments of the drill core, sylvite up to a depth of 5.5 cm, niter only down to 1.5 cm. The salt profile is clearly dominated by chloride salts. Halite represents the prevalent salt phase in the outermost segments, carnallite dominates further to depth. Highest volumes of salt (6–8 cm3/kg stone) are found in the outermost 3.5 cm of the drill core. Here the volume of completely crystallized salt corresponds to a degree of pore filling between 7 and 11 % (comparably low).
5 Merging of Salt Crystallization Models and Climate Data
The approach aimed at quantification of salt crystallization–dissolution processes over depth analogue to the segmentation of the drill core. This necessitated interpolation of climate data for certain depths. A program for such interpolation was developed. Furthermore, the approach was tailored to identification of salt crystallization–dissolution processes by number and intensity, considering total salt as well as individual salt phases. With respect to intensity, a subdivision of six levels according to volume-% of crystallized salt was made for total salt (0 %/salt completely dissolved, 0–25, 25–50, 50–75, 75–100, 100 %/salt completely crystallized). For the individual salt phases a similar subdivision was made. Corresponding limit values were determined for all drill core segments from the ECOS-RUNSALT data. Trend curves of high approximation and related mathematical functions were ascertained for the resulting graphs of the limit values over total range of temperature and relative humidity. On daily basis, climate data were entered in these crystallization models. By use of “If, then…else” functions for each data pair temperature/relative humidity of 1 day the corresponding level of crystallization was determined by the evaluation program. In this way, the distribution of salt crystallization levels over depth was assessed by day for total salt and single salt phases. For more detailed assessment of salt crystallization–dissolution processes, the evaluation scheme was programmed to determine the salt crystallization levels at the beginning and the end of the day as well as minimum and maximum crystallization level. Considering these four parameters, each with six possibilities in case of total salt (6 levels of crystallization), this would mean 1,296 possible constellations regarding salt crystallization–dissolution processes. After removal of non-realistic cases (e.g., “minimum” cannot be higher than “start” or “end”), 196 cases remained regarding total salt. Aiming at clear presentability of results, these were summarized into 36 cases, considering three criteria: particular crystallization levels and total number of crystallization levels passed through, relation between crystallization and dissolution. Corresponding classifications were elaborated for the single salt phases. The evaluation scheme was programmed to identify the corresponding crystallization–dissolution cases by use of “If, then…else” mathematical functions applying to the combination of the parameters “start”, “end”, “minimum” and “maximum”.
6 Results, Discussion and Outlook
Salt crystallization–dissolution processes in the measuring period were found to be limited to the outer 8.5 cm of the profile 770-10. Further to depth, salt (except gypsum) was always completely dissolved. In the whole measuring period gypsum was crystallized all over depth. Crystallization–dissolution processes concerned halite, sylvite, niter, carnallite and calcium nitrate. Nitrocalcite never crystallized. Maximum depth of salt crystallization–dissolution processes decreased from later summer (September–November 2012) to minimum in winter (December 2012–February 2013). It increased again in early summer (March–May 2013) and further in high summer (only data from June 2013 available). Maximum depth of salt crystallization–dissolution processes was reached in high summer. With respect to total salt (except gypsum), 26 of the 36 potential cases of salt crystallization–dissolution processes occurred. The outer 4.5 cm of the depth profile (zone of highest salt loading) were affected by crystallization–dissolution processes almost every day! Frequency of crystallization–dissolution processes significantly decreased further to depth. Highest intensity (here: range of crystallization–dissolution processes over five levels of crystallization) was found in the outermost segment of the depth profile, however, only on few days. Crystallization–dissolution over 3–4 crystallization levels occurred frequently in the front 2.5 cm of the depth profile. Further to depth, intensity of crystallization–dissolution processes decreased. Complete salt crystallization was reached only in the front 3.5 cm of the profile. In the front 1.5 cm complete salt crystallization was reached on more than 100 days, most frequently in early and high summer. Complete salt dissolution was reached rarely in the front 4.5 cm of the profile. Further to depth, frequency of days reaching complete dissolution increased considerably. Further evaluation addressed the identification of active salt phases.
Halite was found as main active salt phase, followed by sylvite, niter, carnallite and calcium nitrate. This concerns likewise frequency and depth of crystallization–dissolution processes. Only in the front 1.5 cm of the profile, the five salt phases were found as jointly active to high extent. The results indicate maximum stress due to salt crystallization processes in the outermost part of the depth profile, being such high to find expression in granular disintegration at the stone surface. This weathering behaviour must be considered as the cumulative result of almost daily crystallization–dissolution processes all over the year, although the results indicate early and high summer as seasons of maximum stress.
An evaluation strategy was worked out, focused on well-directed merging of climate data with data on salt loading. Meaningful quantitative information on salt crystallization–dissolution processes was obtained. The new approach to quantitative analysis of salt crystallization–dissolution processes is transferred to the further case studies included in the “petraSalt” project, thus considering different stone types, monument exposure scenarios, climate regimes and salt load. These results in all are expected to allow reliable rating and interpretation of aggressiveness and damage potential of salt weathering regimes considering their variability.
References
Bionda D (2002–2005) RUNSALT computer program. Online available at: http://science.sdf-eu.org/runsalt/
Heinrichs K (2008) Diagnosis of weathering damage on rock-cut monuments in Petra/Jordan. Environ Geol 56(3): 643–675 (special issue: Monument future: climate change, air pollution, decay and conservation—the Wolf-Dieter-Grimm volume)
Heinrichs K, Azzam R, Krüger M (2012) The use of a wireless sensor network for high-resolution environmental monitoring of stone monuments in context with investigation of salt weathering—exemplified for rock-cut monuments in Petra/Jordan. In: Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone, October 22–26, 2012, New York, USA (in press)
Heinrichs K, Nguyen HT (2011) 3D terrestrial laser scanning of rock-cut monuments in Petra/Jordan. Mitt. Ing.-u. Hydrogeol Lehrstuhl für Ingenieurgeologie und Hydrogeologie, RWTH Aachen 104:27–37
Price C (2000) An expert chemical model for determining the environmental conditions needed to prevent salt damage in porous materials. Protection and Conservation of the European Cultural Heritage, Research report No. 11, Archetype, London
Steiger M, Heritage A (2012) Modelling the crystallization behaviour of mixed salt systems: input data requirements. In: Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone, October 22–26, 2012, New York, USA (in press)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Heinrichs, K., Azzam, R. (2015). Quantitative Analysis of Salt Crystallization–Dissolution Processes on Rock-Cut Monuments in Petra/Jordan. In: Lollino, G., Giordan, D., Marunteanu, C., Christaras, B., Yoshinori, I., Margottini, C. (eds) Engineering Geology for Society and Territory - Volume 8. Springer, Cham. https://doi.org/10.1007/978-3-319-09408-3_89
Download citation
DOI: https://doi.org/10.1007/978-3-319-09408-3_89
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09407-6
Online ISBN: 978-3-319-09408-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)