Abstract
Since the mid-to-late 2000s, synthetic cathinones have gained popularity among drug users due to their psychostimulant effects greater than those produced by cocaine and amphetamine. Among them, 3,4-methylenedioxypyrovalerone (MDPV) and 1-phenyl-2-(pyrrolidin-1-yl)pentan-1-one (α-PVP) are ones of the most popular cathinones available in the clandestine market as “bath salts” or “fertilizers.” Pre-clinical studies indicate that MDPV and α-PVP induced psychomotor stimulation, affected thermoregulation, and promoted reinforcing properties in rodents. However, a direct comparative analysis on the effects caused by MDPV and α-PVP on the behavior and neuronal activation in rodents is still lacking. Behavioral analyses revealed that both MDPV and α-PVP affect spontaneous and stimulated motor responses. In particular, MDPV showed a greater psychomotor effect than α-PVP in line with its higher potency in blocking the dopamine transporter (DAT). Notably, MDPV was found to be more effective than α-PVP in facilitating spontaneous locomotion and it displayed a biphasic effect in contrast to the monophasically stimulated locomotion induced by α-PVP. In addition to the behavioral results, we also found a different modulation of immediate early genes (IEGs) such as Arc/Arg3.1 and c-Fos in the frontal lobe, striatum, and hippocampus, indicating that these drugs do impact brain homeostasis with changes in neuronal activity that depend on the drug, the brain area analyzed, and the timing after the injection. These results provide the first discrimination between MDPV and α-PVP based on behavioral and molecular data that may contribute to explain, at least in part, their toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic cathinones are an emerging class of designer drugs abused for their psychostimulant and empatogenic properties similar to cocaine, methylenedioxymethamphetamine, or other amphetamines (German et al. 2014; Spiller et al. 2011) and commonly commercialized as “bath salts,” “research chemicals,” or “plant food” via the Internet market or smart shops (Meyers et al. 2015; Schneir et al. 2014). Among them, the methylenedioxy derivative of pyrovalerone MDPV (3,4-methylenedioxypyrovalerone) and its closely related derivative α-PVP (1-phenyl-2-(pyrrolidin-1-yl)pentan-1-one) also known as “flakka” or “gravel”) are in the top 5 among the most popular synthetic cathinones on the clandestine market (EMCDDA 2014, 2016; Glennon and Young 2016; WHO 2014, 2015).
These two synthetic cathinones potently and selectively block dopamine (DA) and norepinephrine (NE) transporters with negligible affinity for the serotonin (5-HT) transporter (Baumann et al. 2013; Kolanos et al. 2015; Meltzer et al. 2006; Rickli et al. 2015; Simmler et al. 2013). Consistent with these pharmacodynamic features, acute MDPV and α-PVP treatments produce psychomotor effects in rodents and increased motor activity as well as the appearance of stereotypes (Aarde et al. 2015; Gatch et al. 2015; Gatch et al. 2013; Glennon and Young 2016; Marusich et al. 2012). Moreover, MDPV and/or α-PVP increased heart rate, blood pressure, and differently affected thermoregulation (Glennon and Young 2016; Kiyatkin et al. 2015; Wakabayashi et al. 2015; WHO 2014, 2015). In particular, MDPV’s and α-PVP’s effects on thermoregulation are variable, i.e., they sometimes have no effect, sometimes increase, and sometimes decrease temperature. Kiyatkin et al. (2015) and Miner et al. (2017) found that MDPV had little effect on the brain or body temperature. Aarde et al. (2015) found MDPV and α-PVP decreased temperature. These effects are different from temperature profiles of other methcathinone and psychostimulants, though they share the other properties mentioned of increased heart rate and blood pressure.
Furthermore, as typically reported for psychostimulants, they promoted reinforcing and rewarding properties in rodents (Aarde et al. 2015; Bonano et al. 2014; Fantegrossi et al. 2013; Gatch et al. 2015; Glennon and Young 2016; Naylor et al. 2015).
The major aim of our study was to map the neuronal activation brought about by MDPV and α-PVP in reward-related brain regions such as frontal lobe, striatum, and hippocampus to provide novel information about brain region specificity and the timing of such activation. We focused our attention on two immediate early genes (IEGs), i.e., c-Fos and Arc/Arg3.1. c-Fos is the classic IEG, i.e., a transcription factor which indirectly alters the expression of a given target gene whereas Arc/Arg3.1 is an effector gene, which acts directly on cellular homeostasis and function. Thus, the analysis of these two IEGs could provide us with novel information about the acute profile of cathinone’s actions. To complement the molecular analyses, we performed behavioral measures incorporating a wide range of doses (0.01–20 mg/kg i.p.) of these cathinones to evaluate their behavioral effects in CD-1 male mice on spontaneous and stimulated (drag test and accelerod) motor activity.
Materials and Methods
Animals
Male ICR (CD-1®) mice, 25–30 g (ENVIGO Harlan Italy; S. Pietro al Natisone, Italy), were group-housed (8–10 mice per cage; floor area per animal was 80 cm2; minimum enclosure height was 12 cm) on a 12:12-h light-dark cycle (light period from 6:30 a.m. to 6:30 p.m.), temperature of 20–22 °C, and humidity of 45–55% and were provided ad libitum access to food (Diet 4RF25 GLP; Mucedola, Settimo Milanese, Milan, Italy) and water. Experimental protocols performed in the present study were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the European Communities Council Directive of September 2010 (2010/63/EU) and were approved by Italian Ministry of Health (license no. 335/2016-PR) and by the Ethics Committee of the University of Ferrara. Moreover, adequate measures were taken to minimize the number of animals used and their pain and discomfort.
Drug Preparation
MDPV and α-PVP were purchased from LGC Standards (LGC Standards S.r.l., Sesto San Giovanni, Milan, Italy). Drugs were initially dissolved in absolute ethanol (final concentration was 2%) and Tween 80 (2%) and brought to the final volume with saline (0.9% NaCl). The solution made of ethanol, Tween 80, and saline was also used as the vehicle. For the behavioral analysis, single intraperitoneal injections of five different doses (0.01, 0.1, 1, 10, 20 mg/kg) were tested at a volume of 4 μl/g.
Behavioral Motor Studies and Analysis of Gene Expression
Motor Activity Assessment
Alterations of motor activity induced by MDPV and α-PVP were measured using the drag, the accelerod tests, and the analysis of spontaneous locomotor activity (Canazza et al. 2016; Ossato et al. 2016; Ossato et al. 2015; Vigolo et al. 2015).
Spontaneous locomotor activity was measured by using the ANY-maze video-tracking system (Ugo Basile, application version 4.99 g beta). The mouse was placed in a square plastic cage (60 × 60 cm) located in a sound- and light-attenuated room, and its total distance traveled (m) was monitored for 240 min. Four mice were monitored at the same time in each experiment. The distance covered was analyzed every 15 min for a maximum of 240 min. To avoid mice olfactory cues, cages were carefully cleaned with ethanol solution (5%) and washed with water between animal trials. All experiments were performed between 9:00 a.m. and 1:00 p.m.
In the drag test, the mouse was lifted by the tail, leaving the front paws on the table and dragged backward at a constant speed of about 20 cm/s for a fixed distance (100 cm). The number of steps performed by each paw was recorded by two different observers. For each animal, five to seven measurements were collected. The drag test was performed at 0, 40, 60, 95, 150, 210, 270, 330 min, and 24 h post injection.
In the accelerod test, animals were placed on a rotating cylinder that automatically and constantly (0–60 rotations/min in 5 min) increased velocity. The time spent on the cylinder was measured. The accelerod test was performed at 0, 45, 70, 105, 160, 220, 280, 340 min, and 24 h post injection.
To reduce the animal’s stress induced by manipulation and to confirm the stability and reproducibility over time of the responses of our tests, animals were trained two times per week for 2 weeks before the pharmacological treatment. All experiments were performed between 8:30 a.m. and 2:00 p.m. Experiments were conducted in blind by trained observers working together in pairs (Ossato et al. 2016).
Analysis of Gene Expression
For the molecular analyses, the intermediate dose of 1 mg/kg was chosen based on the behavioral effects. At the scheduled times after the drug injection, mice were killed by cervical dislocation, their brains were quickly removed, and the brain regions of interest were immediately dissected out, frozen on dry ice, and stored at −80 °C until being processed. Dissections were performed according to the mice atlas of Paxinos and Franklin (2004); frontal lobe (approximately from Bregma +2.58 to +1.70) and striatum (approximately from Bregma +1.54 to 0.14) were dissected from 2 mm coronal section while the hippocampi were dissected according to Spijker (2011).
Total RNA was isolated by single step guanidinium isothiocyanate/phenol extraction using PureZol RNA isolation reagent (Bio-Rad Laboratories, Segrate, Milan, Italy) according to the manufacturer’s instructions and quantified by spectrophotometric analysis. Following total RNA extraction, the samples were processed for real-time reverse transcription polymerase chain reaction (real-time RT-PCR) to assess messenger RNA (mRNA) levels. Briefly, an aliquot of each sample was treated with DNase to avoid DNA contamination. RNA was analyzed by TaqMan qRT-PCR instrument (CFX384 Real-Time System, Bio-Rad Laboratories) using the iScript™ one-step RT-PCR kit for probes (Bio-Rad Laboratories). Samples were run in 384 wells formats in triplicate as multiplexed reactions. Data were analyzed with the comparative threshold cycle (∆∆Ct) method using 36B4 as reference gene (Giannotti et al. 2014). The primer efficiencies were experimentally set up for each couple of primers. Thermal cycling was initiated with an incubation at 50 °C for 10 min (RNA retrotranscription) and then at 95 °C for 5 min (retrotranscriptase inactivation). After this initial step, 39 cycles of PCR were performed. Each PCR cycle consisted of heating the samples at 95 °C for 10 s to enable the melting process and then for 30 s at 60 °C for the annealing and extension reaction.
Primers and probe for Arc/Arg3.1 were purchased from Life Technologies (Milan, Italy) (accession 240 number: EF125675, ASSAY ID: Rn02531967_s1). Primers and probes and for c-Fos and 36B4 were purchased from Eurofins MWG-Operon (Ebersberg, Germany), and their sequences are shown below:
-
c-Fos: forward primer 5′-TCCTTACGGACTCCCCAC-3′, reverse primer 5′-CTCCGTTTCTCTTCCTCTTCAG-3′, probe 5′-TGCTCTACTTTGCCCCTTCTGCC-3′.
-
36B4: forward primer 5′-AGATGCAGCAGATCCGCAT-3′, reverse primer 5′-GTTCTTGCCCATCAGCACC-3′, probe 5′-CGCTCCGAGGGAAGGCCG-3′.
Data and Statistical Analysis
Data are expressed in absolute values (m, for distance traveled) in the analysis of spontaneous locomotion and as percentage of basal in drag and accelerod tests. Data represent the mean ± SEM of eight to ten animals for each treatment. All the numerical data are given as mean ± SEM of four independent experimental replications. Data were analyzed by utilizing repeated measures ANOVA. Results from treatments showing significant overall changes were subjected to post hoc Tukey’s tests with significance for p < 0.05. The statistical analysis of the effects of the individual substances at different doses over time was performed by two-way ANOVA followed by Bonferroni’s test for multiple comparisons. The analysis of the overall effect induced by treatments (expressed in histograms) was performed with one-way ANOVA followed by Tukey’s test for multiple comparisons. Molecular data were collected in individual animals (independent determinations) and are presented as means ± standard errors. Changes produced by treatment and time after the injection alone and by their combination were analyzed using a two-way analysis of variance (ANOVA), with treatment and time after injection as independent variables. When appropriate, further differences between groups were analyzed by Fisher’s PLSD test. Statistical significance was assumed at p < 0.05. The statistical analysis was performed with the program Prism software (GraphPad Prism, USA).
Results
Spontaneous Locomotor Activity in Mice
Firstly, we investigated the effect of MDPV and α-PVP (0.01–20 mg/kg i.p.) on spontaneous locomotor activity in mice. Both psychostimulants at high doses (1–20 mg/kg) facilitate the spontaneous locomotor activity in mice within 2 h after treatment in a dose-dependent manner (MDPV, Fig. 1a: significant effect of treatment (F 5,672 = 151.2, p < 0.0001), time (F 15,672 = 68.51, p < 0.0001), and time × treatment interaction (F 75,672 = 4.066, p < 0.0001); α-PVP, Fig. 1b: significant effect of treatment (F 5,672 = 78.93, p < 0.0001), time (F 15,672 = 44.70, p < 0.0001) and time × treatment interaction (F 75,672 = 1.491, p = 0.0064)). Moreover, MDPV was significantly greater than α-PVP at all three doses: 1, 10, and 20 mg/kg (Fig. 1c: significant effect of agonists (F 10,87 = 2316, p < 0.0001)). From Fig. 1a, b, it appears that locomotor activity of mice treated with MDPV and α-PVP was not significantly different from vehicle after 135 and 185 min, respectively.
Effect of the systemic administration of MDPV (0.01–20 mg/kg i.p.) and α-PVP (0.01–20 mg/kg i.p.) on the distance traveled (a, b), the drag test (d, e), and accelerod test (g, h) in mice. The comparison of the total average effect observed was also reported (c, f, i). Data are expressed in absolute values (m, for distance traveled) in the analysis of spontaneous locomotion (a, b) and as percentage of basal in drag (d, e) and accelerod tests (g, h) (see the “Material and Methods” section) and represent the mean ± SEM of −10 determinations for each treatment. Statistical analysis was performed by two-way ANOVA followed by the Bonferroni’s test for multiple comparisons for the dose response curve of each compounds at different times (a, b, d, e, g, h), while the statistical analysis of the comparison of the total average effect of the compounds (panel c, f, i) was performed with one-way ANOVA followed by Tukey’s test for multiple comparisons *p < 0.05, **p < 0.01, ***p < 0.001 versus saline-treated mice and °°°p < 0.001 versus α-PVP at same dosage
Stimulated Locomotor Activity in Mice
Drag Test
Differences were observed between MDPV and α-PVP in the number of steps performed by mice with the front legs: MDPV increased it only at 1 and 10 mg/kg (Fig. 1d: significant effect of treatment (F 5,336 = 8.189, p < 0.0001), time (F 7,336 = 1.062, p = 0.3879), and time × treatment interaction (F 35,336 = 0.6315, p = 0.9504)) while α-PVP only at the dose of 20 mg/kg (Fig. 1e: significant effect of treatment (F 5,336 = 2.786, p = 0.0176), time (F 7,336 = 0.7956, p = 0.5914), and time × treatment interaction (F 35,336 = 0.3993, p = 0.9992)). Effects of both compounds vanished within 24 h (data not shown).
Ultimately, the overall effect observed in 5 h demonstrated that MDPV was more potent and effective than α-PVP in the range doses of 1–10 mg/kg (Fig. 1f: significant effect of MDPV and α-PVP (F 10,87 = 9.208, p < 0.0001)).
Accelerod Test
In the accelerod test, at the lowest doses (0.01 and 0.1 mg/kg i.p.), MDPV induced a prolonged increase in locomotion (Fig. 1g: significant effect of treatment (F 5,336 = 9.038, p < 0.0001), time (F 7,336 = 1.755, p = 0.0956), and time × treatment interaction (F 35,336 = 0.3979, p = 0.9992)). A different situation was observed at the higher doses tested; in fact, MDPV at 1 and 20 mg/kg increased and reduced, respectively, locomotion, with non-significant effects. Conversely, injection of α-PVP evoked a prolonged increase of locomotion only at the highest dose tested (10 and 20 mg/kg) (Fig. 1h: significant effect of treatment (F 5,336 = 8.938, p < 0.0001), time (F 7,336 = 0.7528, p = 0.6274), and time × treatment interaction (F 35,336 = 0.3435, p = 0.9999)). The overall 5 h effect demonstrated that MDPV was more potent than α-PVP in increasing stimulated locomotor activity (Fig. 1i: significant effect of MDPV and α-PVP (F 10,87 = 36.18, p < 0.0001)). Effects of both compounds vanished within 24 h (data not shown).
Molecular Analyses of the Effects of MDPV and α-PVP on Arc/Arg3.1 and c-Fos mRNA Levels
Frontal Lobe
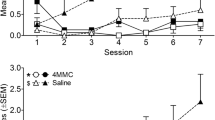
Figure 2a, b shows the effects of a single injection of MDPV and α-PVP (1 mg/kg) on Arc/Arg3.1 and c-Fos mRNA levels in the frontal lobe of mice at three different time points, i.e., 30 min, 2 h, and 24 h after the drug exposure.
Time-dependent changes in Arc/Arg3.1 and c-Fos mRNA levels in the frontal lobe (a, b), striatum (c, d), and hippocampus (e, f) of mice following a single IP injection of MDPV (1 mg/kg) or α-PVP (1 mg/kg). Mice were sacrificed at three different time points, i.e., 30 min, 2 h, and 24 h after the drug administration. Results are expressed as percentages relative to saline-treated group and presented as mean ± standard error of the mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.001 versus saline-treated mice; ## p < 0.01, ### p < 0.001 versus α-PVP-treated mice (two-way ANOVA followed by Fisher’s LSD test)
Arc/Arg3.1 mRNA Levels
Two-way ANOVA showed a main effect of treatment (F 2,48 = 6.2985, p = 0.003724), time after injection (F 2,48 = 4.5014, p = 0.016153), and a significant time × treatment interaction (F 4,48 = 7.0529, p = 0.000131) on Arc/Arg3.1 mRNA levels (Fig. 2a). In detail, post hoc analysis revealed a temporal induction of the IEG, which depends on the time of sacrifice after injection. MDPV upregulates Arc/Arg3.1 mRNA levels 30 min (+47%, p = 0.006321 versus saline-treated mice), 2 h (+53%, p = 0.000479 versus saline-treated mice; +80%, p < 0.000001 versus α-PVP treated mice), but not 24 h after the injection.
A single injection of α-PVP, instead, upregulates Arc/Arg3.1 mRNA levels 30 min after drug exposure (+34%, p = 0.045181 versus saline-treated mice), an effect that disappears within 2 h (−26%, p = 0.068828 versus saline-treated) and is still at control level 24 h later (+9%, p = 0.573214 versus saline-treated mice).
c-Fos mRNA Levels
Two-way ANOVA revealed a main effect of treatment (F 2,49 = 4.9566; p = 0.0110), time after injection (F 2,49 = 11.035; p = 0.0001), and a significant time × treatment interaction (F 4,49 = 4.8629; p = 0.0022) (Fig. 2b). Similarly, mice receiving a single injection of MDPV showed increased c-Fos mRNA levels 30 min later (+79%, p = 0.0002 versus saline-treated mice), an effect that persisted up to 2 h (+33%, p = 0,0432 versus saline-treated mice; +54%, p = 0.0013 versus α-PVP treated mice) but waned after 24 h (−13%, p = 0.4990 versus saline-treated mice).
Similarly to Arc/Arg3.1, a single injection of α-PVP upregulates c-Fos mRNA levels 30 min later (+55%, p = 0.0066 versus saline-treated mice), an effect that disappears within 2 h (−21%, p = 0.1906 versus saline-treated mice) and remains unaltered 24 h later (+5%, p = 0.7847 versus saline-treated mice).
Striatum
Figure 2c, d shows the effects of a single injection of MDPV and α-PVP (1 mg/kg) on Arc/Arg3.1 and c-Fos mRNA levels in the striatum of mice at three different time points, i.e., 30 min, 2 h, and 24 h after the drug exposure.
Arc/Arg3.1 mRNA Levels
Two-way ANOVA revealed a main effect of treatment (F 2,48 = 9.687; p = 0.0003), time after injection (F 2,48 = 4.0982; p = 0.0227), and a significant time × treatment interaction (F 4,48 = 2.9451; p = 0.0295) on Arc/Arg3.1 mRNA levels (Fig. 2c). In detail, multiple comparisons revealed that a single injection of MDPV increases Arc/Arg3.1 mRNA levels after 30 min (+81%, p = 0.0036 versus saline-treated mice), an effect that persists 2 h later (+99%, p < 0.0001 versus saline-treated mice; +96%, p < 0.0001 versus α-PVP treated mice) but subsides after 24 h (+0%, p = 0.9850 versus saline-treated mice). Conversely, Arc/Arg3.1 mRNA levels were not altered by α-PVP injection at the three time points considered.
c-Fos mRNA Levels
Two-way ANOVA revealed a main effect of the treatment (F 2,47 = 7.4041; p = 0.0016), time after injection (F 2,47 = 10.713; p = 0.0001), and a significant time × treatment interaction (F 4,47 = 3.6034; p = 0.0121) for c-Fos mRNA levels (Fig. 2d). Multiple comparisons revealed a marked increase of c-Fos mRNA levels 30 min after a single injection of MDPV (+179%, p = 0.0002 versus saline-treated mice), which persists up to 2 h after the injection (+87%, p = 0.0172 versus saline-treated mice; +91%, p = 0.0120 versus α-PVP treated mice) but disappears 24 h later (+3%, p = 0.9353 versus saline-treated mice). Conversely, a single injection of α-PVP upregulates c-Fos mRNA levels 30 min later (+161%, p = 0.0007 versus saline-treated mice), an effect that fades away within 2 h (−5%, p = 0.8876 versus saline-treated mice) and remains unchanged 24 h later (+14%, p = 0.7404 versus saline-treated mice).
Hippocampus
Figure 2e, f shows the effects of a single injection of MDPV and α-PVP (1 mg/kg) on Arc/Arg3.1 and c-Fos mRNA levels in the hippocampus of mice at three different time points, i.e., 30 min, 2 h, and 24 h after the drug exposure.
Arc/Arg3.1 mRNA Levels
Two-way ANOVA revealed a main effect of treatment (F 2,49 = 6.4063; p = 0.0034) and a significant time × treatment interaction (F 4,49 = 4.8483; p = 0.0023) for Arc/Arg3.1 mRNA levels (Fig. 2e). Interestingly, at variance from the MDPV-induced expression profile observed in the frontal lobe and striatum, in the hippocampus, the effect of the single injection of MDPV is short lasting since it markedly increases Arc/Arg3.1 mRNA levels 2 h later (+111%, p < 0.0001 versus saline-treated mice; +118%, p < 0.0001 versus α-PVP treated mice) but not 30 min after the drug exposure (+32%, p = 0.2451 versus saline-treated mice) and 24 h later (+2%, p = 0.9449 versus saline-treated mice). Of note, no significant changes were observed for α-PVP at the three time points analyzed.
c-Fos mRNA Levels
Two-way ANOVA revealed a main effect of treatment (F 2,49 = 4.5432; p = 0.0155) and a significant time × treatment interaction (F 4,49 = 2.7941; p = 0.0362) for c-Fos mRNA levels (Fig. 2f). Multiple comparisons revealed a selective increase of c-Fos mRNA levels 30 min after MDPV injection (+72%, p = 0.0490 versus saline-treated mice) that further increases after 2 h (+106%, p = 0.0007 versus saline-treated mice; +107%, p = 0.0006 versus α-PVP treated mice) while disappearing 24 h later (−14%, p = 0.7010 versus saline-treated mice). No significant changes were observed for α-PVP at the three time points analyzed.
Discussion
To the best of our knowledge, this is the first study comparing the effects of two cathinones, i.e., MDPV and α-PVP, in CD-1 male mice following a single exposure. Our data show that these cathinones rapidly change the expression of IEGs, with a specific temporal and regional profile, and alter the locomotor response as well differently.
We here demonstrate that MDPV not only was found to be more effective than α-PVP in facilitating both spontaneous and stimulated locomotion (primarily drag and accelerod tests), but also it displayed a biphasic effect as opposed to the monophasically stimulated locomotion induced by α-PVP in both tests. Such difference might depend, at least in part, on the fact that MDPV is more potent than α-PVP in blocking the DAT (Marusich et al. 2014). Accordingly, it could be assumed that the inhibition of motor activity, caused by high doses of the cathinone, may rely on the synaptic overflow of DA in the dorsal striatum that causes the appearance of stereotypies and bizarre behaviors (Aliane et al. 2009). In fact, both cathinones, at the highest dose, induced stereotypies and bizarre behaviors, with MDPV being more potent than α-PVP (data not shown).
MDPV showed also a greater psychomotor effect than α-PVP, in line with its higher potency in blocking the dopamine transporter (DAT): a molecular correlate of such difference might be represented by the more persisting increase of Arc/Arg3.1 and c-Fos elicited by MDPV in striatum, i.e., the brain region with the highest density of DAT (Gainetdinov et al. 1998), while the upregulation promoted by α-PVP’s wanes within 2 h. The psychomotor effects are consistent with the mechanism of action of cathinones that, as other psychostimulants, primarily inhibit monoaminergic transporters thereby increasing extracellular levels of monoamines (Baumann et al. 2013; Glennon and Young 2016). In particular, the increased synaptic levels of dopamine could activate D1 dopamine receptors, leading to increased Arc/Arg3.1 and c-Fos mRNA levels, as previously demonstrated (Fumagalli et al. 2006; Yoshida et al. 1995). However, we cannot rule out the possibility that increased levels of other neurotransmitters, such as glutamate and serotonin, may come into play and drive IEG expression. It can be argued that the increased expression of IEGs may sustain, at least partially, the observed motor activity, given that motor activity over time reflects the time-dependent expression profile of the IEGs analyzed. However, we cannot rule out the possibility that such a difference may reflect the fact that α-PVP is more rapidly degraded to inactive metabolites compared to MDPV.
The picture in the frontal lobe is similar to that observed in the striatum, suggesting that these cathinones modulate dopamine levels also in cortical dopaminergic terminals. Since dopamine-induced activation of cortical regions drive and sustain nervousness, irritability, aggressiveness, and paranoia that approximate schizophrenia (Morton 1999; Seo et al. 2008), it is reasonable to speculate that the increased neuronal activity in the frontal lobe may sustain some of the aggressive and bizarre behaviors herein observed. Of note, MDPV has been recently shown to alter functional connectivity between cortical and subcortical regions (Colon-Perez et al. 2016), further corroborating a drug-induced loss of control over the “top-down” neuronal connection, which is essential for the control of impulsive behaviors (Dalley et al. 2011) and may contribute to explain the high addictive properties of these drugs. Although the different pharmacokinetic profiles do not result in different reinforcing properties of the drugs (Aarde et al. 2013; Watterson and Olive 2014), we cannot rule out the possibility that the more persistent activation of neuronal activity promoted by MDPV may initiate and sustain neuroadaptive changes caused by repeated exposure to the drug: this possibility will be investigated in the future by employing chronic treatment regimens with these two cathinones.
Cathinone-induced profile of IEGs expression is quite different in hippocampus. In fact, in this brain region, MDPV significantly upregulates Arc/Arg3.1 and c-Fos mRNA levels, whereas α-PVP is ineffective. Moreover, it is important to note that c-Fos is immediately activated while Arc/Arg3.1 induction is delayed. This difference, which reflects the different nature of these IEGs, may rely on the fact that this brain region is not the primary target region of MDPV, but instead, it may be involved in secondary drug-related neuroadaptive mechanisms, perhaps involving the glutamatergic neurotransmission, taking place over time. Moreover, since exposure to MDPV induces neuronal hippocampal loss (Adam et al. 2014), the possibility exists that a single dose of MDPV, but not α-PVP, is sufficient to drive a transient increase in neuronal activity as an attempt of the system to counterbalance the initial stage of cytotoxicity reported for these drugs (Wojcieszak et al. 2016).
Taken together, these data indicate that a single exposure to MDPV or α-PVP promotes changes in baseline neuronal activity, an effect that could be used as an early molecular signature of altered brain homeostasis following psychostimulant administration: this may also allow to discriminate between cathinones with different chemical structures and different pharmacodynamic properties.
In conclusion, we provide clear-cut evidence showing that the cathinones MDPV and α-PVP induce changes in locomotor and neuronal activities following a precise temporal and regional profile. Such rapid molecular effects may set the stage for the behavioral response observed and, perhaps, may account for their toxicity, at least partially. Although our analysis is limited to the acute effects of these cathinones, our data show that these drugs can modulate brain activity and functioning. Further studies employing chronic treatment regimens will be performed to determine their long-term effects and the effect of abstinence.
Abbreviations
- α-PVP:
-
1-Phenyl-2-(pyrrolidin-1-yl)pentan-1-one
- MDPV:
-
3,4-Methylenedioxypyrovalerone
- DA:
-
Dopamine
- DAT:
-
Dopamine transporter
- IEGs:
-
Immediately early genes
- NE:
-
Norepinephrine
- 5-HT:
-
Serotonin
References
Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140. doi:10.1016/j.neuropharm.2013.04.003
Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA (2015) In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology 232:3045–3055. doi:10.1007/s00213-015-3944-8
Adam A, Gerecsei LI, Lepesi N, Csillag A (2014) Apoptotic effects of the ‘designer drug’ methylenedioxypyrovalerone (MDPV) on the neonatal mouse brain. Neurotoxicology 44:231–236. doi:10.1016/j.neuro.2014.07.004
Aliane V, Perez S, Nieoullon A, Deniau JM, Kemel ML (2009) Cocaine-induced stereotypy is linked to an imbalance between the medial prefrontal and sensorimotor circuits of the basal ganglia. Eur J Neurosci 30:1269–1279. doi:10.1111/j.1460-9568.2009.06907.x
Baumann MH et al (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. doi:10.1038/npp.2012.204
Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS (2014) Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology 231:199–207. doi:10.1007/s00213-013-3223-5
Canazza I et al (2016) Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology 233:3685–3709. doi:10.1007/s00213-016-4402-y
Colon-Perez LM et al (2016) The psychoactive designer drug and bath salt constituent MDPV causes widespread disruption of brain functional connectivity. Neuropsychopharmacology 41:2352–2365. doi:10.1038/npp.2016.40
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694. doi:10.1016/j.neuron.2011.01.020
EMCDDA (2014) European Monitoring Centre for Drugs and Drug Addiction. European drug report 2014: trends and developments. Publications Office of the European Union, Luxembourg
EMCDDA (2016) European Monitoring Centre for Drugs and Drug Addiction. European drug report 2016: trends and developments. Publications Office of the European Union, Luxembourg
Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC (2013) In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38:563–573. doi:10.1038/npp.2012.233
Fumagalli F, Bedogni F, Frasca A, Di Pasquale L, Racagni G, Riva MA (2006) Corticostriatal up-regulation of activity-regulated cytoskeletal-associated protein expression after repeated exposure to cocaine. Mol Pharmacol 70:1726–1734. doi:10.1124/mol.106.026302
Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG (1998) Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev 26:148–153
Gatch MB, Taylor CM, Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. doi:10.1097/FBP.0b013e328364166d
Gatch MB, Dolan SB, Forster MJ (2015) Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J Pharmacol Exp Ther 354:103–110. doi:10.1124/jpet.115.223586
German CL, Fleckenstein AE, Hanson GR (2014) Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 97:2–8. doi:10.1016/j.lfs.2013.07.023
Giannotti G, Caffino L, Calabrese F, Racagni G, Riva MA, Fumagalli F (2014) Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int J Neuropsychopharmacol 17:625–634. doi:10.1017/S1461145713001454
Glennon RA, Young R (2016) Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and alpha-pyrrolidinovalerophenone (alpha-PVP). Brain Res Bull 126:111–126. doi:10.1016/j.brainresbull.2016.04.011
Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y (2015) Effects of social interaction and warm ambient temperature on brain hyperthermia induced by the designer drugs methylone and MDPV. Neuropsychopharmacology 40:436–445. doi:10.1038/npp.2014.191
Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS, Glennon RA (2015) Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem Neurosci 6:771–777. doi:10.1021/acschemneuro.5b00006
Marusich JA, Grant KR, Blough BE, Wiley JL (2012) Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33:1305–1313. doi:10.1016/j.neuro.2012.08.003
Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87:206–213. doi:10.1016/j.neuropharm.2014.02.016
Meltzer PC, Butler D, Deschamps JR, Madras BK (2006) 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem 49:1420–1432. doi:10.1021/jm050797a
Meyers K, Kaynak O, Bresani E, Curtis B, McNamara A, Brownfield K, Kirby KC (2015) The availability and depiction of synthetic cathinones (bath salts) on the internet: do online suppliers employ features to maximize purchases? Int J Drug Policy 26:670–674. doi:10.1016/j.drugpo.2015.01.012
Miner NB, O'Callaghan JP, Phillips TJ, Janowsky A (2017) The combined effects of 3,4-methylenedioxymethamphetamine (MDMA) and selected substituted methcathinones on measures of neurotoxicity. Neurotoxicol Teratol 61:74–81. doi:10.1016/j.ntt.2017.02.003
Morton WA (1999) Cocaine and psychiatric symptoms prim care companion. J Clin Psychiatry 1:109–113
Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL (2015) Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend 149:280–284. doi:10.1016/j.drugalcdep.2015.02.002
Ossato A, Vigolo A, Trapella C, Seri C, Rimondo C, Serpelloni G, Marti M (2015) JWH-018 impairs sensorimotor functions in mice. Neuroscience 300:174–188. doi:10.1016/j.neuroscience.2015.05.021
Ossato A et al (2016) Effect of JWH-250, JWH-073 and their interaction on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 67:31–50. doi:10.1016/j.pnpbp.2016.01.007
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates, Compact 2nd edn. Elsevier Academic Press, Amsterdam
Rickli A, Hoener MC, Liechti ME (2015) Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol 25:365–376. doi:10.1016/j.euroneuro.2014.12.012
Schneir A et al (2014) Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin Toxicol (Phila) 52:651–658. doi:10.3109/15563650.2014.933231
Seo D, Patrick CJ, Kennealy PJ (2008) Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav 13:383–395. doi:10.1016/j.avb.2008.06.003
Simmler LD et al (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. doi:10.1111/j.1476-5381.2012.02145.x
Spijker S (2011) Dissection of rodent brain regions. In: Li KW (ed) Neuroproteomics. Humana Press, Totowa, pp 13–26. doi:10.1007/978-1-61779-111-6_2
Spiller HA, Ryan ML, Weston RG, Jansen J (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49:499–505. doi:10.3109/15563650.2011.590812
Vigolo A et al (2015) Novel halogenated derivates of JWH-018: behavioral and binding studies in mice. Neuropharmacology 95:68–82. doi:10.1016/j.neuropharm.2015.02.008
Wakabayashi KT, Ren SE, Kiyatkin EA (2015) Methylenedioxypyrovalerone (MDPV) mimics cocaine in its physiological and behavioral effects but induces distinct changes in NAc glucose. Front Neurosci 9:324. doi:10.3389/fnins.2015.00324
Watterson LR, Olive MF (2014) Synthetic cathinones and their rewarding and reinforcing effects in rodents. Adv Neurosci (Hindawi) 2014:209875. doi:10.1155/2014/209875
WHO (2014) World Health Organization. 3,4‐Methylenedioxypyrovalerone (MDPV) critical review report agenda item 4.13 expert committee on drug dependence thirty‐sixth meeting geneva. http://www.who.int/medicines/areas/quality_safety/4_13_Review.pdf
WHO (2015) World Health Organization. 1-Phenyl-2-(pyrrolidin-1-yl)pentan-1-one (α-PVP) critical review report agenda item 5.3 expert committee on drug dependence thirty-seventh meeting geneva. http://www.who.int/medicines/access/controlled-substances/5.3_Alpha-PVP_CRev.pdf
Wojcieszak J, Andrzejczak D, Woldan-Tambor A, Zawilska JB (2016) Cytotoxic activity of pyrovalerone derivatives, an emerging group of psychostimulant designer cathinones. Neurotox Res 30:239–250. doi:10.1007/s12640-016-9640-6
Yoshida H, Ohno M, Watanabe S (1995) Roles of dopamine D1 receptors in striatal fos protein induction associated with methamphetamine behavioral sensitization in rats. Brain Res Bull 38:393–397
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experimental protocols performed in the present study were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the European Communities Council Directive of September 2010 (2010/63/EU) and were approved by Italian Ministry of Health (license no. 335/2016-PR) and by the Ethics Committee of the University of Ferrara.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in the studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Funding
This research has been funded by the Drug Policies Department; Presidency of the Council of Ministers, Italy (project NS-Drugs to M. Marti) by local funds from the University of Ferrara (FAR 2016 to M. Marti); by FIRB 2012 from the Italian Ministry of the University (Grant no. RBFR12LDOW to F. De Giorgio; Institute of Public Health, Section of Legal Medicine, Catholic University of Rome, Italy); and the Zardi Gori Foundation (Zardi Gori Grant to F. Fumagalli).
Rights and permissions
About this article
Cite this article
Giannotti, G., Canazza, I., Caffino, L. et al. The Cathinones MDPV and α-PVP Elicit Different Behavioral and Molecular Effects Following Acute Exposure. Neurotox Res 32, 594–602 (2017). https://doi.org/10.1007/s12640-017-9769-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9769-y