Abstract
As and Pb-contaminated sediment obtained from the Nakdong Lake and Yeongsan River in the Republic of Korea was stabilized using a combination of calcined oyster shell (COS), waste cow bone (WCB) and coal mine drainage sludge (CMDS). The effectiveness of the stabilization treatment was evaluated using the Korean Standard Test (KST). The KST tests were performed using 1 N HCl extraction fluid for As and 0.1 N HCl extraction fluid for Pb. Scanning electron microscopy–energy dispersive X-ray spectroscopy (SEM–EDX) analyses were performed to investigate the mechanism responsible for As and Pb immobilization upon treatment. The treatment results showed that effective stabilization of As and Pb-contaminated sediment was obtained. Specifically, 10 wt% COS–5 wt% CMDS was the best treatment for As immobilization and 5 wt% COS–5 wt% WCB was the best treatment for Pb immobilization. The COS–WCB treatment outperformed the COS–CMDS treatment in immobilizing Pb in the contaminated sediment. SEM–EDX results indicated that Pb immobilization was strongly associated with Ca, Si, Al and P while As immobilization was strongly associated with Fe and O. Therefore, utilization of COS, WCB and CMDS is beneficial for the stabilization of contaminated sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the Republic of Korea, the Four Major Rivers (Han, Yeongsan, Geum, and Nakdong) Restoration Project started November 2009 and will be completed by 2012. This project was designed to prevent natural disasters such as floods and droughts at the cost of 16.9 billion USD (Yum 2010). Also, it was conducted to protect ecosystems and promote cultural and historical tourism. During this ongoing project, 570,000,000 m3 of sediment will be dredged and disposed upland (Yum 2010). Currently, 97 % of the sediments have been dredged and these dredged sediments will be used as fill in farmland soil. However, some of these dredged sediments are contaminated with arsenic and heavy metals because of industrial waste water and sewage (Kim et al. 1999). Therefore, these sediments must be remediated before being used as fill in farmland soils.
Among the various remediation techniques, the stabilization/solidification (S/S) process is one of the most effective methods for dealing with sites contaminated with heavy metals (Yukselen and Alpaslan 2001; Katsioti et al. 2008; Qian et al. 2008; Zhang et al. 2009; Gollmann et al. 2010).
The S/S process is known as the best demonstrated available technology (BDAT) which was implemented for 57 hazardous wastes (USEPA 1993). Diverse S/S agents such as quicklime, hydrated lime, Portland cement, fly ash, hydroxyapatite, etc., have been used to immobilize heavy metals in contaminated soil. Cao et al. (2009) reported on the immobilization of Pb, Zn and Cu in contaminated soils using phosphate rock and phosphoric acid. The phosphate treatment was effective in reducing Pb availability in terms of water solubility, bioaccessibility, and phytoavailability. The reduction of Pb availability was probably due to the formation of insoluble Pb phosphate minerals. Ahmad et al. (2012) investigated Pb immobilization in shooting range soil using eggshell waste. The calcined eggshell treatment was achieved by entrapping Pb into calcium-silicate-hydrate. Navarro et al. (2011) investigated Pb immobilization in mine-contaminated soil using reactive materials such as Portland cement, phosphoric acid and MgO. Effective Pb immobilization was only achieved upon phosphoric acid treatment, which could be due to the precipitation of chloropyromorphite. Zhang et al. (2010) investigated the immobilization of Pb along with Cd in contaminated sediment using nano-hydroxyapatite particles (nHAp). The results indicated that dissolution–precipitation is the main Pb immobilization mechanism. Zupančič et al. (2012) used H3PO4 and hydroxyapatite (HA) of varying ratios to immobilize metals in contaminated soil including As and Pb. A high increase in As mobility and a reduction in Pb bioavailability were observed upon the phosphate treatment.
Moon et al. (2004) also investigated lime-based stabilization/solidification to immobilize As. As immobilization was attained through the formation of Ca–As precipitates. Moon and Dermatas (2007) used fly ash to immobilize As in contaminated soil and tailing samples where a significant reduction in As release was achieved upon fly ash treatment. Kundu and Gupta (2008) showed effective As (III) immobilization using cement and lime by the formation of calcite and non-soluble Ca–As precipitates. An and Zhao (2012) investigated the immobilization of As(III) in soil using polysaccharide stabilized Fe–Mn oxide nanoparticles. They reported a 78 % reduction in the toxicity characteristic leaching procedure (TCLP) leachability of As(III) using a nanoparticle amendment. Duarte et al. (2012) studied As(III) immobilization on gibbsite by combining extended X-ray absorption fine structure (EXAFS) analyses and density functional theory (DFT) calculations. Their results indicated that inner sphere complexation is a viable mechanism for As(III) adsorption onto gibbsite at pH range between 5 and 9.
Even though numerous metal immobilization studies have been conducted, research utilizing the application of calcined oyster shell (COS) to immobilize heavy metals in contaminated sediment is not currently available. COS used in this study as a major S/S agent were obtained from waste oyster shells (WOS) treated by the calcination process at high temperature. The main phase in WOS is calcite (CaCO3) and this phase can be transformed into quicklime (CaO) using the calcination process. WOS are produced at the rate of 250,000 tons per year in the Tong-young, Keoje, and Kosung areas of the Republic of Korea, where about 50 % are used to seed oyster beds and 10 % are recycled as fertilizer (Lee et al. 2005). The rest of the WOS is disposed of in coastal areas, resulting in serious odor problems and degradation of the surrounding environment. Therefore, if the WOS is recycled, two environmental problems could be solved simultaneously. Other minor agents such as waste cow bone (WCB), with high phosphate content, and coal mine drainage sludge (CMDS), with high iron content, were also used to immobilize Pb and As in contaminated sediments, respectively.
With respect to the stabilization mechanism for As and Pb-contaminated soil, effective As immobilization could be achieved upon lime treatment by the formation of Ca–As precipitates, regardless of As speciation (Moon et al. 2004; Bothe and Brown 1999). Also, As5+ could be immobilized by the formation of FeAsO4 upon Fe3+ treatment (Carlson et al. 2002; Sastre et al. 2004). On the other hand, it has been reported that effective Pb immobilization in contaminated soil could be achieved by phosphate treatment with the formation of pyromorphite-like minerals [Pb5(PO4)3(Cl, F, OH)] (Ma et al. 1995; Ryan et al. 2001; Cao et al. 2002). Therefore, the utilization of WCB and CMDS is beneficial for the immobilization of Pb and As in contaminated sediment.
The purpose of this study was to investigate the effectiveness of the COS–CMDS treatment for As immobilization and COS–WCB and COS–CMDS treatment for Pb immobilization in the contaminated sediment. The effectiveness of the stabilization process was evaluated using the Korean Standard Test (KST). The KST methods are used for the evaluation of disposal or reuse criteria for heavy metal-contaminated soils in the Republic of Korea (MOE 2002). However, the regulatory levels for heavy metal-contaminated dredged sediments are not fully established and are currently being developed by the Korean Ministry of Environment. Therefore, the KST standards for heavy metal-contaminated soils established are used as a reference in this study. The residential area warning standard for As is 6 mg/kg and the countermeasure standard for As is 15 mg/kg. In addition, the Pb contamination warning standard is 100 mg/kg and the Pb countermeasure standard is 300 mg/kg (MOE 2002). Also, the effect range-low (ERL) levels for As (8.2 mg/kg) and Pb (46.7 mg/kg) contaminated sediments established by the National Oceanic and Atmospheric Administration (NOAA), USA (NOAA 1999) were used as a reference.
X-ray diffraction (XRD) analyses were performed to investigate the crystalline phases responsible for As and Pb immobilization. Then, scanning electron microscopy–energy dispersive X-ray spectroscopy (SEM–EDX) analyses were utilized to evaluate the mechanism responsible for As and Pb immobilization due to the low detection limit of the XRD device.
Experimental methodology
Contaminated sediments
As and Pb-contaminated sediments were obtained using Petite Ponar Grab sampler (Wildlife Co., 1728-G40) from the Nakdong Lake and the Yeongsan River in South Korea, respectively. The mineralogy of As and Pb-contaminated sediments were investigated using X-ray diffraction analyses. Also, the bulk chemistry of As and Pb-contaminated sediments were measured using X-ray fluorescence (XRF). In this study, the collected As and Pb-contaminated sediments were dewatered, air-dried and sieved using the #10 mesh (2 mm).
Stabilization agents
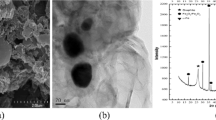
COS and CMDS were used for the stabilization of As-contaminated sediment. The collected WOS were pulverized to pass the #10 (2 mm) sieve. COS was obtained from the calcination of WOS. The calcination process was performed by roasting WOS in an electric furnace (J-FM3, JISICO, South Korea) at 900 °C for 2 h. Calcite (CaCO3) which is the main phase in WOS was almost completely transformed into quicklime (CaO) which is the main phase in COS (Fig. 1). CMDS is produced by the electrolysis process utilized for the treatment of coal mine drainage. The collected CMDS was air-dried and pulverized to pass the #10 (2 mm) sieve. COS, WCB and CMDS were used for the stabilization of Pb-contaminated sediment. The WCB was obtained from restaurants and dried at 105 °C for 2 h and then pulverized to pass the #20 mesh (0.853 mm) sieve.
Treatment of As and Pb-contaminated sediments
The As-contaminated sediment (50 g) was treated with combinations of COS and CMDS concentrations, which ranged from 2.5 to 10 wt% and from 2.5 to 5 wt%, respectively. The Pb-contaminated sediment (50 g) was treated with a combination of COS and WCB and COS and CMDS. The COS, WCB and CMDS treatment contents ranged from 1 to 5 wt%. A liquid to solid (L:S) ratio of 0.2 was used to promote full hydration. The samples were mixed in 300-mL plastic containers by hand with a glass stirring rod or spoon. Following the treatment, all samples were sealed with a cover and cured at room temperature for 7 and 28 days. The specific treatment matrix is presented in Tables 1 and 2.
Physicochemical analyses
The pH and water content were measured according to the KST (MOE 2002). The bulk chemistry of the contaminated sediments was measured using X-ray fluorescence (XRF, ZSX100e, Rigaku, Japan). The contaminated sediments were characterized using a particle size analysis system (Sedigraph 5100, USA). The KST was also applied to evaluate the effectiveness of the stabilization process in more severe conditions. The details of the KST method are as follows: (1) 15 mL of 1 N HCl for As and 0.1 N HCl for Pb was added to each 3 g sample of untreated and stabilized sediments to give a L:S of 5:1; (2) the suspension was shaken at a speed of 100 rpm for 30 min (As) and for 1 h (Pb) at a temperature of 30 °C; (3) 10 mL of the suspension was centrifuged at 3,200 rpm for 20 min; (4) the supernatant was filtered through a 0.45-μm micropore filter; and (5) the filtrate was diluted and acidified with HNO3 until the concentration of HNO3 reached 1 % prior to As and Pb analyses (MOE 2002). The soluble As and Pb concentrations were then analyzed using an inductively coupled plasma-optical emission spectrometer (ICP-OES, Optima 7000DV) (PerkinElmer, CT, USA). Sample analyses were conducted in duplicates or in triplicates, and the average values were reported. The average values were reported only if the individual measurements were within an error range of 10 %. For QA/QC purposes, three different quality control standards were used for every 10 samples.
XRD analyses
In order to investigate mineralogical changes, two treated samples, COS–WCB and COS–CMDS were analyzed by XRD. After the extraction tests, the residue was collected and used for XRD analysis. XRD samples were air-dried and hand-pulverized to pass through a #200 sieve. Step-scanned X-ray diffraction patterns were then collected using a PANalytical XRD instrument (X’Pert PRO MPD). The XRPD analyses were performed at 40 kV and 30 mA using a diffracted beam graphite-monochromator with Cu radiation. The XRPD patterns were collected in the 2θ range of 5°–65° with a step size of 0.02° and a count time of 3 s per step. The qualitative analyses of the XRD patterns were conducted using the Jade software version 7.1 (MDI 2005) and the powder diffraction file (PDF)-2 reference database from the International Center for Diffraction Data database (ICDD) (ICDD 2002).
Scanning electron microscopy–energy dispersive X-ray spectroscopy (SEM–EDX) analyses
SEM–EDX analyses were performed on the KST residue which was obtained after filtration from the KST tests. SEM–EDX analyses were conducted using a Hitachi S-4700 (Hitachi, Japan) equipped with energy dispersive X-ray spectroscopy (EDX), Energy EX-200 (Horiba, Japan). Prior to SEM analyses, the air-dried samples were coated with platinum (Pt).
Results and discussion
Characteristics of As and Pb-contaminated sediments
X-ray diffraction analyses of the As and Pb-contaminated sediments are presented in Fig. 1. In the As-contaminated sediment, quartz (SiO2), muscovite [KAl2(Si3Al)O10(OH, F)2], and magnesium sulfite hydrate (MgSO3H2O) were the main phases identified. Similarly, quartz (SiO2), muscovite [KAl2(Si3Al)O10(OH, F)2], calcite (CaCO3) and clinochlore [(Mg5Al)(Si, Al)4O10(OH)8] were the main phases identified in the Pb-contaminated sediment.
The pH of the As and Pb-contaminated sediments were 5.93 and 7.89, respectively. The As-contaminated sediment was composed of 91.75 % gravel–sand and 8.25 % silt–clay while the Pb-contaminated sediment contained 84.41 % gravel–sand and 12.66 % silt–clay (NIER 2009). The organic matter content of the As and Pb-contaminated sediments were 0.24 and 0.9 %, respectively (NIER 2009). Total As and Pb contents were 63.84 and 72.54 mg/kg obtained by total digestion using a 3:1 HCl/HNO3 solution (aqua regia). The physicochemical properties of the As and Pb-contaminated sediments are presented in Table 3.
Characteristics of stabilizing agents
The physicochemical properties of the COS, CMDS and WCB are presented in Table 3. The COS mainly consisted of 84.3 wt% CaO. The WCB mainly comprised 29.9 wt% CaO and 18.5 wt% P2O5 with a high organic content. The main phase of CMDS was comprised of 7.5 wt% CaO and 66.3 wt% Fe2O3.
KST results
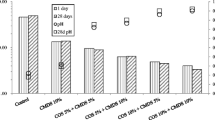
The extracted As concentrations as well as the extraction pH values upon COS–CMDS treatments in accordance with the KST (Ministry of Environment (MOE) 2002) are presented in Fig. 2. The As concentration in the control sample after 28 days of curing was approximately 21 mg/kg and this As concentration was greater than the countermeasure standard of 15 mg/kg. The treatment of only COS was not very effective in reducing As leachability. Specifically, the 3 and 5 wt% COS treatments did not result in a significant reduction in As leachability and the As concentrations were higher than 15 mg/kg, indicating that the amount of COS was not enough to buffer the condition of the 1 N HCl extraction. Previously, effective As immobilization was attained in a contaminated mine tailings sample treated with 25 % COS upon 1 N HCl extraction (Moon et al. 2011). Therefore, a significantly larger amount of COS may be required to immobilize As in the contaminated sediment. However, the treatment of only 5 wt% CMDS was more effective than the treatment of only COS and passed the countermeasure standard of 15 mg/kg. For the samples treated with 5 wt% CMDS, there was an approximate 45 % reduction in As leachability as compared to the control sample. This suggests that the existing iron in the CMDS and the presence of Ca (Table 3) played an important role in reducing As leachability. It has been reported that As mobility can be reduced by the formation of amorphous iron(III) arsenate (FeAsO4·H2O) (Carlson et al. 2002) and scorodite (FeAsO4·2H2O) (Sastre et al. 2004). Also, it has been reported that the precipitation of scorodite was identified at a low pH and in highly oxidizing conditions (Magalhāes 2002; Porter et al. 2004) while the formation of highly insoluble Fe3(AsO4)2 was observed at a pH of around 5 with moderately oxidizing conditions (Porter et al. 2004). Therefore, it could be expected that the form of As in the contaminated sediment should be the 5+ form which could explain why the iron in the CMDS was effective in immobilizing the arsenate (As5+). Therefore, the combination treatment of COS and CMDS could be expected to be very effective in reducing As leachability. The lowest As concentration obtained by the 1 N HCl extraction was observed upon 10 wt% COS and 5 wt% CMDS addition where a concentration of 7.87 mg/kg (62.82 % reduction) was attained after 28 days of curing. This As concentration passed the effect range-low (ERL) level of 8.2 mg/kg for As-contaminated sediment established by the NOAA, USA (NOAA 1999). Moreover, the As concentration in the leachate after a curing period of 28 days was generally greater than the As concentration after a curing period of 7 days. This could be due to the carbonation effects at longer curing periods. Some of the Ca sources that contributed to the formation of calcium arsenate, which is responsible for As5+ immobilization, may have been consumed in the carbonation reaction. This could have resulted in the higher As leachability at longer curing periods. According to Akhter et al. (1997), calcium arsenate is not stable in the stability region of CaCO3 in the presence of CO2. Similarly, TCLP As concentrations increased with increasing curing time for some treated samples. However, CO2 penetration was not clearly evident after 3 years of curing. Therefore, the stability of As immobilization at longer curing periods is worth investigating. Overall, all the treatments passed the countermeasure standard of 15 mg/kg, but none of them passed the warning standard of 6 mg/kg. It has been reported that in order to pass the warning standard, more than 20 % Portland cement and an extraction pH value higher than 3 was needed (Yoon et al. 2010). Therefore, to pass the warning standard, a higher percentage of immobilizing agent is most probably needed and/or the pH value should be increased.
The extracted Pb concentrations obtained by the 0.1 N HCl extraction as well as the extraction pH values upon COS–WCB and COS–CMDS treatments are presented in Fig. 3. A Pb concentration of 9 mg/kg was attained from the control samples. This Pb concentration was lower than the warning standard of 100 mg/kg for contaminated soil. At 46.7 mg/kg, the NOAA effect range-low (ERL) level for Pb is even lower than 100 mg/kg (NOAA 1999). Even though the control sample Pb concentration is low, the treatments could be beneficial if the Pb sediment quality guideline concentration is set lower in the near future. No significant reduction in Pb leachability was observed upon 1 wt% COS treatment. Also, the extraction pH value upon 1 wt% COS treatment was less than 1.5. This indicated that any Pb compounds formed upon treatment with 1 wt% COS could not be totally immobilized at this low extraction pH condition. Ok et al. (2010) have reported that a significant reduction in Pb leachability in Pb-contaminated soil obtained adjacent to abandoned mines was attained upon treatment with 5 wt% COS. This indicated that higher amounts of COS could be used to immobilize Pb effectively if COS is used alone. On the other hand, the combination treatment of COS–WCB and COS–CMDS was very effective in reducing Pb leachability after 28 days of curing. Specifically, more than 90 % reduction of Pb leachability was attained. Pb leachability upon COS–WCB and COS–CMDS treatments was less than 1 mg/kg after 28 days of curing. The extraction pHs of the samples upon COS–WCB and COS–CMDS treatments were higher than 3. This shows that in order to achieve effective Pb immobilization, the pH condition should be buffered and the extraction pH value should be higher than 3. Similar results were obtained by Lee et al. (2011). This study achieved more than a 99 % reduction in Pb leachability in firing range soil upon treatment with 5 wt% COS and 3 wt% WCB, after 28 days of curing. Overall, the COS–WCB treatment was more effective than the COS–CMDS treatment. This finding supports the theory that effective Pb immobilization was strongly associated with the formation of pyromorphite-like minerals [Pb5(PO4)3(Cl, F, OH)] (Ma et al. 1995; Ryan et al. 2001; Cao et al. 2002).
SEM–EDX analyses
The mechanism responsible for Pb immobilization was evaluated by SEM–EDX analysis on the Pb-contaminated sediment treated with 5 wt% COS and 5 wt% WCB, because this sample resulted in the lowest Pb concentration tested by 0.1 N HCl extraction fluid. To determine the mechanism responsible for As immobilization, the As-contaminated sediment treated with 10 wt% COS and 5 wt% CMDS was analyzed because this sample resulted in the lowest As leachability with the highest pH value by the 1 N HCl extraction fluid.
The SEM–EDX results of the sample treated with 5 wt% COS and 5 wt% WCB for Pb immobilization showed that effective Pb immobilization was strongly associated with Ca, Si, Al, O and P (Fig. 4). This suggests that both CS/AH and pyromorphite formation was probably associated with effective Pb immobilization upon the combination treatment of COS and WCB. In the case of As immobilization, the sample treated with 10 wt% COS and 5 wt% CMDS showed that effective As immobilization was strongly linked to Fe and O (Fig. 5). This suggests that Fe–As precipitates are the most probable compounds responsible for effective As immobilization, upon the combination treatment of COS and CMDS. Also, Ca–As precipitates are expected to be the key compounds responsible for effective As immobilization since various Ca–As precipitates have been reported as the main compounds for As immobilization by numerous researchers upon lime treatment (Dutré and Vandecasteele 1995, 1998; Moon et al. 2004). It could be expected that the Fe–As precipitates are more important in reducing As leachability than the Ca–As precipitates, since the sole treatment of CMDS was more effective than the sole treatment of COS.
Conclusion
In this study, As and Pb-contaminated sediments were stabilized using a combination of COS, WCB and CMDS. KST test was conducted to evaluate the effectiveness of the stabilization process. The treatment results showed that effective As and Pb immobilization in the treated samples were obtained. The most effective As immobilization results were obtained from the sample treated with 10 wt% COS and 5 wt% CMDS while the 5 wt% COS and 5 wt% WCB treatment was the most effective for Pb immobilization. Moreover, WCB treatment was more effective than the CMDS treatment in the presence of COS for the immobilization of Pb in the contaminated sediment. SEM–EDX results suggested that Pb immobilization was strongly associated with Ca, Si, Al, O and P while As immobilization was strongly linked to Fe and O. Therefore, utilization of COS, WCB, and CMDS is beneficial for the immobilization of heavy metals in the contaminated sediment.
References
Ahmad M, Hashimoto Y, Moon DH, Lee SS, Ok YS (2012) Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated mechanistic approach. J Hazard Mater 209–210:392–401
Akhter H, Cartledge FK, Roy A, Tittlebaum ME (1997) Solidification/stabilization of arsenic salts: effects of long cure times. J Hazard Mater 52:247–264
An B, Zhao D (2012) Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe–Mn oxide nanoparticles. J Hazard Mater 211–212:332–341
Bothe JV, Brown PW (1999) Arsenic immobilization by calcium arsenate formation. Environ Sci Technol 33:3806–3811
Cao X, Ma LQ, Chen M, Singh SP, Harris WG (2002) Impacts of phosphate amendments on lead biogeochemistry at a contaminated site. Environ Sci Technol 36:5296–5304
Cao X, Wahbi A, Ma L, Li B, Yang Y (2009) Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J Hazard Mater 164:555–564
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36:1712–1719
Duarte G, Ciminelli VST, Dantas SS, Duarte HA, Vasconcelos IF, Oliveira AF, Osseo-Asare K (2012) As(III) immobilization on gibbsite: investigation of the complexation mechanism by combining EXAFS analyses and DFT calculations. Geochim Cosmochim Ac 83:205–216
Dutré V, Vandecasteele C (1995) Solidification and stabilization of arsenic-containing waste: leach tests and behavior of arsenic in the leachate. Waste Manag 15:55–62
Dutré V, Vandecasteele C (1998) Immobilization mechanism of arsenic in waste solidified using cement and lime. Environ Sci Technol 32:2782–2787
Gollmann MAC, da Silva MM, Masuero ÂB, dos Santos JHZ (2010) Stabilization and solidification of Pb in cement matrices. J Hazard Mater 179:507–514
International Centre for Diffraction Data (2002) Powder diffraction file, PDF-2 database release
Katsioti M, Katsiotis N, Rouni G, Bakirtzis D, Loizidou M (2008) The effect of bentonite/cement mortar for the stabilization/solidification of sewage sludge containing heavy metals. Cement Concrete Comp 30:1013–1019
Kim J-Y, Chung C-H, Oh K-H, Koh Y-K, Moon J–J, You K-A (1999) Heavy metal contamination of stream sediments from the Yeongsan River and Kwangju stream, Kwangju. J Korean Earth Sci Soc 20(1):96–100
Kundu S, Gupta AK (2008) Immobilization and leaching characteristics of arsenic from cement and/or lime solidified/stabilized spent adsorbent containing arsenic. J Hazard Mater 153:434–443
Lee JY, Hong CO, Lee CH, Lee DK, Kim PJ (2005) Dynamics of heavy metals in soil amended with oyster shell meal. Korean J Environ Agric 24:358–363
Lee K-Y, Moon DH, Kim K-W, Cheong KH, Kim T-S, Khim J, Moon KR, Choi SB (2011) Application of waste resources for the stabilization of heavy metals (Pb, Cu) in firing range soils. J Korean Soc Environ Engineers 33(2):71–76 (Korean)
Ma LQ, Logan TJ, Traina SJ (1995) Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ Sci Technol 29:1118–1126
Magalhāes MCF (2002) Arsenic, an environmental problem limited by solubility. Pure Appl Chem 74(10):1843–1850
Material’s Data Inc (2005) Jade, Version 7.1, California, USA
Ministry of Environment (MOE) (2002) The Korean Standard Test (KST) Methods for soils (in Korean). Korean Ministry of Environment, Gwachun, Kyunggi
Moon DH, Dermatas D (2007) Arsenic and lead release from fly ash stabilized/solidified soils under modified semi-dynamic leaching conditions. J Hazard Mater 141:388–394
Moon DH, Dermatas D, Menounou N (2004) Arsenic immobilization by calcium–arsenic precipitates in lime treated soils. Sci Total Environ 330:171–185
Moon DH, Kim K-W, Yoon I-H, Grubb DG, Shin D-Y, Cheong KH, Choi H-I, Ok YS, Park J-H (2011) Stabilization of arsenic-contaminated mine tailings using natural. Environ Earth Sci 64:597–605
National Institute of Environmental Research (NIER) (2009) Preliminary monitoring of river and lake sediments (II)
National Oceanic and Atmospheric Administration (NOAA) (1999) Sediment Quality Guidelines
Navarro A, Cardellach E, Corbella M (2011) Immobilization of Cu, Pb and Zn in mine-contaminated soils using reactive materials. J Hazard Mater 186:1576–1585
Ok YS, Oh S-E, Ahmad M, Hyun S, Kim K-R, Moon DH, Lee SS, Lim KJ, Jeon W-T, Yang JE (2010) Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ Earth Sci 61:1301–1308
Porter SK, Scheckel KG, Impellitteri CA, Ryan JA (2004) Toxic metals in the environment: thermodynamic considerations for possible immobilisation strategies for Pb, Cd, As, and Hg. Crit Rev Env Sci Tec 34:495–604
Qian GR, Shi J, Cao YL, Xu YF, Chui PC (2008) Properties of MSW fly ash–calcium sulfoaluminate cement matrix and stabilization/solidification on heavy metals. J Hazard Mater 152:196–203
Ryan JA, Zhang P, Hesterberg D, Chou J, Sayers DE (2001) Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite. Environ Sci Technol 35:3798–3803
Sastre J, Hernandez E, Rodriguez R, Alcobe X, Vidal M, Rauret G (2004) Use of sorption and extraction tests to predict the dynamics of the interaction of trace elements in agricultural soils contaminated by a mine tailing accident. Sci Total Environ 329:261–281
USEPA (1993) Technology resource document-solidification/stabilization and its application to waste materials, USEPA, June (EPA/530/R93/012)
Yoon I-H, Moon DH, Kim K-W, Lee K-Y, Lee J-H, Kim MG (2010) Mechanism for the stabilization/solidification of arsenic-contaminated soils with Portland cement and cement kiln dust. J Environ Manag 91:2322–2328
Yukselen A, Alpaslan B (2001) Leaching of metals from soil contaminated by mining activities. J Hazard Mater B37:289–300
Yum K (2010) The roles and effects of the four rivers restoration project to cope with climate change. J Korean Soc Civil Eng 58(9):10–15 (Korean)
Zhang J, Liu J, Li C, Jin Y, Nie Y, Li J (2009) Comparison of the fixation effects of heavy metals by cement rotary kiln co-processing and cement based solidification/stabilization. J Hazard Mater 165:1179–1185
Zhang Z, Li M, Chen W, Zhu S, Liu N, Zhu L (2010) Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ Pollut 158:514–519
Zupančič M, Lavrič S, Bukovec P (2012) Metal immobilization and phosphorus leaching after stabilization of pyrite ash contaminated soil by phosphate amendments. J Environ Monit 14(20):704–710
Acknowledgments
This study was financially supported by investigation of basic environmental R&D Project from Yeongsan River Environment Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moon, D.H., Oh, DY., Wazne, M. et al. Immobilization of As and Pb in contaminated sediments using waste resources. Environ Earth Sci 69, 2721–2729 (2013). https://doi.org/10.1007/s12665-012-2094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-2094-0