Abstract
Heavy metal accumulation due to industrial activities has become a very sensitive issue for the survival of the aquatic life. Therefore, distributions of several heavy metals have been studied in the surface sediments of Tapti–Hazira estuary, Surat, to assess the impact of anthropogenic and industrial activities near estuary. Totally 60 sediment samples were collected from four different sites at Tapti–Hazira estuary, Surat from January 2011 to May 2011 and examined for metal contents. The average heavy metal load in the study area are found to be 43.28–77.74 mg/kg for Pb, 48.26–72.40 mg/kg for Cr, 117.47–178.80 mg/kg for Zn, 71.13–107.82 mg/kg for Ni, 123.17–170.52 mg/kg for Cu, 0.74–1.25 mg/kg for Cd, 14.73–21.69 mg/kg for Co. Calculated enrichment factors (EF) reveal that enrichment of Pb and Cd is moderate at all sites, whereas other metals Cr, Ni, Zn, Co, and Cu show significant to very high enrichment. Geo-accumulation index (I geo) results revealed that the study area is nil to moderately contaminated with respect to Cd, moderately to highly polluted with respect to Pb, Zn, and Cu and high to very highly polluted with respect to Co and Cr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The physical and chemical characteristics of any water body are of ecological significance especially in estuarine ecosystems that are prone to daily tidal physico-chemical fluctuations (Chapman and Wang 2001). An estuary is a dynamic semi-enclosed coastal body of water which has a free connection with the open sea and within which sea water is measurably diluted with fresh water derived from land drainage of river in flow. There are basically three reservoirs of metals in aquatic environment: water, sediment and biota. Among these three, the sediment is the major source for metals, in some cases, holding over 99 % of the total amount of metal present in the system (Renfro 1973). Sediments are preferred monitoring tools, since concentration of contaminants are higher than that in water, and show less variation with time and space, allowing more consistent assessment of spatial and temporal contamination (Krishna et al. 2011).
Sediments exist as complex and dynamic mixtures of mineral particles, particular organic material (detritus), microbes and flocculated chemicals. Contaminants in sediment may be associated with any of sediment fractions; therefore, it is important that upper crust contamination (UCC) in sediments by heavy metals must be evaluated (Chapman and Wang 2001). Heavy metals are deposited on to sediment particles (especially silt and clay due to high surface area) and move together with them and subsequently release to overlying water column either due to physical disturbance or digenesis. Sediment may persist as source of pollutants long after the cessation of direct discharge. The adsorption–desorption process is a prime action through which pollutants are transferred from unsaturated dissolved phase into solid phase (Kumar and Edward 2009). The degree of adsorption depends on properties of metals, viz. valency, radius, degree of hydration, co-ordination with oxygen, etc. The majority of sediment metal concentrations exceed interim sediment quality guideline’s threshold effect level (TEL) (Smith et al. 1996) in many of the estuaries (Pope and Langston 2011).
Heavy metals in marine waters and their corresponding sediments have become a significant topic of concern for man and aquatic life (Melegy et al. 2011). Heavy metals are natural constitutes of the earth crust. A number of elements are biologically essential and are introduced into aquatic enrichment by various anthropogenic activities (Turekian and Wedepohl 1961). Main anthropogenic sources of heavy metals exist in various industrial sources and are present as mining activities, foundries, smelters, and diffuse sources such as piping, constituents of products, combustion of by product, and human activities (Govil and Krishna 2007; Al-Khashman 2012).
Heavy metals pollutants are of high ecological significance. They are not removed from water by self-purification, but accumulate in suspended particulates and sediment, then enter into the food chain via passing to higher consumer (Cevik et al. 2009). Heavy metals may enter into aquatic ecosystems from natural and anthropogenic sources, such as industrial wastewater discharges, sewage wastewater, fossil fuel combustion, and atmospheric deposition (Sekabira et al. 2010). The most important heavy metals from the point of view of water pollution are Cu, Zn, Pb, Cd, Cr, Ni, and Hg. Metal passes from the water to sediment and vice versa under certain conditions by different processes, such as ion exchange, metal substitution, adsorption, and dissolution.
Heavy metal pollution in sediments has been studied by many groups. A potential ecological risk assessment of heavy metals in sediments of Yangtze River, China (Fu et al. 2009) was conducted. Saha et al. (2001) investigated the nutrient status and pollution load for six heavy metals (Cu, Zn, Fe, Mn, Pb and Cd) in the sediments of Jagannath canal, West Bengal. Kozhenkova et al. (2000) has monitored the heavy metal pollution in sea water using brown algae. Begum et al. (2009) carried out the analysis of water, plankton, fish, and sediments of Cauvery River. Sundaray et al. (2011) observed the pollution load due to heavy metals in the Mahanadi estuarine sediments. Heavy metal contamination in lagoon sediments was also studied by Uluturhan et al. (2011). Bentum et al. (2011) has assessed the heavy metal pollution in the sediments of Fosu Lagoon, Ghana. An assessment of heavy metals in sediments of Indian rivers has been made by many research groups (Ramanathan et al. 2006; Jain et al. 2007, 2008; Nair and Laluraj 2006; Singh et al. 2005; Raju et al. 2011). Quantitative estimation of mercury in sediments and organisms from two estuarine regions around Bombay island has been done (Srinivasan and Mahajan 1988).
The present study was aimed to assess the distribution, transportation, and statistical approach of lead, chromium, zinc, nickel, copper, cadmium, cobalt, and iron in sediments of Tapti estuary and to assess the pollution status as well as the possible influence of anthropogenic activities. The certified reference material (CRM) was used to validate the analytical results of experimental work. This work has very good scope in studying the environmental impact since number of industries, e.g., Kribhco, NTPC, Reliance, Essar, L&T, Shell and the dock are growing on the study area in recent times. Also the dock is situated between Essar and L&T, where various shipping activities are being done. Thus, it has direct impact of metal pollution in the estuary sediment.
Materials and methods
Study area
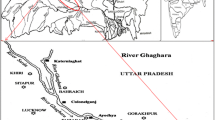
The Tapti River is one of the major rivers of peninsular India with the length of around 724 km, originates in the eastern ‘SATPURA RANGE’ of the southern part of Madhya Pradesh (District Betul) and flows from east to west. It then flows westwards, draining Madhya Pradesh’s Nirmal region, Maharatra’s Kandesh, East Vidarbh region in the North West corner of the Deccan plateau and South Gujarat before emptying into the Gulf of Cambay of the Arabian Sea. The Tapti River basin encompasses an area of 65.145 km2, nearly 2 % of the total area of India. The river serves a major source of domestic, irrigation and industrial water supply. The Tapti River gets heavily polluted in Gujarat and Maharashtra due to discharge of domestic sewage and industrial solid waste at Surat, Akola, Amravati, Dhuke, Bhusawal, and Malegaon. The study area shown in Fig. 1 is situated between longitude 72º39′41″–72º40′30″ E and latitude 21º05′30″–21º09′31″ N, covers the mouth of the estuary to Hazira beach.
Sediment sampling and pretreatment
After complete survey along the estuary coast, the surface sediment samples were collected once in a month (January 2011–May 2011) from 12 locations at four different sites (Fig. 1), covering the mouth of the estuary at Hazira beach. Samples were collected using Van veen grab sampler. Four sites were selected, S1 to S4, and the distance between the two sampling sites is about 2 km. The samples were collected from the back sides of the industries at the distance of about 50–100 m from the sea shore. At each site, about 100 g of sample was collected in triplicate at three different locations D1–D3 as shown in Table 1. The sediment samples were collected in polythene bag previously cleaned with dilute HNO3 to prevent metal precipitation. Samples were transferred to laboratory for further treatment and analysis.
Analytical method
Sediment samples were dried in a laboratory using an air circulating oven to a constant drying weight at 120 °C. By applying cone-quartz method (Vogel 1989), sediment samples were homogenized and passed through a 2 mm nylon sieve. The sediment fraction of <2 mm size were ground in agate mortar and passed through 63 μm sieve to obtain silt-like samples. The pH of sediment suspension (4 % in water) was measured using a calibrated Equip-Tronics pH meter (Model: EQ-614A, India). The pH of the suspension of sediment ranged from 8.0 to 8.59 for all samples. The chemicals used were of analytical reagent grade (Merck, Darmstadt, Germany). Laboratory equipments and glasswares were carefully cleaned with dilute HCl to minimize metal contamination. To determine the concentration of heavy metals, 1.0 g of dried sediment sample was digested in PTFE (Polytetrafluoroethylene) vessel with 9 mL of HNO3, 3 mL of HCl, 3 mL of HF (40 % W/V), and 2 mL of H2O2 (30 % W/V) in microwave oven (Q-ProM, Germany) for 15 min in distorted time periods USEPA-3052 (1996). Each suspension was filtered through Whatman filter paper No-42 and diluted to make stock solution. Concentrations of heavy metals (Pb, Cr, Zn, Ni, Cu, Cd, Co, and Fe) in different extracts were determined using atomic absorption spectrophotometer (AAS) (GBC Avanta version 1.32, Australia) with air–acetylene flame. Operational conditions were adjusted according to manufacturer’s guide lines to yield optimal determination. All analyses were done in triplicate by aspirating solution into AAS against the standard concentration of particular metal. Background value of the used acid mixtures was considered in computation of results. The CRM is a material whose one or more properties are adequately homogeneous and used for the calibration of an AAS instrumental results (Beg and Ali 2008). The certified standard reference material LGC-6137 was procured from the LGC Promochem India Private Litmited, Mumbai branch, Powai, Mumbai, and the analytical results are presented in Table 2 (LGC-6137 2008).
Statistical analysis
Enrichment factor (EF)
Enrichment factor is an effective tool to evaluate the magnitude of contaminants in the environment (Mohiuddin et al. 2011). EF is a relative abundance of chemical element in sediments compared to background and it is an effective tool to differentiate the metal sources between anthropogenic and naturally occurring source (Valdes et al. 2005). EF is an important parameter for the assessment of heavy metals (Qi et al. 2010). EF value is calculated by the ratio of the elemental concentration in the sediment to its natural background (Turekian and Wedepohl 1961). A reference element is impelled in the formula of EF to counterbalance lithogenic influences of granulometic and mineralogical variations of sediments.
where C n (sample) is the concentration of heavy metal ‘n’ in the examined sample, C ref (sample) is the concentration of reference element in the sample, B n (background) is the concentration of heavy metal ‘n’ in background environment, B ref (background) is the concentration of reference element in background environment. In this study, Fe is selected as a reference element due to its low occurrence of variability (Schiff and Weisberg 1999). Five contamination degrees are recognized on the basis of the enrichment factor shown in Table 3.
Geo-accumulation index (I geo)
The geo-accumulation index introduced by Muller (1969) was used to assess the metal pollution load in aquatic sediments (Thangaradjou et al. 2010). I geo is expressed as follows:
where C n is the concentration of metal ‘n’ in sample and B n is the geochemical back ground value of metal ‘n’. The constant 1.5 allows for natural fluctuations in the content of a given substance in the environment and very small anthropogenic influences (Kumar and Edward 2009). Six classes of the I geo have been distinguished as given in Table 3 (Muller 1969).
Results and discussion
Heavy metal distribution
Table 2 represents the average concentrations of heavy metals in sediments collected during the January 2011–May 2011. The results of the heavy metal analyses show that Fe is found to be maximum with respect to other metals in the concentration ranging from 5.66–9.0 %. Other metal concentrations had wider variations: Pb, 43.28–77.74; Cr, 48.26–72.40; Zn, 117.47–178.80; Ni, 71.13–107.82; Cu, 123.17–170.52; Cd, 0.74–1.25; and Co, 14.73–21.69 mg/kg. In Table 2, the highest values of Fe, Zn, and Cu were found at sampling station C1D3 (S1 site); Ni and Cd were found at C4D3 and C4D2, respectively (S4 site); Pb and Co were found at C1D2 (S1 site); and the highest concentration value of Cr was found at C2D2 (S2 site). Above results show that Fe, Zn, Cu, and Co may have originated from same source. The maximum concentration of Pb, Cd, Cu, Zn, and Fe are (77.74, 1.25, 170.52, 178.80 mg/kg, and 9 %, respectively) exceeding world average shale values by 3.87, 4.17, 3.78, 1.88, and 1.96 times, respectively (Turekian and Wedepohl 1961). Considering the distance from the seashore to all four sites (S1 to S4), it was observed that the metal distribution of Co and Cd were minimum irrespective of the distance from sea, whereas metal distribution of Zn and Cu were maximum in all the four sites. Mean concentration of Cu, Cr, Zn, and Ni exceeds the effect range low (ERL) values, but below effect range median (ERM) value (except Ni) (Long and Morgan 1991).
Contamination can be evaluated via I geo and EF. Both I geo and EF depend on the particular background data used. Average values of EFs for each site throughout the study period for metals are shown in Table 4. Average data reveal that Cr shows extreme enrichment, Ni and Co show very high enrichment. In case of Zn, Cu, and Pb, significant enrichment observed whereas Cd shows moderate enrichment throughout the study. According to Zhang and Liu (2002), EF value between 0.5 and 1.5 indicates that the metal is entirely from crustal material or natural process, whereas EF value >1.5 suggests that the sources are more likely to be anthropogenic. The high EF values (>1.5) show environmental contamination by these metals instead of weathering processes. Overall average I geo values for each metal were found in following order: Cd < Pb < Cu < Zn < Ni < Co < Cr. The high values of I geo indicate a significant anthropogenic effect on the sediment of Tapti estuary. Zn, Co, and Ni exhibit very high contamination. I geo for Pb and Cd ranged from 1.38 to 1.85 and 0.41 to 0.48, respectively, and the I geo classes were 2 and 1, respectively. Thus, Pb and Cd contamination level was classified as slightly contaminated level.
Lead and chromium
The average lead (Pb) and chromium (Cr) levels ranged from 43.28 to 77.74 and 48.26 to 72.40 mg/kg in the study area, respectively. Normal TEL and probable effect level (PEL) for Pb are 35.0 and 91.3 mg/kg whereas for Cr are 37.3 and 91.3 mg/kg, respectively (Smith et al. 1996). It is clearly seen that Pb and Cr concentrations in the study area exceed the TEL but below the PEL. According to sediment quality guideline (SQG) (Long et al. 1995), all sites are moderately polluted due to Pb and Cr. The highest concentration of Pb, observed at S4 site (near Reliance Ind. Ltd.) was could be due to discharge from NTPC, Kawas. The estuary gets discharged wastewater containing Cr from industries located at Hazira belt. The maximum concentration for Pb exhibits 185.11 mg/kg in April-2011 at C3D2 location, whereas it is 153.68 mg/kg for Cr in the month of March-2011 at C2D3 location (Supplementary material Fig. S1).
The lead EF varied from 4.49 to 5.49 within all sampling sites, providing maximum value at a site S4, indicating that all sampling sites (except S4) exhibit significant enrichment of Pb. The EF values for Cr were found in the range from 39.87 to 64.5. It suggests extreme enrichment of Cr at all sampling sites. The highest I geo values for Pb and Cr were found to be 1.85 and 4.95, respectively. From I geo values, it is concluded that all sites are moderately contaminated by Pb, high to very highly contaminated by Cr.
Copper and nickel
The average distribution of copper (Cu) and nickel (Ni) in the study area ranged from 123.17 to 170.52 and 71.13 to 107.82 mg/kg, respectively. The study areas exceed the TEL values for Cu and Ni (35.7 and 18 mg/kg) at all sites. Concentration of Cu is below the PEL value of Cu (197 mg/kg) whereas concentration of Ni exceeds its PEL value (36.0 mg/kg). Maximum concentration of Cu was found at C1D3 station (S1 site) that confirms its origin from Essar industry. Such high values of Cu in study area may be from anthropogenic sources as well as geogenic activities. The high concentration of Ni may arise from petroleum industry discharge. The maximum level of Cu is observed to be 283.54 mg/kg in the month of April 2011 at C4D2 location and for Ni, it was about 166.74 mg/kg in the month of May 2011 at C4D3 location (Supplementary material Fig. S2). As per SQG, all sites are heavily polluted due to Cu and Ni.
The maximum EFs and I geo values for Cu enrichment are significant at all sites, whereas contamination due to Cu is moderate to high at S2 and S3 sites and high at S1 and S4 sites. Ni shows very high enrichment at all sampling sites with the maximum EF value of 24.53. I geo for Ni is almost same at all sites with an average value of 3.79, exhibits high contamination at all sites.
Cadmium and cobalt
The average concentrations of cadmium (Cd) and cobalt (Co) in the study area were found in the range from 0.74 to 1.25 and 14.73 to 21.69 mg/kg, respectively. The concentration of Cd exceeds the TEL value (0.596 mg/kg) but below the PEL value (3.53 mg/kg). A maximum concentration of Cd was found at C4D2 station (S4 site) and that of Co at C1D2 station (S1 site). Co occurs in association with other metals such as Cu, Ni, and Zn. It is associated with sulfides and gets released during the processing of sulfides thus turning harmful to environment (Onianwa 2001). According to SQG, all sites are non-polluted by Cd. The Cd exhibited maximum concentration of about 2.5 mg/kg in the month of February 2011 at C4D2 location and Co observed maximum concentration of 29.65 mg/kg at C1D2 location in the month of January 2011(Supplementary material Fig. S3).
The EF values for Cd were found in the range 1.93–2.80, indicate moderate enrichment at sites, whereas Co shows very high enrichment at all sites with range 33.30–35.74. The high enrichment of Co at S2 site may be due to in cooperation of oxides of Co on the base material present in sediment, e.g., Al2O3, quartz, etc. I geo values for Cd were found in the range of 0.41–0.78 revealing nil to moderate contamination for all sites. I geo values for Co suggest high to very high contamination at all sites with the maximum value of 4.40.
Zinc and iron
The average concentrations of zinc (Zn) and iron (Fe) are found to be in the range from 117.47 to 178.80 mg/kg and 5.66 to 9.0 %, respectively. Concentration of Zn exceeds the TEL value at all stations except C1D1 and C3D3, whereas it is below the PEL value. The maximum concentrations of both metals, Zn and Fe, were found at station C1D3 (S1 sites). Zn is widely used in the galvanization of Iron, and both metals are found maximum at same S1 site located near Essar industry Ltd. as shown in Fig. 1. It is well established that iron hydroxide acts as an excellent scavenger for other suspended metals (Duan et al. 2010). Zn and Fe show maximum concentrations 345.34 mg/kg and 16.09 %, respectively, in the month of January 2011 at C1D3 location (Supplementary material Fig. S4). According to SQG, all sites are moderately to heavily polluted due to Zn.
The EF values of Zn ranged from 12.54 to 19.50. Zn shows significant enrichment at all sites. I geo values for Zn indicate high contamination with range of 3.02–3.33.
Inter-element relationships
Pearson’s correlation coefficients are calculated to determine relationships among different metals (Garcia and Millan 1998), presented in Table 5. Results show that concentrations obtained for metals in the month of January 2011 are relatively good correlation among themselves, than those in four months. Element pairs Zn versus Co, Ni versus Co, Cu versus Co, and Fe versus Co show negative correlation whereas remaining pairs show positive correlation, out of Fe versus Ni, Fe versus Cd, Fe versus Cr, Ni versus Cr, Zn versus Cd, Zn versus Cu, Ni versus Cd and exhibit significant to very high correlation (0.80 > r > 0.95). Metal pairs Cu versus Fe, Cr versus Fe, Ni versus Fe, Cr versus Cu, Cr versus Ni, and Ni versus Cu exhibit good positive correlation (0.80 > r > 0.95) in the month of April 2011. All metals exhibit negative to low correlation with pH. Though elemental association may signify that each paired element has identical sources or common sink in upper crust sediments (Singh et al. 2002), yet it indicates that there is no mutual relationship among the metals. Hence, the prediction of level of any metal having the same source point as the other metals is impossible. Negative correlation or lack of correlation reflects an anthropogenic contribution due to urban development near Tapti estuary.
Transportation of heavy metals
The transportation of heavy metals in riverine estuary system associated with physical and chemical processes such as weathering process and chemical-phase transformation such as carbonate to oxide. Once they enter the aquatic environments, heavy metals would be adsorbed on sediment particles. The extent of metal sorption and its transportation depends on sediment characteristics (grain size, chemical components, organic carbon contents, mineral phases, and texture), sedimentary environmental characteristics (salinity, pH, redox potential, presence of ligand in sediment), and sources whereby heavy metals entered into the environment (Marcussen et al. 2008). Figure 2 shows the month wise metal transportations at each main sampling site S1–S4. Fe, Cd, and Co show no transportation throughout the site. Since Fe was found in abundance around study area, Cd and Co was found in lowest range as compared to other metals. Fe, Cd and Co shows no transportation throughout the sites whereas Pb, Cr, Zn, and Ni show significant transportations at each site. The possible mechanisms of explaining this mobilization include adoption–desorption and flocculation on Fe–Al Oxides at different salinity levels. Heavy metals mobilize as soluble chloride complexes. The stability and solubility of heavy metal complexes were relatively high while affinity for sorption to the sediments was low. Upon the formation of these complexes, the activity of free heavy metal in water would decrease and desorption of sediments would increase. With the increase in salinity, alkalinity also increases, resulting in precipitation and adsorption of heavy metals (Williams et al. 1994).
Comparison with the other estuaries
The concentrations of metals and average shell values in the sediments of Tapti estuary were compared with those of other estuaries around the world, such as, Turkey, China and Spain. Apart from Cr and Fe, the concentration level of all metals studied herein were mostly higher than the average shell values with Pb 6-fold, Zn 2.5-fold, Ni 1.4-fold, Cu 3-fold, Cd 6-fold approximately higher than those of the earth’s crust (Turekian and Wedepohl 1961). This increase of concentrations of Pb, Zn, Ni, Cu, and Cd may due to anthropogenic as well as industrial solid waste and wastewater discharge. The metal concentrations in the sediments of Tapti estuary reported herein were compared with those published by other groups of researchers on various estuaries of the world (Table 6). The assessment informations of Seyhan River, Turkey (Davutluoglu et al. 2011); Bohai-Bay, North China (Duan et al. 2010); Manakudy estuary, Southwest coast, India (Kumar and Edward 2009); Arosa estuary, New Spain (Fernandez et al. 2006); Nanliu River estuary, Beibu Gulf of South China (Xia et al. 2011); Vembanad estuary, Southwest coast, India (Padmalal et al. 1997); Tambaraparni River estuary, Tamil Nadu, India (Jayaraju et al. 2011) are provided in the Table 6. Tapti estuary was found to be more polluted than Bohai-Bay, North China and Nanliu estuary, China based on Pb, Cr, Zn, and Cd compared to those in the Table 6.
Conclusions
The upper crust metal contamination does not show contaminations due to single source as the results do not coincide with the natural structure of the region due to growing industries. Nevertheless, it is suggested that more detailed study should be carried out to determine whether heavy metal pollution affects the marine ecosystems or not. Obtained data showed that site S1 (Essar Industry Ltd.) is relatively more polluted than other sites, S2, S3, and S4. On the basis of the calculated I geo values, sediments of Tapti estuary are nil to moderately contaminated with respect to Cd, moderate to strongly polluted with respect to Pb, Zn, Ni, Cr, Cu, and Co, and extremely contaminated with respect to Fe at all sampling sites. By considering average concentration of metals, SQG suggests that this study area is moderately polluted throughout the study period. Monthly monitoring of heavy metals indicates high fluctuations in the concentrations of metals (except Cd) and at particular site reveals metal transportations. The metal transportation is also confirmed by negative to very low correlation among metals. Since only two major metal industries lying on the Tapti estuarine region, high fluctuations in concentration among the metals were observed. The obtained metal concentrations levels in Tapti estuary were found to be higher than that of many other estuaries. The levels of heavy metals studied should be kept under strict control, since they exhibit potential biological risks. There should be a provision to measure toxic metals in industrial effluents before discharging them into estuarine river.
References
Al-Khashman OA (2012) Assessment of heavy metal accumulation in urban soil around potash industrial site in the east of the dead sea and their environmental risks. Soil Sediment Contam 21:276–290
Beg KR, Ali S (2008) Toxicity of reference sediment samples. Am J Environ Sci 4(4):347–352
Begum A, Ramaiah M, Harikrishna, Khan I, Veena K (2009) Heavy metal pollution and chemical profile of Cauvery river water. E-J Chem 6(1):47–52
Bentum JK, Anang M, Boadu KO, Koranteng-Addo EJ, Antwi EO (2011) Assessment of heavy metals pollution of sediments from Fosu Lagoon in Ghana. Bull Chem Soc Ethopia 25(2):191–196
Cevik F, Goksu MZL, Derici OB, Findik O (2009) An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geoaccumulation index and statistical analyses. Environ Monit Assess 152:309–317
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22
Davutluoglu OI, Seckin G, Ersu CB, Yilmaz T, Sari B (2011) Assessment of metal pollution in water and surface sediments of the Seyhan River, Turkey, using different indexes. Clean-Soil Air Water 39(2):185–194
Duan L, Song J, Xu Y, Li X, Zhang Y (2010) The distribution, enrichment and source of potential harmful elements in surface sediments of Bohai Bay, North China. J Hazard Mater 183:155–164
Fernandez RS, Cave MR, Hill SJ (2006) Trace metal distribution in the Arosa estuary (N.W. Spain): the application of a recently developed sequential extraction procedure for metal partitioning. Anal Chim Acta 557:344–352
Fu C, Guo J, Pan J, Qi J, Zhou W (2009) Potential ecological risk assessment of heavy metal pollution in sediment of the Yangtze River within the Wanzhou section, China. Biol Trace Elem Res 129:270–277
Garcia R, Millan E (1998) Assessment of Cd, Pb and Zn contamination in roadside soils and grasses from Gipuzkoa (Spain). Chemosphere 37:1615–1625
Govil PK, Krishna AK (2007) Soil contamination due to heavy metals from an industrial area of Surat, Gujarat, Western India. Environ Monit Assess 124:263–275
Jain CK, Malik DS, Yadav R (2007) Metal fractionation study on bed sediments of Lake, Nainital, Uttaranchal, India. Environ Monit Assess 130:129–139
Jain CK, Gupta H, Chakrapani GJ (2008) Enrichment and fractionation of heavy metals in bed sediments of River Narmada, India. Environ Monit Assess 141:35–47
Jayaraju N, Reddy BCSR, Reddy KR (2011) Anthropogenic metal pollution in surface sediments of the Tambaraparni River Estuary. Chem Ecol 27(4):337–350
Kozhenkova SI, Khristoforova NK, Chernoval EN (2000) Long-term monitoring of sea water pollution by heavy metals in Northern Primorye with the use of Brown Algae. Russ J Ecol 31(3):211–215
Krishna AK, Mohan KR, Murthy NN (2011) A multivariate statistical approach for monitoring of heavy metals in sediments: a case study from Wailpalli Watershed, Nalgonda District, Andhra Pradesh, India. Res J Environ Earth Sci 3(2):103–113
Kumar SP, Edward JKP (2009) Assessment of metal concentration in the sediment cores of Mankudy estuary, south west coast of India. Indian J Mar Sci 38(2):235–248
LGC-6137 (2008) Certified Reference Material of estuarine sediment—extractable metals, LGC Standards, LGC Limited., UK. Accessed 25th July 2012 https://www.lgcstandards.com/epages/LGC.sf/en_GB/?ObjectPath=/Shops/LGC/Products/LGC6137
Long ER, Morgan LG (1991) The potential for biological effects of sediments-sorbed contaminants tested in the National Status and Trends Program. Technical Memorandum NOS OMA 52, National Oceanic and Atmospheric Administration, Seattle, WA, 175 pp + appendices
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19(1):81–97
Marcussen H, Dalsgaard A, Holm PE (2008) Content, distribution and fate of 33 elements in sediments of rivers receiving wastewater in Hanoi, Vietnam. Environ Pollut 155:41–51
Melegy A, El Wahab MA, Helal S (2011) Distribution and enrichment of heavy metals in recent sediments of Safaga bay, Egypt. Mar Georesour Geotechnol 29:364–375
Mohiuddin KM, Ogawa Y, Zakir HM, Otomo K, Shikazono N (2011) Heavy metals contamination in water and sediments of an urban river in a developing country. Int J Environ Sci Technol 8(4):723–736
Muller G (1969) Index of geoaccumulation in the sediments of the Rhine river. Geol J 2:108–118
Nair SM, Laluraj CM (2006) Geochemical index of trace metals in the surficial sediments from the western continental shelf of India, Arabian Sea. Environ Geochem Health 28:509–518
Onianwa PC (2001) Roadside topsoil concentrations of lead and other heavy metals in Ibadan, Nigeria. Soil Sediment Contam 10(6):577–591
Padmalal D, Maya K, Seralathan P (1997) Geochemistry of Cu, Co., Ni, Zn, Cd and Cr in the surficial sediments of a tropical estuary, southwest coast of India: a granulometric approach. Environ Geol 31(1/2):85–93
Pope ND, Langston WJ (2011) Sources, distribution and temporal variability of trace metals in the Thames estuary. Hydrobiologia 672:49–68
Qi S, Leipe T, Rueckert P, Di Z, Harff J (2010) Geochemical sources, deposition and enrichment of heavy metal in short sediment cores from the Pearl river estuary, Southern China. J Mar Syst 82:S28–S42
Raju K, Vijayaraghavan K, Seshachalam S, Muthumanickam J (2011) Impact of anthropogenic input on physicochemical parameters and trace metals in marine sediments of Bay of Bengal off Chennai, India. Environ Monit Assess 177:95–114
Ramanathan AL, Prasad MBK, Shrivastav SK, Anshumali, Saxena R (2006) Metal fractionation studies in surfacial and core sediments in the Achankovil river basin in India. Environ Monit Assess 121:77–102
Renfro WC (1973) Transfer of 65Zn from sediments by marine polychaete worms. Mar Biol 21:305–316
Saha SB, Mitra A, Bhattacharyya SB, Choudhury A (2001) Status of sediment with special reference to heavy metal pollution of a brackishwater tidal ecosystem in northern Sundarbans of West Beangal. Trop Ecol 42(1):127–132
Schiff KC, Weisberg SB (1999) Iron as a reference element for determining trace metal enrichment in Southern California coastal shelf sediments. Mar Environ Res 48:161–176
Sekabira K, Origa HO, Basamba TA, Mutumba G, Kakudidi E et al (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int Environ Sci Technol 7(3):435–446
Singh M, Muller G, Singh IB (2002) Heavy metals in freshly deposited stream sediments of rivers associated with urbanization of the Ganga plain, India. Water Air Soil Pollut 141:35–54
Singh VK, Singh PK, Mohan D (2005) Status of heavy metals in water and bed sediments of river Gomti- A tributary of the Ganga River, India. Environ Monit Assess 105:43–67
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638
Srinivasan M, Mahajan BA (1988) Mercury pollution in the estuarine region around Bombay Island. Environ Technol Lett 9:331–336
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: mahanadi basin, India. J Hazard Mater 186:1837–1846
Tailor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285
Thangaradjou T, Nobi EP, Dilipan E, Sivakumar K, Kanan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Est Coast Mar Sci 87:253–264
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Bull Geol Soc Am 72:175–192
Uluturhan U, Kontas A, Can E (2011) Sediment concentrations of heavy metals in the Homa Lagoon (Eastern Aegean Sea): assessment of contamination and ecological risks. Mar Pollut Bull 62:1989–1997
USEPA-3052 (1996) Microwave assisted digestion of siliceous and organically based matrices, Method 3052, Rev 0, In SW-846: test methods for evaluating solid waste, physical/chemical methods. US Environmental Protection Agency Office of Solid Waste, Washington, DC, pp 1–20
Valdes J, Vargas G, Sifeddine A, Ortlieb L, Guinez M (2005) Distribution and enrichment evaluation of heavy metals in Mejillones Bay (23 S), Northern Chile: geochemical and statistical approach. Mar Pollut Bull 50:1558–1568
Vogel AI (1989) A text book of quantitative analysis, 5th edn. ELBS publication, London
Williams TP, Bubb JM, Lester JN (1994) Metal accumulation within salt marsh environments: a review. Mar Pollut Bull 28:277–290
Xia P, Meng X, Yin P, Cao Z, Wang X (2011) Eight-year sedimentary record of heavy metal inputs in the intertidal sediments from the Nanliu River estuary, Beibu Gulf of South China Sea. Environ Pollut 159:92–99
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-Weathering features, anthropogenic impact and chemical fluxes. Est Coast Mar Sci 54:1051–1070
Acknowledgments
The authors are grateful to University Grants Commission (UGC) (File No. 37-474/2009(SR)), New Delhi, India and Gujarat Council on Science and Technology (GUJCOST) (File no. 201426/09-10/3051-3052), Ghandhinagar, Gujarat, India for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, B.A., Shah, A.V., Mistry, C.B. et al. Assessment of heavy metals in sediments near Hazira industrial zone at Tapti River estuary, Surat, India. Environ Earth Sci 69, 2365–2376 (2013). https://doi.org/10.1007/s12665-012-2066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-2066-4