Abstract
Cd–Se thin films of varying thicknesses were prepared on glass substrate by chemical bath deposition technique at room temperature in which selenium dioxide is source of Se2− while cadmium acetate is source of Cd2+ ions. Ammonia was used as complexing agent. Nickel doping is achieved by adding Ni(NO3)2 solution to the bath. Approximate thickness was measured by gravimetric method. Band gap of the films was determined by measuring percent transmittance of film at various wavelengths. The band gap and thickness of the film were found to increase with increase of Ni concentration in films. The composition and structural characterization was carried out by using EDAX and SEM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cadmium selenide (CdSe) is a II–VI group semiconductor compounds having suitable band gap (1.7 eV), high absorption coefficient and can be utilized in a number of opto-electronic devices such as PEC cells [1–3], hybrid solar cells [4], transistors [5], light emitting diodes [6], etc. A variety of deposition techniques such as electrode deposition [7, 8], vacuum evaporation [9–11] and chemical bath deposition (CBD) [12–14] have been used to deposit CdSe thin films. Among them, CBD method is the best suited for thin film deposition because of its convenience, least expenses and its ability to deposit solid solutions without the aid of sophisticated instrumentation [15]. We have used this method successfully for getting uniform, well adherent, nearly stoichiometric CdSe films, with fewer defects [12]. In recent year’s major attention have been given to the investigation of electrical and optical properties of CdSe thin films in order to improve the performances of the devices and for finding new applications [16–20]. Various researchers demonstrated the higher energy band gap ternary system (doped with other metal) for increasing the short circuit current in the solar cell [21]. Investigation have also been made on the improved properties of CdSe thin film doped with Al [22], In [23], Hg [24], Cu [25], Sn [26], Fe [27]. Literature survey shows that there is no work on Ni doped CdSe films. The objective of present work is to study the effect of Ni doping on optical and structural properties of CdSe thin films prepared by CBD technique at room temperature on glass substrate. Recently there are many reports on various thin films prepared by different techniques [28–34].
2 Experimental details
2.1 Thin film deposition
The thin films of CdSe were deposited onto glass substrates with dimensions (76 mm × 26 mm × 1 mm) of a standard microscope glass. Glass substrates were immersed in chromic acid solution for 1 h then washed with distilled water and acetone and dried in oven.
The adhesion with the substrate is of prime importance for the quality of the deposited thin films. To improve the film adhesion, the glass substrates were immersed in a diluted solution of tin chloride (SnCl2) prior to the deposition process and afterwards thermally treated at 200 °C. Chemical deposition of CdSe film is carried out at room temperature. As precursor of selenide ions, sodium selenosulphate (Na2SeSO3) was used. 0.05 M Na2SeSO3 solution was prepared by refluxing the mixture of 0.05 M sodiumsulphate (Na2SO3) and 0.05 M selenium dioxide (SeO2) solutions in equal proportion with constant stirring for 5 h at 110 °C. The deposition bath is prepared by taking 30 ml freshly prepared 0.02 M cadmium acetate (CH3COO)2Cd·2H2O and 50 ml 0.05 M Na2SeSO3 in 250 ml beaker. pH of the bath solution is adjusted to 9.0 by adding excess of liquor ammonia. The glass substrates were immersed in solution. The solution was continuously stirred using mechanical stirrer. The substrates with CdSe coating were taken out of the bath after deposition period of 16 and 18 h, washed with water and dried using hot air blower. For Ni doping, to above solution Ni(NO3)2 solution was added in varying quantities (0.5, 1.0, 1.5 and 2.0 ml). Ni(NO3)2 solution was prepared by dissolving 1 mg of pure nickel metal in 25 ml of concentrated HNO3 under vigorous stirring.
2.2 Characterization of thin film
Approximate thickness of the film was measured by gravimetric method (weight difference method). In gravimetric method, the thickness of film was determined by weighing the glass substrate before and after deposition on the microbalance of sensitivity 0.0001 g, assuming the density of film to be same as that of bulk material, thickness may be calculated by using the relation.
where A is the surface area of the films, M is mass of the films, ρ is the density of the CdSe.
The microstructure of the films was studied by using a scanning electron microscope (JEOL Model-6360), attached with an energy dispersive X-ray analysis (EDAX) to measure quantitatively the sample composition. The film was coated with 10 nm gold layer using Polaron SEM sputter coating unit, before taking micrographs and EDAX. The elemental composition of the CdSe and Ni doped CdSe deposited films were carried out by using the EDAX technique The optical absorbance/transmittance of the films were studied at room temperature in the spectral range of 500–1,000 nm using UV Visible spectrophotometer (Chemito, spectroscan UV 2700) from which the band gap is calculated by using the formula
where h is plank constant, c is velocity of light, and λ is wavelength.
3 Result and discussion
3.1 Reaction mechanism
The deposition of CdSe thin film takes place when the ionic product (IP) of Cd2+ and Se2− ions exceeds the solubility product (SP) of CdSe (i.e. IP ≥ SP (10−33). The CdSe deposition takes place according to the following steps [28]:
The hydrolysis of sodium selenosulphate (Na2SeSO3) in solution to give Se2− ions is according to the chemical reaction
Similarly, the hydrolysis of ammonia in water to give OH− ions,
When the ammonia is added to the Cd2+ salt solution, Cd(OH)2 starts precipitating when the SP of Cd(OH)2 is exceeded,
The Cd(OH)2 precipitate dissolves in excess ammonia solution to form the complex cadmium tetra-amine ions [Cd(NH3)4]2+ as follows,
Finally, the CdSe thin film formation takes place,
Ordinarily nickel metal was dissolved in concentrated nitric acid (HNO3) the doping of nickel was carried by adding the different volumes of nickel nitrate (NiNO3) solution to the above chemical bath. The reaction mechanism is as follows:
Finally we get CdNiSe
3.2 Growth mechanism
The growth mechanism of thin films in the CBD method can take place either in the bulk of the solution (homogeneous precipitation process) or at the substrate surface (heterogeneous process) [35]. Froment and Lincot have proposed that the first process is associated with the agglomeration of colloids formed in the solution by the homogeneous reaction. It can be considered as a “cluster-by cluster” growth, leading to particulate film. The second process is the growth mechanism involving the reaction of atomic species at the surface; it corresponds to an atom-by-atom process commonly known as an “ion-by-ion” nucleation and growth mechanism. The predominance of one given mechanism is governed by classical laws of homogeneous versus heterogeneous nucleation on a solid surface, involving the super saturation ratio in the solution and the catalytic activity of the substrate and the rate of stirring [36]. In short, the growth mechanism depends on the experimental conditions.
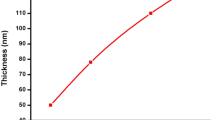
In the present study, due to the absence of thermal and mechanical agitation, a strong [Cd(NH3)4]2+ complex, and a stable Na2SeSO3 precursor, the Cd2+ and Se2− ions were slowly released in the solution, which then condense with an “ion by ion” basis on the substrates that are suitably mounted in the solution. CBD of semiconductor thin films involves a nucleation/incubation phase followed by a growth phase and terminal phase [36]. In the present study the film thickness was linearly increased with time (Table 1). CdSe films were extremely adherent to the substrate and did not peel off although the deposition was carried out for prolonged time intervals. After a certain time interval or terminal thickness some powdery CdSe material was deposited over the adherent film surface that could be removed with water. The Ni doped CdSe thin film were prepared by using same technique. After addition of NiNO3 in a bath of CdSe a heterogeneous reaction and precipitation occurs to form CdNiSe thin film [37]. The film thickness lies between 1.55 and 1.78 μm and it increases with increases in the concentration of Ni as well as deposition time (Table 1).
3.3 Optical properties
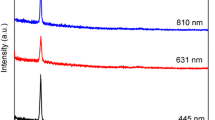
Figure 1 shows transmittance spectra of the CdSe and Ni doped CdSe films. Band gap value is calculated by using Eq. (2) for wavelength at which Percentage transmittance is minimum or zero. The value of this wavelength decreases as the concentration of Ni in the film increases. The band gap value of the undoped CdSe film (1.77 eV) was found somewhat smaller than the Ni doped CdSe thin film (1.88–2.38 eV). Also as the Ni concentration in the film increases band gap increases (Table 2). This may be due to change in structure and stoichiometry of film as Ni content in the film changes as can be seen from SEM images. As the structure changes the lattice constants are also changing which are related to band gap [38–40].
3.4 Structural and Elemental properties
Scanning electron microscopy (SEM) is a convenient technique to study microstructure of thin films. Figure 2a–d shows the SEM pictures of the CdSe and Ni doped CdSe thin films of various atomic percent of Ni 0.49, 0.83, 1.32, respectively at room temperature. Figure 2a shows irregular shape crystallites and some of them are agglomerated and chank type crystals, in Fig. 2b, c most of the crystals are rectangular with smooth surface, in Fig. 2d cubic crystals with large surface area with uniform coating are seen.
From images, it is clear that the all films were compact and homogeneous with well defined, fine grain background. As the concentration of Ni increases, there is a sharp change in structure as well as thickness of the film. Increase in the thickness of the films shows a substantial granular growth and dense porous network structure. Increase in overgrowth of surface particles with thickness is well known for chemically deposited films [41]. Figure 3 shows a typical EDAX pattern and the details of the relative analysis are depicted in Table 3. The strong peaks for Cd and Se were found in the spectrum, and no impurity peaks were detected in the EDAX spectrum. The average atomic percentage ratio of Cd:Se was found to be 50.5:49.5, showing that the film was in good stoichiometric ratio. The EDAX analysis results indicated that the Cd:Se ratios are very close to a 1:1 stoichiometric composition as shown in (Table 3) and the Cd:Ni:Se films having the average atomic percentage ratio were found to be 43.99:0.49:55.52, 43.65:0.83:53.51, 60.30:1.32:38.37 for 0.5, 1, 1.5 ml Ni concentration, respectively.
4 Conclusions
CdSe and Ni doped CdSe thin films were successfully deposited by using CBD technique from aqueous medium at room temperature. Thickness of the films was measured by gravimetric method and it was found to be increased with the deposition time as well as the increased Ni concentration. The structure of the films consists of fine and highly oriented grains. The crystallites size, i.e. the thickness increases when the Ni content and the deposition time increases. A direct optical band gap of was found from transmittance spectra. As the concentration of Ni in the film increases the band gap of the film increases.
References
K Rajeshwar Adv. Mater. 4 23 (1992)
W Lee, W-C Kwak, S Ki Min, J-C Lee, W-S Chae, Y-M Sung and S-H Han Electrochem. Commun. 10 1699 (2008)
A Kongkanand, K Tvrdy, K Takechi, M Kuno and P V Kamat, J. Am. Chem. Soc. 130 4007 (2008)
L Han, D Quin, X Jiang, Y Liu, L Wang, J Chen and Y Cao Nanotechnology 17 4737 (2006)
C J Panchal Indian J. Phys. 79 1269 (2005)
S Nizamoglu, T Ozel, E Sari and H V Demir Nanotechnology 18 065709 (2007)
S M Pawar, A V Moholkar and C H Bhosale Mater. Lett. 61 1034 (2007)
C M Shen, X G Zhang and H L Li Appl. Surf. Sci. 240 34 (2005).
K N Shreekanthan, B V Rajendra, V B Kasturi and G K Shivakumar Cryst. Res. Technol. 38 20 (2003)
S Velumani, X Mathew, P J Sebastian, S K Narayandass and D Mangalaraj Sol. Energy Mater. Sol. Cells 76 347 (2003)
Z Aneva, D Nesheva, C Main, S Reynolds, A G Fitzgerald and E Vateva Semicond. Sci. Technol. 23 095002 (2008)
H E Esparza-Poncea, J Hernandez-Borja, A Reyes-Rojas, M Cervantes-Sancheza, Y V Vorobiev, R Ramirez-Bon, J F Perez-Robles and J Gonzalez-Hernandez Mater. Chem. Phys 113 824 (2009)
R B Kale and C D Lokhande Semicond. Sci. Technol. 20 1 (2005)
S Bhushan and A Shrivastava Indian. J. Phys. 84 1517 (2010)
S Erat, H Metina and M Ari Mater. Chem. Phys. 111 114 (2008)
O Oduor and R D Gould Thin Solid Films 270 387 (1995)
K P Mohanchandra and J Uchil Thin Solid Films 305 124 (1997)
M J Lee and S-C Lee Solid State Electron. 43 833 (1999)
D Samanta, B Samanta, A K Chaudhuri, S Ghorai and U Pal Semicond. Sci. Technol. 11 548 (1996)
U Pal, D Samata, S Ghorai and A K Chaudhuri J. Appl. Phys. 74 6368 (1993)
R Chandran and G Suresh Chalcogenide Lett. 8 689 (2011)
T O Poehler and D Abraham Appl. Phys. Letters. 6 125 (1965)
T Mahalingam, R Mariappan, V Dhanasekaran, S M Mahan, G Ravi and J P Chu Chalcogenide Lett. 7 669 (2010)
V M Bhuse Mater. Chem. Phys. 91 60 (2005)
K Subba Ramiah, Y K Su, S J Chang, F S Juang, K Ohdaira, Y Shiaki, H P Liu, I G Chen and A K Bhatnagar J. Cryst. Growth 224 74 (2001)
E U Masumdar, V B Gaikwad, V B Pujari, P D More, and L P Deshmukh Mater. Chem. Phys. 77 669 (2003)
S Thanikaikarasan, K Sundaram, T S Velumani and J-K Rhee Mater. Sci. Eng B 174 242 (2010)
P Saikia, A Borthakur and P K Saikia Indian J. Phys. 85 551 (2011)
A T M K Jamil and H Noguchi Indian J. Phys. 85 737 (2011)
T P Rao, M C Santhosh Kumar and V Ganesan Indian J. Phys. 85 1381 (2011)
L Daoyong, O Yu, C Li, C Weiran and S Shaohua Indian J. Phys. 85 293 (2011)
D Kalhar, R Zahiri, S A Ketabi and A Ebrahimzad Indian J. Phys. 84 539 (2010)
B Saha, R Thapa, N S Das and K K Chattopadhyay Indian J. Phys. 84 681 (2010)
B Saha, R Thapa, S Jana and K K Chattopadhyay Indian J. Phys. 84 1341 (2010)
R B Kale and C D Lokhande J. Phys. Chem. B 109 20288 (2005)
M Froment and D Lincot Electrochim. Acta 40 1293 (1995)
D A Johnston, M H Carletto, K T R Reddy, I Forbes and R W Miles Thin Solid Films 102 403 (2002)
I Vurgaftman and J R Meyer J. Appl. Phys. 94 3675 (2003)
R Andreichin, A Ivanova and U Stanislavova Proceedings 5th IMEKO-symposium on photon detractors (pt. II) (ed.) J. Schanda (Bulgaria: Varna) (1971)
M C Santhosh Kumar and B Pradeep Indian. J. Phys. 85 401 (2011)
G Hodes, A Albu-Yaran, F Decker and P Matsuke Phys. Rev. B 36 4215 (1987)
Acknowledgments
The authors are thankful to principal M.S.G. College, Malegaon Camp and General Secretary M. G. Vidyamandir for providing laboratory facilities and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pawar, R.R., Bhavsar, R.A. & Sonawane, S.G. Structural and optical properties of chemical bath deposited Ni doped Cd–Se thin films. Indian J Phys 86, 871–876 (2012). https://doi.org/10.1007/s12648-012-0140-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-012-0140-0