Abstract
A novel treatment mix was designed for the simultaneous immobilization of As, Cu, and Pb in contaminated soils using natural (waste oyster shells (WOS)) and industrial (coal mine drainage sludge (CMDS)) waste materials. The treatments were conducted using the standard U.S. sieve size no. 20 (0.85 mm) calcined oyster shells (COS) and CMDS materials with a curing time of 1 and 28 days. The As immobilization treatments were evaluated using the 1-N HCl extraction fluid, whereas the Pb and Cu immobilization treatments were evaluated using the 0.1-N HCl extraction fluid based on the Korean leaching standards. The treatment results showed that the immobilization of As, Cu, and Pb was best achieved using a combination mix of 10 wt% COS and 10 wt% CMDS. This treatment mix was highly effective leading to superior leachability reductions for all three target contaminants (>93 % for As and >99 % for Cu and Pb) for a curing period of 28 days. The X-ray absorption near-edge structure (XANES) results showed that As was present in the form of As(V) in the control sample and that no changes in As speciation were observed following the COS-CMDS treatments. The scanning electron microscopy (SEM)-energy dispersive X-ray spectroscopy (EDX) sample treated with 10 wt% COS and 10 wt% CMDS indicated that As immobilization may be associated with the formation of Ca–As and Fe–As precipitates while Pb and Cu immobilization was most probably linked to calcium silicate hydrates (CSHs) and calcium aluminum hydrates (CAHs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pb and Cu soil contamination in civil and military firing ranges is a serious problem that often requires immediate remedial action. Typical military-grade bullets, made of Pb alloy slugs enclosed in a Cu alloy jacket, may result in substantive accumulation of Pb and Cu in shooting range soils (Dermatas et al. 2004). On the other hand, As contamination in chromated copper arsenate (CCA)-contaminated soil may be a public health concern. In the past years, CCA has been used as a timber-preserving agent against insect or microbial (fungal or bacterial) decay. However, in recent years, many countries around the world have applied restrictions in CCA use, particularly for timber destined for residential applications. For example, the use of wood with a As2O5 content higher than 0.1 % was banned on 8 October 2007 by the Korean Ministry of Environment (Koo et al. 2008), leaving a legacy of CCA-contaminated soil sites. In this study, the simultaneous immobilization of As, Cu, and Pb in a contaminated soil was explored for a composite soil sample made of army firing range soil and CCA-contaminated soil. The study was commissioned by the Korean Ministry of the Environment to simultaneously treat metalloid and heavy metal-contaminated soil using a stabilization process. The composite soil sample was created to ensure the presence of all metals of concern in the treated soil sample. In addition to the technical feasibility (effectiveness of the method for all target contaminants), cost and sustainability were the main considerations for the proposed remedial treatment scheme. In that sense, the study earmarked to consider nontraditional stabilization materials of minimal cost, preferably waste materials with a recycle/reuse potential.
A novel mixture composed of natural waste materials (waste oyster shells (WOS)) and an industrial waste (coal mine drainage sludge (CMDS)) were considered as immobilizing agents. More than 250,000 tons of calcium-rich WOS are produced annually in the Republic of Korea, and 40 % are dumped in coastal areas, causing serious nuisance and odor problems and instigating degradation of the surrounding environment. Recently, WOS (as the Ca source) was applied as the main stabilizing agent to immobilize As by formation of Ca–As precipitates and heavy metals by the formation of pozzolanic reaction products in contaminated soils (Moon et al. 2011b, 2015). However, treatment using only WOS in its natural or calcined state showed limited effectiveness with respect to As immobilization, unless larger quantities of WOS were applied (Moon et al. 2011b). Specifically, concentrations higher than 25 % calcined oyster shell (COS) were needed in order to obtain effective As immobilization in contaminated mine tailings when a 1-N HCl extraction fluid was applied (Moon et al. 2011b). Therefore, the selection of CMDS was made because of its high iron content, which may prove to be beneficial for As immobilization due to As–Fe complexation. Effective As immobilization upon iron treatment has been reported in the literature by various researchers (Chung et al. 2001; Kim et al. 2003; Lee 2006; Kumpiene et al. 2008; Wilopo et al. 2008; Drahota and Filippi 2009; Moon et al. 2015). CMDS is an iron-rich industrial waste byproduct resulting from coal mine industrial operations. Thus, there is strong evidence that the combination treatment by COS and CMDS, could potentially lead to the development of an effective technology for simultaneous immobilization of As and heavy metals.
In the case of heavy metals immobilization, treatment of only COS showed a significant reduction in Pb and Cu leachability with 5 and 10 % COS content upon 0.1 N HCl extraction (Moon et al. 2013). Therefore, when As and heavy metals co-exist in contaminated soil, treatment of only COS may provide mixed results. A combination treatment of COS and another agent may be able to solve this problem and provide effective immobilization of As and heavy metals simultaneously with a small quantity of admixture. In this study, a combination treatment of COS and CMDS was investigated for the simultaneous immobilization of As and heavy metals in contaminated soil.
The purpose of this study is to investigate the feasibility of beneficially using waste materials (WOS and CMDS) for the simultaneous immobilization of As, Pb, and Cu in contaminated soil. The effectiveness of immobilization is evaluated based on the 1-N HCl extraction method for As and the 0.1-N HCl extraction method for Pb and Cu in accordance with the Korean Standard Test (KST) methods (MOE 2002). The KST methods are used for the evaluation of disposal or reuse criteria for heavy metal-contaminated soils (MOE 2002). X-ray absorption near-edge structure (XANES) and analyses scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDX) analyses are conducted to evaluate the immobilized metal speciation upon COS-CMDS treatment.
Experimental methodology
Contaminated soil
Soil samples from a military firing range and a CCA-contaminated site, both located in Busan City, Republic of Korea, were obtained at a depth of 0∼30 cm from the soil surface. The collected soils were air dried and passed through a through a standard U.S. sieve size no. 10 (2 mm) to remove large particles such as cobbles and gravel. A composite soil sample was produced by mixing the shooting range soil and CCA-contaminated soil at a ratio of 4:1 in order to obtain realistic concentrations for all target contaminants. The total concentrations of As, Pb, and Cu in composite soil measured by aqua regia (1 ml of HNO3 (65 %, Merck) and 3 ml of HCl (37 %, J.T. Baker)) were 189, 8588, and 719 mg/kg, respectively. The composite soil was classified as silty clay loam by a particle size analyzer (PSA) in accordance with the US Department of Agriculture (USDA). The soil pH was determined to be 7.64 using ASTM method D 4980-89 (ASTM 2000). The physicochemical and mineralogical properties as well as total concentrations of As and heavy metals in the soil are presented in Table 1. The bulk chemistry of the As- and heavy metal-contaminated soils measured by using X-ray fluorescence (XRF; ZSX100e, Rigaku, Japan) are presented in Table 2.
Stabilizing agents
The waste oyster shells (WOS) used in this study were obtained from Tong-young City in the Republic of Korea. The collected WOS were pulverized and powdered using a hammer mill and a ball mill to obtain a fine and homogeneous powder that passes the standard U.S. sieve size no. 20 (0.85 mm). In order to perform the calcination process, the WOS was heated at 900 °C for 2 h in an electric furnace (J-FM3, JISICO, Republic of Korea) to transform the calcite (CaCO3) into quicklime (CaO). The calcined WOS was given the designation COS. CMDS was collected from an electric purification facility which treats acidic mine drainage located in the Kangwon Province in the Republic of Korea and sieved using the standard U.S. sieve size no. 20. The major chemical compositions of the COS and CMDS as determined by XRF are presented in Table 2. The major compound in the COS sample was CaO (78.9 wt%), whereas the CMDS mainly consisted of 81.8 wt% of Fe2O3 with 9.25 wt% of CaO (Table 2).
Stabilization treatments
The As-, Cu-, and Pb-contaminated composite soil sample was treated with combinations of the three levels of each of the stabilizing materials (COS, CMDS), namely 0, 5, and 10 %. The control treatment, used for benchmarking purposes, contained no stabilizing materials (0 % COS, 0 % CMDS). A water content of 20 % was applied to the treated samples to enable the full hydration process. All treated samples were subjected to two curing periods, namely, 1 and 28 days. The detailed treatment conditions based on the percent binder/soil ratio (dry basis) and sample designations are presented in Table 3. The effectiveness of the stabilization process was evaluated using 1 N HCl extraction fluid for As and 0.1 N HCl extraction fluid for Pb and Cu, respectively, in accordance with the KST methods (MOE 2002). The soluble As, Cu, and Pb concentrations were analyzed using an inductively coupled plasma-optical emission spectrometer (ICP-OES; Optima 7000DV; PerkinElmer, CT, USA). Sample analyses were conducted in duplicate or in triplicate, and the average values were reported with an error range of 10 %. For QA/QC purposes, three different quality control standards were used for every ten samples.
X-ray absorption near-edge structure analyses
The oxidation state of As is worth investigating since As toxicity and mobility depends significantly on oxidation state. It has been reported that As(III) is more mobile and toxic than As(V) (Pantsar-Kallio and Manninen 1997; Stronach et al. 1997). Moreover, As(III) is 25–60 times more toxic than As(V) (Dutré and Vandecasteele 1995; Pantsar-Kallio and Manninen 1997). In order to evaluate the oxidation state of As for the control and treated samples after 28 days of curing, XANES analyses were performed. The XANES samples were ground to a fine powder (<74 μm) using an agate mortar and pestle. The samples were then packed in the recession of a metal sample holder with a uniform thickness and sealed with the polyimide film. Arsenic K-edge XANES spectra were collected at the beam line BL10C (wide XAFS) at the Pohang light source (PLS-II) with a ring current of 300 mA at a storage ring of 3 GeV. A Si(311) double crystal monochromator was used to monochromatize the X-ray photon energy. Spectra were collected in fluorescence mode for all samples using pure N2 gas-filled ionization chambers as gas detectors. Prior to the XANES measurement, all samples were kept in an argon (Ar) atmosphere in order to prevent sample oxidation that is caused by any air-borne contamination. Three arsenic compounds As(0) powder (As, 99.997 %, Aldrich 267961), sodium (meta) arsenite (NaAsO2, 99.0 %, Sigma S7400), and sodium arsenate (Na2HAsO4·7H2O, 98.0 %, Sigma 9663) were used as the standard references for As(0), As(III), and As(V), respectively.
SEM-EDX analyses
The SEM-EDX sample treated with 10 wt% COS and 10 wt% CMDS was air dried, and subsamples were prepared using double-sided carbon tape and coated with platinum (Pt). SEM-EDX analyses were performed using a Hitachi S-4800 SEM instrument equipped with an ISIS 310 EDX system.
Results and discussion
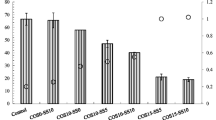
The As, Cu, and Pb concentrations and pH values, in the control samples and CMDS- and COS-CMDS-treated soil samples are presented in Figs. 1, 2, and 3. For As, an approximate reduction of 72 % in As leachability is attained upon treatment with 10 wt% CMDS. This is a significant achievement in terms of As immobilization with a treatment of only CMDS. The CMDS mainly consists of approximately 82 wt% of Fe2O3 with 9 wt% of CaO according to the XRF data. Therefore, As immobilization is most likely achieved by the formation of Fe–As precipitates (Moon et al. 2015) with possible contributions from Ca–As precipitates (Moon et al. 2004). The pH of the extraction fluid in sample CMDS10 after a curing period of 28 days is about 0.3, which is higher than the control samples’ pH of 0.18. This indicates that the Ca content available in the CMDS plays a role in increasing the extraction fluid pH, which may safeguard the Fe–As precipitates against dissolution. All COS-CMDS combination treatments improve As immobilization even more than CMDS alone. The best As immobilization (a reduction of >93 % in As leachability compared with the control sample) is achieved for a curing period of 28 days with the 10 wt% COS and 10 wt% CMDS combination treatment.
In the case of Pb and Cu, treatment with 10 wt% CMDS alone is not as effective in reducing Pb and Cu leachability as for As. Application of 10 % CMDS alone causes Pb and Cu leachability reductions of approximately 61 and 27 %, respectively, compared with the control for a curing period of 28 days. As in the case of As, combination treatments of COS and CMDS lead to significant reductions (>99 %) in Pb and Cu leachability. Based on the extraction results for all treatments attempted in this study, it appears that curing period does not play a prominent role on the stabilization effectiveness. For the most part, the extraction levels for all three contaminants and treatment conditions appear to differ only slightly for the attempted curing periods of 1 and 28 days. The extraction fluid pH values ranged between 1.42 and 12.09 after 28 days of curing. Significantly, high extraction fluid pH values of 11.50 and 12.09 were observed for samples COS10-CMDS5 and COS10-CMDS10, respectively, where very low Pb and Cu leachability was achieved. Similar to As immobilization, the best Pb (>99 %) and Cu (>99 %) immobilization was obtained after 28 days of curing upon the treatment with 10 wt% COS and 10 wt% CMDS. Pb and Cu immobilization may be associated with pozzolanic reaction products such as calcium silicate hydrates (CSHs) and calcium aluminum hydrates (CAHs).
XANES analyses
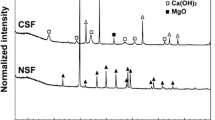
The XANES spectra of the control and selected treated samples along with As standards are presented in Fig. 4. The XANES results indicated that the As speciation in the control samples is As(V) which most probably resulted from the use of As2O5 from CCA. Moreover, As(V) was predominantly detected in samples from CMDS10, COS10-CMDS5, and COS10-CMDS10 without reduction. This suggests that no changes in As speciation were achieved following the COS-CMDS treatments.
SEM-EDX analyses
The SEM-EDX results for the 10 wt% COS and 10 wt% CMDS treatment (COS10-CMDS10) producing a superior immobilization performance for As, Pb, and Cu are presented in Fig. 5. Elemental dot map results show that As immobilization is most likely associated with Ca, Fe, and O (Fig. 5a). This suggests that Ca–As and Fe–As precipitates may be the compounds responsible for effective As immobilization. Ca–As precipitates have been reported by numerous researchers as the main compounds responsible for As immobilization in lime-treated soils (Dutré and Vandecasteele 1996, 1998; Moon et al. 2004, 2011b). Similarly, Fe–As precipitates have been widely reported as the responsible compounds for As immobilization (Chung et al. 2001; Kim et al. 2003; Lee 2006; Kumpiene et al. 2008; Wilopo et al. 2008; Drahota and Filippi 2009; Moon et al. 2015). The form of As was identified as arsenate [As(V)] based on the XANES analyses. Therefore, a specific Fe–As precipitate such as FeAsO4 reported by a previous study (Sims et al. 1986; Cui et al. 2012) may well be the compound responsible for effective As immobilization in the contaminated soil.
In the case of Pb, elemental dot map results show that Pb immobilization may be associated with Ca, Al, Si, and O due to the formation of pozzolanic reaction products such as CSHs and CAHs (Fig. 5b). It has been reported that Pb could be incorporated within the CSH structure through linkages to Si–O chains upon the hydration of tricalcium silicate, which is the main compound in Portland cement (Rose et al. 2000). Moulin et al. (1999) also reported that Pb can be retained through Si–O–Pb bonds. Moreover, effective Pb immobilization was achieved by the formation of CSH in quicklime-treated samples (Dermatats and Meng 2003). Specific types of CSHs such as, CaH4Si2O7 and Ca5Si6O16(OH)2 were observed in the presence of montmorillonite as the phases strongly associated with Pb immobilization upon quicklime treatment (Moon et al. 2006).
Similar to the Pb immobilzation mechanism, elemental dot map results showed that Cu immobilization may also be associated with Ca, Al, Si, and O due to the formation of CSHs and CAHs (Fig. 5b). Johnson (2002) reported that effective Cu immobilization by CSH was based on incorporation rather than substitution. Also, it has been reported that when the Cu source is blended with Portland cement, Cu immobilization could be achieved by the formation of Cu6Al2O8CO3·12H2O (Qiao et al. 2007). A recent study also showed that Cu immobilization was strongly associated with both CSH/CAH and ettringite (Moon et al. 2011a).
Conclusions
The As-, Cu-, and Pb-contaminated soil collected from an army firing range site and a CCA-treated wood site was immobilized using COS and CMDS. The dosage of COS ranged from 0 to 10 wt% and either 5 or 10 % CMDS was used. Upon treatment with COS and CMDS, the treated samples were subjected to curing periods of 1 and 28 days. The effectiveness of immobilization was evaluated using 1 N HCl extraction for As and 0.1 N for Pb and Cu. The immobilization results showed that treatment of only CMDS was very effective for As (72 % reduction in As leachability), but it was not for Pb and Cu after 28 days of curing. The lowest leachability of As (>93 % reduction in As leachability), Pb (>99 % reduction in Pb leachability), and Cu (>99 % reduction in Cu leachability) in the contaminated soil was obtained upon the combined treatment of 10 wt% COS and 10 wt% CMDS after 28 days of curing. The XANES analyses indicated that the As speciation in the control samples was As(V), and no changes in As speciation were observed following the COS-CMDS treatments. The SEM-EDX sample treated with 10 wt% COS and 10 wt% CMDS showed that As immobilization may be associated with the formation of Ca–As and Fe–As precipitates. In the case of Fe–As precipitates, FeAsO4 could be the compound likely resposible for As immobilization since the form of As in the contaminated soil confimed by XANES analyses was As(V). The SEM-EDX results also showed that pozzolanic reaction products such as CSHs and CAHs may be the most probable compounds responsible for effective Pb and Cu immobilization. Therefore, it could be concluded that the COS-CMDS treatment was very effective in simultaneously immobilizing As, Cu, and Pb and that the COS-CMDS mixture can be utilized as a cost-effective stabilizing agent for contaminated soils.
References
American Society for Testing and Materials (ASTM) (2000) Annual Book of ASTM Standards, Soil and Rock, vol. 4.08
Ball DF (1964) Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soil. J Soil Sci 15:84–92
Chung IJ, Choi YS, Hong SW, Park HM (2001) Immobilization of arsenic in tailing by using iron and hydrogen peroxide. Environ Technol 22(7):831–835
Cui M, Na S, Khim J, Jang M (2012) Stabilizatin of heavy metal contaminated paddy soils (in Korean). J Korean Soc Hazard Mitigation 12(2):287–292
Dermatas D, Meng X (2003) Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng Geol 70(3):377–394
Dermatas D, Menounou N, Dutko P, Dadachov M, Arienti P, Tsaneva V (2004) Lead and copper contamination in small arms firing ranges. Global NEST J 6(2):141–148
Drahota P, Filippi M (2009) Secondary arsenic minerals in the environment: a review. Environ Int 35:1243–1255
Dutré V, Vandecasteele C (1995) Solidification/stabilization of arsenic-containing waste; leach tests and behavior of arsenic in the leachate. Waste Manage 15(1):55–62
Dutré V, Vandecasteele C (1996) An evaluation of the solidification/stabilization of industrial arsenic containing waste using extraction and semi-dynamic leach tests. Waste Manage 16(7):625–631
Dutré V, Vandecasteele C (1998) Immobilizationmechanism of arsenic in waste solidified using cement and lime. Environ Sci Technol 32:2782–2787
FitzPatrick EA (1983) Soils: their formation, classification and distribution. Longman Science & Technical, London, p 353
Johnson CA (2002) Metal binding in the cement matrix: an overview of our current knowledge. Department of Water Resources and Drinking Water, Water-Rock Interaction Group, EAWAG, for Cemsuisse, Switzerland
Kim JY, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ Sci Technol 37:189–195
Koo J, Song B, Kim H (2008) Characteristics of the release of chromium, copper, and arsenic from CCA-treated wood exposed to the natural environment. Anal Sci Technol 21(1):1–8
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manage 28:215–225
Lee KC (2006) Immobilization characteristics of arsenic contaminated soil using soluble phosphate and arsenic coagulant. Master thesis, Sangmyung University, Republic of Korea
MDI (2005) Jade version 7.1. Material’s Data Inc, Livermore, California, USA
MOE (2002) The Korean Standard Test (KST) methods for soils (in Korean). Korean Ministry of Environment, Gwachun, Kyunggi
Moon DH, Dermatas D, Menounou N (2004) Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Sci Total Environ 330(1–3):171–185
Moon DH, Dermatas D, Grubb DG (2006) The effectiveness of quicklime-based stabilization/solidification on lead (Pb) contaminated soils, In: Environmental Geotechnics (5th ICEG), Thomas H.R. (ed.), Thomas Telford Publishing, London, 1, 221–228
Moon DH, Cheong KH, Khim J, Grubb DG, Ko I (2011a) Stabilization of Cu-contaminated army firing range soils using waste oyster shells. Environ Geochem Health 33:159–166
Moon DH, Kim KW, Yoon IH, Grubb DG, Shin DY, Cheong KH, Choi HI, Ok YS, Park JH (2011b) Stabilization of arsenic-contaminated mine tailings using natural and calcined oyster shells. Environ Earth Sci 64:597–605
Moon DH, Park J-W, Cheong KH, Hyun S, Koutsospyros A, Park J- H, Ok YS (2013) Stabilization of lead and copper contaminated firing range soil using calcined oyster shells and fly ash. Environ Geochem Health 35:705–714
Moon DH, Wazne M, Cheong KH, Chang Y-Y, Baek K, Ok YS, Park J-H (2015) Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environ Sci Pollut Res 22:11162–11169
Moulin I, Stone WE, Sanz J, Bottero J-Y, Mosnier F, Haehnel C (1999) Lead and zinc retention during hydration of tri-calcium silicate: a study by sorption isotherms and 29Si nuclear magnetic resonance spectroscopy. Langmuir 15:2829–2835
Pantsar-Kallio M, Manninen PKG (1997) Speciation of mobile arsenic in soil samples as a function of pH. Sci Total Environ 204:193–200
Qiao XC, Poon CS, Cheeseman CR (2007) Investigation into the stabilization/solidification performance of Portland cement through cement clinker phases. J Hazard Mater B139:238–243
Rose J, Moulin I, Hazemann J-L, Masion A, Bertsch PM, Bottero J-Y, Mosnier F, Haehnel C (2000) X-ray absorption spectroscopy study of immobilization processes for heavy metals in calcium silicate hydrates: 1. Case of lead. Langmuir 16:9900–9906
Sims R, Sorenson D, Sims J, McLean J, Mahmood R, Dupont R, Jurinak J, Wagner K (1986) Contaminated surface soils in-place treatment techniques. Noyes Publications, Park Ridge, NJ
Stronach SA, Walker NL, Macphee DE, Glasser FP (1997) Reactions between cement and As(III) oxide: the system CaO-SiO2-As2O3-H2O at 25 °C. Waste Manage 17(1):9–13
Wilopo W, Sasaki K, Hirajima T, Yamanaka T (2008) Immobilization of arsenic and manganese in contaminated groundwater by permeable reactive barriers using zero valent iron and sheep manure. Mater Trans 49(10):2265–2274
Acknowledgments
This study was supported by the Korean Ministry of Environment as the GAIA (Geo-Advanced Innovative Action) project (no. 2014000540011). The authors would like to thank the members of Pohang Accelerator Laboratory (PAL), Republic of Korea for their integral help with XANES analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Moon, D.H., Cheong, K.H., Koutsospyros, A. et al. Assessment of waste oyster shells and coal mine drainage sludge for the stabilization of As-, Pb-, and Cu-contaminated soil. Environ Sci Pollut Res 23, 2362–2370 (2016). https://doi.org/10.1007/s11356-015-5456-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5456-9