Abstract
Novel psychoactive substances (NPSs) are new psychotropic drugs designed to evade substance regulatory policies. 25E-NBOMe (2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine) has recently been identified as an NPS, and its recreational misuse has been reported to be rapidly increasing. However, the psychopharmacological effects and mechanisms of 25E-NBOMe have not been studied. We examined the abuse potential of 25E-NBOMe using the conditioned place preference in male mice and self-administration paradigms in male rats. Additionally, immunoblot assay, enzyme-linked immunosorbent assay, and microdialysis were used to determine the molecular effects of 25E-NBOMe in the nucleus accumbens (NAc). Our data demonstrated that 25E-NBOMe induces conditioned place preference, and the dopaminergic signaling in the NAc mediates these. Following 25E-NBOMe administration, expression of dopamine transporter and dopamine D1 receptor (D1DR) were enhanced in the NAc of male mice, and NAc dopamine levels were reduced in both male mice and rats. Induction of intracellular dopaminergic pathways, DARPP32, and phosphorylation of CREB in the NAc of male mice was also observed. Significantly, pharmacological blockade of D1DR or chemogenetic inhibition of D1DR-expressing medium spiny neurons in the NAc attenuated 25E-NBOMe-induced conditioned place preference in male mice. We also examined the hallucinogenic properties of 25E-NBOMe using the head twitch response test in male mice and found that this behavior was mediated by serotonin 2A receptor activity. Our findings demonstrate that D1DR signaling may govern the addictive potential of 25E-NBOMe. Moreover, our study provides new insights into the potential mechanisms of substance use disorder and the improvement of controlled substance management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Novel psychoactive substances (NPSs) are chemical derivatives of established drugs and can induce psychological effects, such as relaxation, euphoria, and hallucination. NPS can be flexibly synthesized to evade substance regulation. This flexibility has resulted in the continual emergence of these substances and a rapid increase in NPS abuse worldwide (Chatwin 2017; UNODC 2022). The accelerating speed of NPS emergence is causing a severe problem in society, such as adverse health effects, morbidity, or mortality, and identifying the psychoactive and pharmacological properties of NPSs to respond is challenging (Corkery et al. 2017). NPSs can be divided into several classes based on their pharmacological properties, including phenethylamines, piperazines, tryptamines, synthetic cathinones, synthetic cannabinoids, and arylcyclohexylamines. In particular, phenethylamines activate serotonin (5-HT) receptors or inhibit monoamine reuptake, producing hallucinogenic and psychostimulant effects (Miliano et al. 2016). N-(2-methoxybenzyl)phenethylamine (NBOMe) drugs are phenethylamine derivatives used recreationally to induce psychedelic experiences (Halberstadt 2017; Zawilska et al. 2020). The basic structure of NBOMe drugs, 2,5-dimethoxyphenethylamine, has a high structural similarity with ecstasy and mescaline, which produce psychological effects through their action on the serotonergic system (Passie and Benzenhöfer 2018). The structure-activity relationship analysis revealed that the N-methoxybenzyl group in NBOMe drugs remarkably increases their binding affinities to the serotonin 2A receptor (5-HT2AR), enhancing hallucinogenic effects (Hansen et al. 2014; Halberstadt 2017; Eshleman et al. 2018). In particular, three NBOMe drugs commonly featured in case reports, 25B-, 25C-, and 25I-NBOMe, exert potent sub-nM binding affinities for the 5-HT2AR, with Ki = 0.5, 0.7, and 0.6 nM respectively (Rickli et al. 2015).
25E-NBOMe (2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine) is an NBOMe drug with an ethyl substitution at the para- position of the benzene ring (Fig. 1A). In the United Kingdom, Sweden, and South Korea, 25E-NBOMe is a controlled substance under their respective narcotic laws (UK Statutory Instruments 2014; Swedish Code of Statutes 2018; Korean Law Information Center 2019). In the United States, NBOMe drugs, 25B-, 25C-, and 25I-NBOMe, are listed on Schedule I (Drug Enforcement Administration 2016). In addition, 25E-NBOMe is considered a controlled substance analogue of NBOMe drugs and is treated under Federal law in Schedule I (United States Code 2022). In case studies, NBOMe drug users were reported to suffer mild psychological side effects, such as confusion and agitation, to severe adverse effects, including renal failure, rhabdomyolysis, seizure, and death (Suzuki et al. 2015; Halberstadt 2017). A growing body of evidence supports the psychotropic impacts of NBOMe drugs. It has been reported that 25I-NBOMe exerts distinct serotonergic psychedelic behavior, such as head twitch responses (Halberstadt and Geyer 2014; Elmore et al. 2018), and induces sensorimotor impairments and decreased acoustic responses mediated by the 5-HT pathway (Miliano et al. 2019). Moreover, NBOMe drugs, such as 25I-, 25N-, 25B-, 25H-, and 25D-NBOMe, have been identified to exhibit abuse potential in rodents (Jeon et al. 2019; Seo et al. 2019; Custodio et al. 2020; Jo et al. 2022; Lee et al. 2023). Although 25E-NBOMe has great potential for abuse, its psychopharmacological effects and molecular mechanisms have not yet been elucidated.
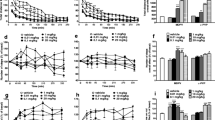
The effect of 25E-NBOMe on CPP, self-administration, and locomotor activity. A Chemical structure of 25E-NBOMe (2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine). B The binding affinity of ketanserin and 25E-NBOMe at 5-HT2A receptor (n = 3). C Experimental scheme for the 25E-NBOMe CPP test and NAc dissection. CPP score of each male mouse group (saline, 25E-NBOMe 0.03, 0.1, 0.3, 1, 3 mg/kg, and methamphetamine 1 mg/kg) in the 25E-NBOMe CPP test (n = 10). D Experimental scheme for the 25E-NBOMe self-administration test. E Number of infusions, F active lever presses, and G inactive lever presses of each male rat group (saline, 25E-NBOMe 0.01, 0.03, and 0.1 mg/kg/infusion) from 7 days of 25E-NBOMe self-administration test (n = 5). H 30 min of total locomotor activity of each male mouse group (saline, 25E-NBOMe 0.1, 0.3, 1, and 3 mg/kg) following acute 25E-NBOMe administration (n = 6–7). Data are presented as means ± standard error of the mean. ∗P < 0.05 and ∗∗P < 0.01 versus control. Sal saline, METH methamphetamine, NAc nucleus accumbens, inf infusion
Substance use disorder is a debilitating brain disorder characterized by an overwhelming compulsion to use drugs and loss of control over drug intake (Koob and Volkow 2010). The dopamine system is highly associated with substance use disorder. Exposure to addictive drugs reorganizes the neurocircuitry involving the dopamine system, resulting in long-lasting adaptations that lead to substance use disorder (Volkow et al. 2002, 2017). The nucleus accumbens (NAc) in the ventral part of the mesolimbic region is a crucial structure in reward development (Di Chiara et al. 2004). Dopamine from dopaminergic neurons in the midbrain binds to dopamine receptors in the NAc to transmit reward signals. To date, various NPSs such as ecstasy, MDPV (synthetic cathinone), and JWH-018 (synthetic cannabinoid) have been identified to affect dopaminergic signaling in the NAc, suggesting a correlation with promoting abuse potential (Miliano et al. 2016). Moreover, previous studies reported that 25I- and 25B-NBOMe induce dopamine contents in the NAc (Miliano et al. 2019; Custodio et al. 2020; Herian et al. 2021; Wojtas et al. 2023). Dopamine receptors are G protein-coupled membrane receptors and are divided into two types, the dopamine D1 receptors (D1DR) and the dopamine D2 receptors (D2DR), based on their pharmacological properties. D1DR activates Gsα proteins to stimulate cyclic adenosine monophosphate (cAMP) production, while D2DR activates Giα proteins to inhibit cAMP-dependent signaling (Beaulieu and Gainetdinov 2011). Both D1DR and D2DR activation are associated with the establishment of reward-related behaviors in drug use (Self 2010).
Despite these insights, the mechanisms by which 25E-NBOMe promotes substance use disorder and its action on the dopamine system remain largely unknown. In this study, we first confirmed whether 25E-NBOMe exerts binding affinity to 5-HT2AR using an in vitro binding assay and examined the abuse potential of 25E-NBOMe using the conditioned place preference (CPP) in male mice and self-administration paradigms in male rats to evaluate the rewarding and reinforcing properties of the drug, respectively. We then investigated the influence of 25E-NBOMe on dopaminergic signaling in the NAc of both male mice and rats. We applied pharmacological approaches and designer receptors exclusively activated by designer drugs (DREADD)-based chemogenetic tools in male mice to determine the neuronal mechanisms underlying the acquisition of 25E-NBOMe reward. Finally, we examined the hallucinogenic effect of 25E-NBOMe by measuring head twitch responses in male mice and determining its association with serotonergic activity.
Materials and methods
Animals
Experimental animals were selected based on previous studies with consideration of the sensitivity and advantage of each experiment model (Ellenbroek and Youn 2016; Homberg et al. 2017). Male C57BL/6J mice were purchased from Dae Han Biolink Co., Ltd. (Eumseong, Korea) and were used in the CPP test, locomotor test, immunoblot analysis, enzyme-linked immunosorbent assay (ELISA), and head twitch response test. Male Sprague Dawley rats were purchased from Orient Bio Co., Ltd. (Seongnam, Korea) and were used in self-administration and microdialysis tests. Male D1R-Cre bacterial artificial chromosome (BAC) transgenic mice (B6.FVB(Cg)-Tg(Drd1-cre) EY217Gsat/Mmucd) were obtained from the Mutant Mouse Resource and Research Center (MMRRC) and were used to investigate the cell type-specific effects on reward-related behaviors. Only male animals were used in experiments for the facilitation of data interpretation. All experiments were performed in 8- to 10-week-old mice or rats. Ten mice were housed per cage (26 × 42 × 18 cm), and the rats were housed individually. Both mice and rats were acclimatized in the laboratory animal facility at a temperature of 24 ± 1 °C and 55 ± 5% humidity under a 12/12 h light/dark cycle. Animals were allowed access to food and water ad libitum, except during the food training period for rats. All animal care procedures and experimental protocols carried out in this study were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Sungkyunkwan University (SKKUIACUC2019-01-26-2, SKKUIACUC2019-12-08-1, and SKKUIACUC2022-01-01-1).
Drugs
25E-NBOMe hydrochloride (97.57% HPLC purity) was synthesized and provided by Professor Yong-Sup Lee at the Laboratory of Medicinal Chemistry, College of Pharmacy of Kyung Hee University (Seoul, Korea). Methamphetamine hydrochloride, SCH23390 hydrochloride (D1DR antagonist), and raclopride tartrate (D2DR antagonist) were purchased from Sigma Aldrich (St. Louis, MO, USA). CNO (Clozapine N-oxide, DREADD ligand) and ketanserin tartrate (5-HT2AR antagonist) were purchased from Tocris Bioscience (Bristol, UK). All drugs used in vivo studies were dissolved in saline (0.9% NaCl) or vehicle (5% DMSO, 5% Tween 80, 90% saline).
5-HT2AR binding assay
5-HT2AR binding assay was performed in HEK-293 cells, which stably express 5-HT2AR. Human embryonic kidney-293 (HEK-293) cells were cultured in a minimal essential medium supplemented with 8% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified atmosphere of 5% CO2. Using the calcium phosphate method, the cells were transfected with a 5-HT2AR cDNA in pRC/CMV. After 24 h of transfection, the medium was removed, and the cells were then incubated for 120 min at 4 °C with serum-free media containing the test drugs (0–1 µM) and 1 nM [3H]-methylspiperone. To measure binding affinity, the cells were rinsed with 1 ml of ice-cold serum-free media three times and then solubilized in 0.5 ml of 1% SDS. Subsequently, the radioactivity was measured by liquid scintillation counting (Wallac 1450, Perkin Elmer, Waltham, MA, USA).
Stereotaxic surgery
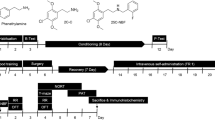
Stereotaxic surgery for the implantation of microdialysis probes was performed in 8-week-old rats. Animals were anesthetized with pentobarbital (50 mg/kg, i.p.) and placed on a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA). Microdialysis probe guide cannula (CMA microdialysis, Kista, Sweden) was implanted unilaterally into the NAc (AP + 1.7 mm, ML + 0.8 mm, and DV − 6.0 mm from the bregma; 0° angle). Two screws were anchored into the skull at the rear of the guide cannula to help fix its position, and dental cement was applied around the cannula and screws. The rats were allowed to recover for six days before the experiments. Stereotaxic surgery for cell type-specific viral manipulation was performed in 8-week-old D1R-Cre mice. Anesthesia was induced with pentobarbital (50 mg/kg, i.p.), and the mice were placed on a stereotaxic frame. For selective inactivation of D1R- medium spiny neurons (MSNs) in the NAc with CNO, 0.5 µl of AAV1-hSyn-DIO-hM4D(Gi)-mCherry (7 × 1012 vg/ml; #44362, Addgene, Watertown, MA, USA) was injected bilaterally into the NAc (AP + 1.5 mm, ML ± 1.0 mm, and DV − 4.5 mm from the bregma; 0° angle) of D1R-Cre mice at a rate of 0.1 µl/min using a 33-gauge syringe needle (Hamilton, Reno, NV, USA). The needle was left in place for an additional 10 min to allow virus diffusion and then slowly removed. The mice were allowed to recover and express the virus for at least 3–4 weeks before the experiments. Seven days post-surgery, mice were administered daily injections of the antibiotic gentamicin sulfate (0.32 mg/kg, i.p.; Shin Poong Pharm Co., Ltd., Seoul, Korea).
Conditioned place preference (CPP) test
The CPP apparatus comprised two chambers (15 cm × 15 cm × 15 cm) separated by a closable guillotine door. One chamber had white walls with a stainless steel grid floor, while the other had black walls with a striped floor. Both chambers were illuminated with dim lighting (12–13 lx). The CPP procedure included a baseline test (day 1), a conditioning phase (days 2–9), and a preference test (day 10). For the baseline and preference tests, mice were allowed free access to both chambers, and the amount of time spent on each side was recorded for 20 min using video tracking software (NeuroVision, Busan, Korea). The baseline test data were used to assign mice into groups to ensure equal mean times spent in each chamber across groups. Subsequently, the conditioning phase was carried out using a biased procedure and performed daily for eight consecutive days. Each mouse was randomly placed in a drug-paired chamber for 45 min. For the 25E-NBOMe conditioning phase, mice were injected with saline or 25E-NBOMe (0.03, 0.1, 0.3, 1, or 3 mg/kg, i.p.) and put in the drug-paired chambers every other day. The mice received saline on alternate days and were placed in the opposite unpaired chambers. The CPP score was calculated as the difference between the time spent in the drug-paired chamber during the baseline and preference tests.
CPP test with inhibition of the dopamine D1 or D2 receptor
For the 25E-NBOMe CPP with dopamine D1 or D2 receptor inhibition, mice were first injected with SCH23390 (0.5 mg/kg, i.p.), raclopride (1 mg/kg, i.p.), or saline and placed in their home cages. After 30 min, mice were injected with saline or 25E-NBOMe (0.1 mg/kg, i.p.) and placed in the drug-paired chambers for 45 min. The mice received saline treatments on alternate days and were put in the opposite unpaired chambers. The CPP test was performed as described above, except the amount of time spent on each side was recorded using Ethovision software (Noldus, Wageningen, Netherlands).
CPP test with DREADD-induced inhibition of D1R-MSNs
For the 25E-NBOMe CPP with DREADD-induced inhibition of D1R-MSNs, D1R-Cre mice were microinjected with AAV1-hSyn-DIO-hM4D(Gi)-mCherry into the NAc and were allowed to recover for 3–4 weeks before CPP conditioning. Mice were first injected with CNO (5 mg/kg) or saline and placed in a home cage. After 30 min, mice were injected with saline or 25E-NBOMe (0.1 mg/kg, i.p.) and placed in the drug-paired chambers for 45 min. The mice received saline treatments on alternate days and were placed in the opposite unpaired chambers. The CPP test was performed as described above, except the amount of time spent on each side was recorded using Ethovision software. Following the preference test, mice were anesthetized with pentobarbital (50 mg/kg, i.p.) and intracardially perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were rapidly dissected and fixed for 4 h with ice-cold 4% paraformaldehyde in 0.1 M PBS. The brains were then dehydrated in 30% sucrose in 0.1 M PBS for 2 days at 4 °C, after which they were frozen at − 80 °C. The NAc regions were sectioned coronally into 50-µm-thick sections using a cryostat (Leica, Nussloch, Germany). NAc slices were washed once with PBS and mounted onto microscope slides with H-1400 mounting medium (Vector Laboratories, Burlingame, CA, USA) to confirm the location of expression of the Cre-inducible DREADD virus. Digital images were acquired using a DP digital camera (Olympus Optical, Tokyo, Japan) connected to a fluorescence microscope (BX51, Olympus).
Self-administration test
Self-administration tests were conducted using operant conditioning chambers (28 × 26 × 20 cm), placed inside light- and sound-attenuating cubicles (Med Associates, ST. Albans, VT, USA). A house light, two response levers (active and inactive levers), and two cue lights above each response lever were placed in the operant chambers. For food training, rats were initially trained to perform active lever presses to dispense 45-mg food pellets (F0021, Bio-Serv, Flemington, NJ, USA) on a fixed-ratio 1 (FR1) schedule until they obtained 80 food pellets during a 1-h session. Each animal had one session daily for three consecutive days. After food training, rats were implanted with a venous catheter. Rats were anesthetized with pentobarbital (50 mg/kg, i.p.), and the catheter (0.3 mm ID × 0.64 mm OD; Dow Corning, Midland, TX, USA) was inserted into their jugular vein. The distal end of the catheter was connected to the back of the rat and exited the skin through a 22-gauge guide cannula (P1 Technologies, Roanoke, VA, USA) anchored with Mersilene surgical mesh (Ethicon Inc., Somerville, NJ, USA). During recovery, rats were intravenously injected daily with 0.2 ml of heparin (20 IU/ml) and the antibiotic gentamicin sulfate (0.32 mg/ml) for seven days. Following the recovery period, rats were trained to self-administer 25E-NBOMe via 2 h daily saline or 25E-NBOMe (0.01, 0.03, or 0.1 mg/kg/infusion, i.v.) self-administration sessions with an FR1 schedule for seven consecutive days. An active lever press resulted in the presentation of a drug infusion and then a 20 s time-out period during which lever presses were recorded but had no consequences. All behavioral data were recorded using Med Associates software (Med Associates).
Locomotor activity test
Locomotor activity was measured in opaque black plastic boxes (30 cm × 30 cm × 30 cm). For the acute 25E-NBOMe locomotor activity test, mice were treated with saline or 25E-NBOMe (0.1, 0.3, 1, or 3 mg/kg, i.p.) and placed in the test box. Immediately after treatment, locomotor activity was recorded for 30 min using video tracking software (EthoVision).
Immunoblot analysis
For immunoblotting, the mouse brains were rapidly dissected immediately after 25E-NBOMe CPP tests, and the NAc was sectioned coronally to produce 800-µm-thick sections using a cryostat (Leica). The NAc was isolated with a 17-gauge stainless steel punch, and NAc tissues were homogenized in a MagNA Lyser (Roche Diagnostics, Indianapolis, IN, USA) with ice-cold lysis T-PER (Tissue Protein Extraction Reagent, Thermo Fisher Scientific, Rockford, IL, USA) containing phosphatase and protease inhibitor cocktails (Roche Diagnostics). The supernatant was collected after centrifugation at 13,000×g for 15 min. Protein concentrations were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), 4 µg of protein was separated and transferred onto a polyvinylidene difluoride membrane (Merck Millipore, Burlington, MA, USA). Each membrane was blocked in Tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% nonfat milk for 1 h at room temperature. The membranes were then incubated with primary antibodies against DAT (rabbit, 1:3000, AB2231, Merck Millipore), D1DR (rabbit, 1:2000, ab20066, Abcam, Cambridge, UK), D2DR (rabbit, 1:1000, ab85367, Abcam), DARPP32 (rabbit, 1:1000, Cat#2306, Cell Signaling, Danvers, MA, USA), CREB (rabbit, 1:1000, ab32515, Abcam), p-CREB (rabbit, 1:500, E-AB-20849, Elabscience, Houston, TX, USA), and β-actin (mouse, 1:2000, SC-47778, Santa Cruz) overnight at 4 °C. After washing the membranes five times with TBST, the blots were incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 1 h at room temperature. After washing five times with TBST, antibody binding was visualized using the enhanced chemiluminescence detection method (Dongin LS., Seoul, Korea), and the membranes were exposed to a photographic film. Protein bands were quantified by densitometry analysis using ImageJ software (NIH, Bethesda, MD, USA).
Dopamine enzyme-linked immunosorbent assay (ELISA)
For dopamine ELISA, mice received saline or 25E-NBOMe (0.1 mg/kg, i.p.) injections on alternating days for eight days following the experimental scheme used in the CPP test. The brains were rapidly dissected 30 min after the last injection. The isolated NAc tissues were homogenized with ice-cold lysis T-PER (Thermo Fisher Scientific) containing phosphatase and protease inhibitor cocktails (Roche Diagnostics). To prevent the degradation of catecholamines, 1 mM EDTA and 4 mM sodium metabisulfite were added. After centrifugation at 13,000×g for 15 min, the supernatant was collected, and the protein concentrations were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Dopamine level was determined quantitatively using a dopamine ELISA kit (BA E-5300, ImmuSmol, Talence, France). 4 µg of protein was extracted and acylated. 90 µl of extracted samples were placed into wells of a microtiter plate, and 25 µl of enzyme solution (mixture of enzyme and coenzyme) were added to each well. The plate was incubated for 2 h at 37 °C. 100 µl of supernatant was transferred into pre-coated dopamine microtiter plates, and 50 µl of dopamine antiserum was added. The plate was incubated for 20 h at 4 °C. After washing the plate four times with wash buffer, the plate was incubated with 100 µl of enzyme conjugate for 30 min on a shaker (600 rpm) at room temperature. After four washes with wash buffer, the plate was incubated with 100 µl of substrate for 30 min on a shaker at room temperature and without exposure to light. After adding 100 µl of stop solution, the absorbance of the solution was measured at 450 nm on a microplate reader (SpectraMax M2, Molecular Devices, San Jose, CA, USA). Dopamine levels were quantified using a corresponding standard curve.
Microdialysis
The microdialysis tests were performed using the experimental method of the previous studies with minor modifications (Kim et al. 2016, 2017, 2019; Lee et al. 2023). For dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) microdialysis, rats were implanted with a microdialysis probe guide cannula into the NAc and were allowed to recover for six days. A microdialysis probe (6 kD cut-off molecular weight, 2 mm membrane length; CMA 11, CMA microdialysis) was inserted into the NAc through a guide cannula. Artificial cerebral spinal fluid (150 mM NaCl, 3 mM KCl, 1.4 mM CaCl2, 0.8 mM MgCl2 in 10 mM phosphate buffer, pH 7.4; aCSF) was perfused at a flow rate of 1.5 µl/min using CMA 100 microinjection pump (CMA microdialysis) for 2 h of stabilization. Six baseline samples were collected at 20-minute intervals into microcentrifuge tubes over 2 h. Vehicle or 25E-NBOMe was injected every 60 min with a gradually increasing dose (0.3, 1, and 3 mg/kg, i.p.), while microdialysis samples were simultaneously collected every 20 min. The location of the microdialysis probe was verified histologically at the end of the microdialysis experiment. For the quantification of microdialysates, 25 µl of collected microdialysis samples from each rat were added to 5 µl of working internal standard solution (30 ng/ml Dopamine-d4, 20 ng/ml DOPAC-d5, and 800 ng/ml HVA-d5) and analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) system. The LC–MS/MS system consisted of 1260 Infinity LC system and 6460 triple quadrupoles MS/MS (Agilent Technologies, Santa Clara, CA, USA) coupled with a 1260 Infinity extra binary pump and degasser (Agilent Technologies). The XBridge BEH HILIC Sentry Guard Cartridge 130 Å (4.6 × 20 mm, 3.5 μm; Waters, Milford, MA, USA) and the Atlantis T3 column (2.1 × 100 mm, 3 μm; Waters) were employed as sample enrichment and separation columns, respectively. The mobile phases (A, 5 mM ammonium formate, 0.1% formic acid in water; B, 0.1% formic acid in acetonitrile) were passed through both the enrichment and separation columns with the following gradient conditions: 0–1.0 min, 5% B; 1.0–6.5 min, 5–90% B; 6.5–7.5 min, 90% B; 7.5–7.6 min, 90–5% B; 7.6–11.5 min, 5% B. The MS/MS system was operated using electrospray ionization in the polarity-switching mode (Dopamine, positive; DOPAC and HVA, negative). The MS/MS conditions were optimized as follows: drying gas temperature, 350 °C; drying gas flow, 10 l/min; nebulization pressure, 35 psi; capillary voltage, 4.5 kV; the temperature of sheath gas, 250 °C; and sheath gas flow, 5 l/min. Each analytical stock solution (1 mg/ml) was prepared in 1 mM ascorbic acid in a 1:1 solution of water and methanol to prevent oxidation. The LC–MS/MS data were processed by Mass Hunter software (Agilent Technologies). The baseline value was determined from three consecutive microdialysis samples with less than 20% fluctuations in the dopamine, DOPAC, and HVA concentrations. The areas under the dose-time curve (AUC) for dopamine were calculated for each period (0–60 min, 60–120 min, and 120–180 min), accompanied by changes in the dose of the drug (0.3, 1, and 3 mg/kg).
Head twitch response test
A head twitch response was defined as a rapid rotational jerk of the head that is not involved in any grooming or scratching behaviors (Kim et al. 2000b; Fantegrossi et al. 2004). Head twitch responses were measured in opaque black plastic boxes (30 cm × 30 cm × 30 cm). Mice were administered saline or 25E-NBOMe (0.1, 0.3, 1, or 3 mg/kg, i.p.) and placed in the test box. Behavior was recorded for 33 min immediately after drug administration, and the head twitch responses were manually counted and totaled by blinded observers. The number of responses was counted at 10–13, 20–23, and 30–33 min. For the 25E-NBOMe head twitch response test with inhibition of the 5-HT2A receptor, mice were first injected with ketanserin (0.1 mg/kg, i.p.) or saline and put in their home cages. After 15 min, mice were injected with saline or 25E-NBOMe (1 mg/kg, i.p.) and placed in the test box. The head twitch response test was performed as described above.
Statistical analysis
All experimental data are presented as means ± standard error of the mean (SEM) and were analyzed using GraphPad Prism software (version 6.0; GraphPad Software, San Diego, CA, USA). Data from the CPP, locomotor activity, and head twitch response tests were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s or Tukey’s post hoc tests. Data from the CPP test with DREADD-induced inhibition of D1R-MSNs were analyzed by one-way ANOVA with Fisher’s Least Significant Difference (LSD) post hoc test. Data from self-administration and microdialysis were analyzed using two-way repeated-measures ANOVA with drug treatment as a between-subject factor and time as a within-subject factor, followed by Dunnett’s or Fisher’s LSD post hoc tests. A two-tailed unpaired Student’s t test was used to determine differences between the two groups. For the 5-HT2AR binding assay, IC50 values were defined as the dose at 50% of the maximum response. IC50 values were calculated from the fitting curve of the dose-response data using nonlinear regression. Statistical significance was set at P < 0.05 in all statistical analyses.
Results
25E-NBOMe induces rewarding properties
We first measured the binding affinity of 25E-NBOMe to 5-HT2A receptors in vitro using 5-HT2AR-transfected HEK-293 cells. The IC50 value of the positive control ketanserin was 187.5 nM, while 25E-NBOMe demonstrated a higher binding affinity for the 5-HT2A receptor, with an IC50 of 7.719 nM (Fig. 1B). To assess the abuse potential of 25E-NBOMe in male rodents, we tested the rewarding effects of the drug using a CPP paradigm in male mice. Administration of 25E-NBOMe (0.1 mg/kg) significantly increased place preference compared to saline treatment, indicating that 25E-NBOMe induced drug reward in mice. For the positive control, methamphetamine (1 mg/kg) also significantly increased place preference in the mice (Fig. 1C). We next investigated the reinforcing effect of 25E-NBOMe using a self-administration paradigm in male rats. The number of infusions, active lever presses, and inactive lever presses were counted during the 2 h daily FR1 sessions (Fig. 1D). Rats administered with 25E-NBOMe (0.03 mg/kg/infusion) received more infusions compared to the control group only on day 2 (Fig. 1E). However, no difference in active lever pressing was observed between the saline and 25E-NBOMe groups (Fig. 1F). 25E-NBOMe (0.01 mg/kg/infusion) group showed an increase in inactive lever pressing on day 1, with no difference in inactive lever presses in the other groups (Fig. 1G). Locomotor activity was measured for 30 min immediately after acute 25E-NBOMe administration. No differences in locomotor activity were observed in the saline and all 25E-NBOMe groups (Fig. 1H).
25E-NBOMe modulates the expression of dopamine signaling proteins in the NAc
The abuse potential of drugs is mainly governed by dopaminergic activity in the NAc, the critical target of the midbrain for reward (Phillips et al. 2003). To further investigate the functional effect of 25E-NBOMe administration on the molecular and neuronal mechanisms associated with the acquisition of drug reward, we examined the expression changes in dopamine-related proteins in the NAc of 25E-NBOMe 0.1 mg/kg group following the preference test session of CPP test (Fig. 2A), which showed the induction of CPP scores. We found that 25E-NBOMe administration significantly increased dopamine transporter (DAT) levels in the NAc compared to saline (Fig. 2B). This was accompanied by elevated D1DR NAc levels (Fig. 2C) but not D2DR levels (Fig. 2D). Moreover, the 25E-NBOMe-injected group exhibited a significant increase in dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP32) expression in the NAc (Fig. 2E). We determined whether 25E-NBOMe affects the regulation of prominent transcription factors, including cAMP response element-binding protein (CREB), which is highly implicated in substance use disorder. 25E-NBOMe administration increased phosphorylated CREB (p-CREB) levels in the NAc compared to the control (Fig. 2G, H), while the total CREB levels remained unchanged (Fig. 2F).
The effect of 25E-NBOMe on dopamine-related proteins in the NAc of male mice. A Representative images of immunoblots from each protein assayed in the NAc of saline and 25E-NBOMe (0.1 mg/kg) groups immediately following the 25E-NBOMe CPP test. B–H Immunoblot analysis of protein expression in the NAc of each male mouse group. B DAT (n = 7), C D1DR (n = 7), D D2DR (n = 7), E DARPP32 (n = 7), F CREB (n = 7), G p-CREB (n = 7) and H Relative ratio of p-CREB to total CREB (n = 7). Data are presented as the means ± standard error of the mean. ∗∗P < 0.01 and ∗∗∗P < 0.001 versus control. Sal saline, 25E 25E-NBOMe
25E-NBOMe regulates the levels of dopamine in the NAc
To further investigate whether the 25E-NBOMe modulates dopaminergic signaling, we determined dopamine levels in the NAc tissue using dopamine ELISA following 25E-NBOMe (0.1 mg/kg) injections on alternate days for 8 days, 30 min after the last injection. 25E-NBOMe markedly reduced dopamine levels in the NAc compared to saline (Fig. 3A). Moreover, we used microdialysis to measure extracellular dopamine concentrations in the NAc of freely-moving animals following vehicle or sequential 25E-NBOMe administration at 0.3, 1, and 3 mg/kg 60 min apart. The basal dopamine levels were 0.235 ± 0.082 ng/ml. Relative dopamine levels were determined by dividing the basal values for each subject. Significant decreased dopamine concentrations were observed in the NAc after injection of 25E-NBOMe at a dose of 0.3 mg/kg at 40 and 60 min, 1 mg/kg at 100 min, and 3 mg/kg at 180 min compared to the control vehicle group, respectively (Fig. 3B). In addition, we observed same trends in 25E-NBOMe-injected group as decrease dopamine compare to vehicle group in the results presenting the AUC of each period (0–60 min, 60–120 min, 120–180 min) (Fig. 3C). Although the dopamine concentrations were decreased by 25E-NBOMe injections, both DOPAC and HVA, a metabolites of dopamine, did not change significantly in the NAc in all time points (Fig. 3D, E).
The effect of 25E-NBOMe on the dopamine levels in the NAc. A Experimental scheme for the 25E-NBOMe injections and NAc dissection. Dopamine levels were measured using ELISA in the NAc of each male mouse group after saline or 25E-NBOMe (0.1 mg/kg) injections on alternated days for 8 days, 30 min after the last injection (n = 11–12). B Changes in the concentrations of dopamine in microdialysis samples collected in the NAc of each male rat group after vehicle or progressively increasing doses of 25E-NBOMe (0.3, 1, and 3 mg/kg) injections (n = 4 for only vehicle injections, 5 for 25E-NBOMe injections). C The areas under the dose-time curve for dopamine concentrations in the NAc of each rat group at each period (0–60 min, 60–120 min, and 120–180 min) (n = 4 for only vehicle injections, 5 for 25E-NBOMe injections). D Changes in the concentrations of DOPAC in microdialysis samples collected in the NAc of each rat group after vehicle or progressively increasing doses of 25E-NBOMe (0.3, 1, and 3 mg/kg) injections (n = 4 for only vehicle injections, 5 for 25E-NBOMe injections). E Changes in the concentrations of HVA in microdialysis samples collected in the NAc of each rat group after vehicle or progressively increasing doses of 25E-NBOMe (0.3, 1, and 3 mg/kg) injections (n = 4 for only vehicle injections, 5 for 25E-NBOMe injections). Each arrowhead represents drug treatment. Data are presented as the means ± standard error of the mean. ∗P < 0.05 versus control. Sal saline, Veh vehicle, 25E 25E-NBOMe, NAc nucleus accumbens
Dopamine 1 receptor signaling mediates 25E-NBOMe-induced rewarding effect
To determine the impact of dopamine receptor inhibition on the rewarding effects of 25E-NBOMe, we performed a CPP test in male mice pretreatment with D1DR or D2DR antagonists 30 min before 25E-NBOMe conditioning sessions. Consistent with earlier experiments, 25E-NBOMe (0.1 mg/kg) increased CPP compared to saline treatment. Pretreatment with the D1DR-selective antagonist SCH23390 (0.5 mg/kg) significantly blocked the expression of 25E-NBOMe-induced CPP, while pretreatment with the D2DR-selective antagonist raclopride (1 mg/kg) did not (Fig. 4A). Previous work has demonstrated that MSNs in the NAc are divided into two groups. D1R-MSNs mainly express D1DR, whereas D2R-MSNs express D2DR. The contributions of these two populations of MSNs on addiction-related behaviors are distinct (Bertran-Gonzalez et al. 2008; Lobo and Nestler 2011). Our pharmacological test data have shown that D1DR antagonist pretreatment blocked the acquisition of 25E-NBOMe CPP. To further elucidate the role of D1R-MSNs on 25E-NBOMe reward, we expressed hM4D(Gi)-mCherry selectively in D1R-MSNs and administered DREADD ligand CNO to selectively inhibit D1 activity during 25E-NBOMe conditioning sessions (Fig. 4B). Vehicle-pretreated 25E-NBOMe (0.1 mg/kg) group showed an increase in CPP. Surprisingly, pretreatment with CNO (5 mg/kg) significantly blocked the expression of 25E-NBOMe-induced CPP (Fig. 4C).
The effect of D1DR signaling on 25E-NBOMe-induced CPP in male mice A CPP score of each male mouse group in the 25E-NBOMe (0.1 mg/kg) CPP test with pharmacological blockade of D1DR or D2DR (n = 7–10). B Experimental scheme for the injection of AAV1-hSyn-DIO-hM4D(Gi)-mCherry into the NAc of D1R-Cre mice and 25E-NBOMe CPP test. Representative image of a coronal slice showing injection sites of AAV1-hSyn-DIO-hM4D(Gi)-mCherry (red) into the NAc. Scale bar: 200 μm. C CPP score of each male mouse group in the 25E-NBOMe (0.1 mg/kg) CPP test with DREADD-induced inhibition of D1R-MSNs (n = 7–8). Data are presented as the means ± standard error of the mean. ∗P < 0.05 versus control; #P < 0.05 versus 25E-NBOMe group. NAc nucleus accumbens, ac anterior commissure
5-HT2A receptor signaling mediates 25E-NBOMe-induced head twitch response
In addition to the abuse potential of 25E-NBOMe, we assessed the serotonin-dependent hallucinogenic activity of 25E-NBOMe. Behaviorally, male mice treated with a single administration of 25E-NBOMe (0.1, 0.3, 1, and 3 mg/kg) exhibited significantly greater head twitch responses compared to saline-treated mice, indicating that 25E-NBOMe possesses hallucinogenic properties (Fig. 5A). To determine the impact of 5-HT2A receptor inhibition on the head twitch response, male mice were given a 5-HT2A receptor antagonist 15 min before 25E-NBOMe (1 mg/kg) injection. Pretreatment with the 5-HT2A receptor antagonist ketanserin (0.1 mg/kg) significantly attenuated the 25E-NBOMe-induced head twitch response (Fig. 5B).
The effect of 25E-NBOMe on head twitch response in male mice. A Head twitch response of each male mouse group (saline, 25E-NBOMe 0.1, 0.3, 1, and 3 mg/kg) following single 25E-NBOMe administration (n = 6–7). B Head twitch response of each male mouse group in the 25E-NBOMe (1 mg/kg) head twitch response test with pharmacological blockade of 5-HT2A receptor (n = 6). Data are presented as the means ± standard error of the mean. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 versus control; #P < 0.05 versus 25E-NBOMe group. Sal saline
Discussion
We investigated the abuse potential of 25E-NBOMe and the involved molecular and neuronal mechanisms that lead to 25E-NBOMe addiction-related behaviors in male rodents. First, we demonstrated that 25E-NBOMe exhibits rewarding effects using the CPP paradigm. 25E-NBOMe showed relatively low reinforcement in the self-administration test and no impact on locomotor activity. Changes in dopaminergic protein expression were observed in the NAc of male mice following 25E-NBOMe administrations. Moreover, 25E-NBOMe decreased the level of dopamine in the NAc, particularly extracellular dopamine. We found that the pharmacological blockade of D1DR and chemogenetic inhibition of D1R-MSNs reversed 25E-NBOMe-induced reward. Furthermore, we also demonstrated that 25E-NBOMe exhibits hallucinogenic properties mediated by 5-HT2AR activity.
The CPP and self-administration paradigms are typically utilized in animal models to evaluate drug reward and reinforcement, respectively, as indicators of abuse potential (Spanagel 2017; Green and Bardo 2020). Phenethylamine structure-based psychostimulants, such as amphetamine and methamphetamine, exhibit psychotropic effects and robustly induce substance use disorder. Similarly, NBOMe drugs, including 25E-NBOMe, have a phenethylamine structure, and chemical modifications of this core induce potent psychedelic activity (Hansen et al. 2014). Since NBOMe drugs show higher potency than classical hallucinogens, high rates of NBOMe use have been reported in case studies (Wood et al. 2015). Although studies of several NBOMe drugs (25I-, 25N-, 25B-, 25H-, and 25D-NBOMe) have shown that NBOMe drugs induce either CPP or self-administration in rodents (Jeon et al. 2019; Seo et al. 2019; Custodio et al. 2020; Jo et al. 2022; Lee et al. 2023), the abuse potential of 25E-NBOMe has not been scientifically demonstrated. Our results showed that 25E-NBOMe (0.1 mg/kg) significantly induced place preference in mice, suggesting that 25E-NBOMe exerts a rewarding effect. The place preference increased dose-dependently and decreased above 0.3 mg/kg doses. Furthermore, we found that rats self-administered 25E-NBOMe (0.03 mg/kg/infusion), but the differences in the number of infusions between 25E-NBOMe and the control group were seen only on day 2. Considering our results and several studies reporting that the NBOMe drugs either fail to induce a number of infusions or only occur in a few sessions in the self-administration paradigm (Jeon et al. 2019; Seo et al. 2019; Lee et al. 2023), NBOMe drugs, including 25E-NBOMe, may have moderate or low reinforcing properties. Moreover, we measured the locomotor activity immediately after the single 25E-NBOMe injection to determine whether 25E-NBOMe treatment results in motor stimulation or sedative effects. However, we observed no changes in locomotion in the drug and control groups.
To investigate the mechanisms by which 25E-NBOMe produces reward-related behaviors, we tested the hypothesis that 25E-NBOMe administration would alter dopamine signaling in the NAc to cause rewarding effects. First, we observed the rapid increase in DAT expression in the NAc of 25E-NBOMe (0.1 mg/kg)-conditioned mice following the CPP test. In addition, our data revealed that 25E-NBOMe induced D1DR levels in the NAc, while D2DR levels did not change. These results suggest that 25E-NBOMe may affect DAT function and D1DR-mediated synaptic transmission in NAc neurons. Evidence of disturbance in signaling was also manifested in the concentrations of the neurotransmitter dopamine. We determined that 25E-NBOMe decreased tissue and extracellular dopamine levels in the NAc, while we injected progressively increasing doses in the microdialysis test, the effect of higher dose (1, 3 mg/kg) was similar to those of lower dose (0.3 mg/kg). The observed alterations may suggest that a low dose of 25E-NBOMe is sufficient to induce saturation or maximal efficacy, such as a potential ceiling effect on extracellular dopamine reduction in the NAc. Moreover, phenethylamine hallucinogens, 25I-NBOMe, DOI, and DOB are established to rapidly induce tolerance, which is the state that adapts to the drug’s action and requires higher doses to achieve the same initial effect in head twitch response test and leads to down-regulation of 5-HT2AR in the frontal cortex (Buchborn et al. 2015; Herian et al. 2021; de la Fuente Revenga et al. 2022). Tolerance of phenethylamine drug was also reported in short-interval injections (60–90 min) (Buchborn et al. 2018). Similarly, it can be suggested that 25E-NBOMe efficiently induces rapid tolerance of 5-HT2AR, and sequential administration may not have resulted in a further dose-dependent decrease in dopamine. Moreover, we observed no changes in DOPAC and HVA levels in the NAc upon 25E-NBOMe exposure. The degradation of dopamine by metabolism enzymes, such as monoamine oxidase and catechol-O-methyl transferase, produces DOPAC and HVA in the cytosol of presynaptic dopaminergic neurons (Meiser et al. 2013). Thus, microdialysis results, in which 25E-NBOMe reduced dopamine in the synaptic space but did not alter DOPAC and HVA, may imply that 25E-NBOMe exposure improves dopamine turnover and metabolism, maintaining DOPAC and HVA concentrations unchanged from the basal level even though dopamine concentration is reduced. While our reduced dopamine results are promising, there are potent limitations in isolating the effect of a single dose of 25E-NBOMe, as the gradually increasing dose regimen may have potential lingering effects from previous doses. While we found that 25E-NBOMe reduced dopamine, previous studies have shown that several NBOMe drugs increase dopamine levels in the NAc (Miliano et al. 2019; Custodio et al. 2020; Herian et al. 2021; Wojtas et al. 2023), and the mechanisms are not precise in how 25E-NBOMe exerts its effects. One possible explanation for these observations is that massive increased DAT levels lead to increased dopamine uptake from the extracellular space into the presynaptic neuron (Foster and Vaughan 2017). Previous evidence indicates that 5-HT inhibited dopamine in the striatum (Muramatsu et al. 1988), and 5-HT receptor activation through the administration of serotonin reuptake inhibitor led to a decrease in dopamine (Dewey et al. 1995; Kapur and Remington 1996). Moreover, it has been established that 5-HT2CR activity encodes an inhibitory role in dopamine concentration in the NAc (Howell and Cunningham 2015). Interestingly, a study on the binding affinity of the 5-HT receptors has reported that 25E-NBOMe exerts more potency to 5-HT2CR than other NBOMe drugs (Eshleman et al. 2018). Considering these, different pharmacological properties of 25E-NBOMe from other NBOMe drugs likely occur in discrepancies of dopamine.
Recent studies have demonstrated that dopamine depletion enhanced striatal D1DR sensitivity and examined increased neuronal firing and locomotor responses to D1DR agonist treatment (Kim et al. 2000a; McKinley et al. 2019). Also, repeated exposure to addictive drugs disrupt basal dopamine-releasing, resulting in enhanced sensitivity of dopamine receptors, and re-exposure causes hyperactive signaling in proportion to the expressed compulsive drug intake (Volkow et al. 2004). In line with these, dopamine receptor activity, especially D1DR, may be increased to compensate for the decrease in dopamine in the NAc by 25E-NBOMe administration. It has been reported that seven days of 25B-NBOMe injection increased D1DR expression in the NAc, while D2DR was reduced (Custodio et al. 2020). Some studies have reported that the down-regulation of D2DR is associated with a response to addictive drugs (Ru et al. 2022). In contrast, other studies have pointed to no changes in D2DR expression levels in the NAc (Gong et al. 2021), implying the controversial effects on D2DR changes depending on experimental conditions such as administration schedules. Despite this, our results strongly link increased D1DR levels and downstream dopamine signaling in response to 25E-NBOMe. DARPP32 is a prominent mediator of intracellular response in dopamine receptor signaling pathways and is required to induce drug-related effects (Nairn et al. 2004; Gould and Manji 2005). It has been reported that mice lacking DARPP32 showed reduced cocaine-induced place preference and expression of neuronal transcription factor (Fienberg et al. 1998; Zachariou et al. 2002). In addition, although the activity of D1DR and D2DR are known to transduce dopaminergic signals via the DARPP32 pathway, pharmacological D1DR stimulation activates DARPP32, while D2DR stimulation negatively blocks DARPP32 activity in the mouse striatum (Bateup et al. 2008). Our data revealed that 25E-NBOMe administration significantly increases DARPP32 expression and phosphorylation of CREB, one of the significant target transcription factors of DARPP32 in the NAc. Despite NBOMe drugs, including 25E-NBOMe, exhibiting low binding affinity for proteins in the dopaminergic systems, such as D1DR, D2DR, and DAT (Rickli et al. 2015; Eshleman et al. 2018), recent evidence indicates that drugs that stimulate 5-HT2AR may modulate dopaminergic signaling directly or indirectly (Howell and Cunningham 2015). In the ultrastructural study, it has been demonstrated that 5-HT2AR is localized in dopaminergic neurons of the ventral tegmental area (VTA) region. Moreover, activation of 5-HT2AR may modulate the activity of dopaminergic neurons in the VTA by enhancing the dopamine synthesis process and affecting dopamine release in the NAc through dopaminergic neuron projection (Nichols 2016). It is reported that repeated 25B-NBOMe administration reduced the DAT level in the VTA (Custodio et al. 2020). The effect of 25E-NBOMe within 5-HT2AR of the VTA may contribute to the alteration in neuronal activity or dopamine transport in the VTA, thereby altering dopamine concentration in the NAc. 5-HT2AR is also abundantly expressed in the neurons of the NAc (Bubar and Cunningham 2006). Some studies have reported that treatment with 5-HT or DOI, a 5-HT2AR agonist, increased the firing of dopaminergic neurons in the mesolimbic system, an effect that a selective 5-HT2AR antagonist can reverse (Pessia et al. 1994; Bortolozzi et al. 2005). Moreover, neuronal function in the NAc can be increased by 25B-NBOMe administration (Custodio et al. 2020). Taken together with changes in DAT, D1DR expression, and dopamine levels, these suggest that 25E-NBOMe activates 5-HT2AR, which modulates the dopaminergic system and eventually leads to addictive behaviors. Since desensitization of 5-HT2AR by repeated 25E-NBOMe injections over several days may cause different effects on dopamine signaling or content in the NAc compared to a single dose, future studies are needed to examine the alternative possibility compared to an acute effect. Furthermore, it cannot be assumed that D1DR expression levels reflect actual activity. Thus, whether D1DR activity plays a critical role in 25E-NBOMe-induced reward remains to be demonstrated.
To further investigate whether 25E-NBOMe reward-related behaviors are mediated by dopamine receptor activity, we treated the mice with dopamine receptor antagonists during CPP conditioning. We found that pretreatment with a selective D1DR antagonist inhibited 25E-NBOMe place preference, while a selective D2DR antagonist did not affect drug reward. The distinct roles of D1DR and D2DR in reward-related behaviors of drugs have been noted in many studies. Previous studies reported that pharmacological blockade of D1DR attenuated cocaine or methamphetamine-induced reward in male rats (Cervo and Samanin 1995; Nazarian et al. 2004; Gu et al. 2020), while blockade of D2DR did not influence cocaine reward (Cervo and Samanin 1995; Pruitt et al. 1995; Nazarian et al. 2004). Furthermore, evidence from several biochemical studies showed that drugs of abuse facilitate different functional responses in NAc cell types. For example, cocaine-induced p-ERK and ΔFosB expression, which are regulated by neuronal activation, was observed only in D1R-MSNs of the NAc, not in D2R-MSNs (Bertran-Gonzalez et al. 2008; Lobo et al. 2013). Using viral-mediated targeting of specific cell types, recent studies reported that optogenetic activation of D1R-MSNs in the NAc enhanced cocaine CPP relative to controls, whereas stimulation of D2R-MSNs reversed this effect (Lobo et al. 2010; Soares-Cunha et al. 2020). Furthermore, DREADD-induced inhibition of D1R-MSNs in the NAc blocked the expression of cocaine place preference (Calipari et al. 2016). To confirm the causal role of D1DR in the 25E-NBOMe reward, we applied DREADD to inhibit D1R-MSNs selectively during CPP conditioning. We observed that a DREADD ligand CNO-induced selective inhibition of D1R-MSNs blocked the development of 25E-NBOMe reward. These suggest that D1DR signaling in the NAc plays a crucial role in acquiring 25E-NBOMe reward.
The head twitch response is the rhythmic paroxysmal head rotation widely used to examine the hallucinogenic effect of drugs in animal models. It is well established that the classical hallucinogens LSD, psilocybin, N, N-Dimethyltryptamine, and mescaline induce potent head twitch responses, which are correlated to their hallucinogenic potency in humans (Halberstadt and Geyer 2011; Canal and Morgan 2012). Previous studies employing pharmacological inhibition or genetic deletion of each serotonin receptor subtype have demonstrated that the 5-HT2A receptor mediates the head twitch response (Vickers et al. 2001; González-Maeso et al. 2007). Our data show that 25E-NBOMe elicited greater binding affinity to 5-HT2AR and significantly induced head twitch responses in male mice. Furthermore, we confirmed that the pretreatment of ketanserin inhibited head twitch responses, indicating that the hallucinogenic activity of 25E-NBOMe is responsible for the 5-HT2AR activity. In the previous studies, NBOMe drugs exhibited a characteristic biphasic dose-response effect, in which head twitch responses gradually increased as the dose increased and then levels descended at higher doses (Elmore et al. 2018; Custodio et al. 2020). This biphasic effect has also been reported in CPP tests (Jeon et al. 2019; Custodio et al. 2020). Consistent with these findings, 25E-NBOMe produced an inverted U-shaped dose-response curve in the head twitch response as well as CPP score, suggesting the precise details of 25E-NBOMe’s action at higher doses are more complicated due to the non-specific binding of 25E-NBOMe. Previous reports have demonstrated that 5-HT2AR-expressing cells in the medial prefrontal cortex area commonly co-expressed 5-HT2CR (Nocjar et al. 2015). In addition, in the medial prefrontal cortex, 5-HT2CR is expressed mainly on GABAergic neurons, which release the inhibitory neurotransmitter GABA (Nocjar et al. 2015; Santana and Artigas 2017). Notably, 5-HT2AR activation versus 5-HT2CR activation may oppositely affect the behavioral responses. It is reported that activation of 5-HT2CR inhibits the 5-HT2AR-mediated head twitch responses (Vickers et al. 2001). These might suggest that the higher doses of 25E-NBOMe cause not only 5-HT2AR activation but also 5-HT2CR activation, which may result in suppressed behavioral responses through exerting inhibitory signal transmission by activating inhibitory GABAergic neurons (Elmore et al. 2018). Nevertheless, the complex interplay of 5-HT2AR and 5-HT2CR-mediated neuronal mechanisms on the induction of abuse potential and hallucinogenic properties needs further investigation.
In conclusion, our findings indicate that 25E-NBOMe has abuse potential via dopaminergic signaling, particularly the D1DR-mediated signaling pathway in the NAc. This signaling may induce neuroadaptations and contribute to the rewarding properties of 25E-NBOMe. In addition, 25E-NBOMe induces hallucinogenic effects mediated by 5-HT2A receptor signaling. These results provide new insights into the potential mechanisms of NPS use disorder and can contribute to improving the management of controlled substances. However, although the addictive behavior of drugs and drug-induced bio-molecular changes may differ depending on gender (Calipari et al. 2017), it is a limitation of our research that we did not determine whether the pharmacological action of 25E-NBOMe varies in both male and female animals. Further study is warranted to determine whether 25E-NBOMe induces consistent responses in males and females to conduct a comprehensive and exhaustive investigation. Also, to intensively investigate the pharmacological action of 25E-NBOMe in a dose-dependent manner, further study of 25E-NBOMe on neuronal systems such as dopamine, glutamate, and serotonin with single-dose experimental groups is needed.
References
Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P (2008) Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11(8):932–939. https://doi.org/10.1038/nn.2153
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63(1):182–217. https://doi.org/10.1124/pr.110.002642
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28(22):5671–5685. https://doi.org/10.1523/JNEUROSCI.1039-08.2008
Bortolozzi A, Díaz-Mataix L, Scorza MC, Celada P, Artigas F (2005) The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem 95(6):1597–1607. https://doi.org/10.1111/j.1471-4159.2005.03485.x
Bubar MJ, Cunningham KA (2006) Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem 6(18):1971–1985. https://doi.org/10.2174/156802606778522131
Buchborn T, Schröder H, Dieterich DC, Grecksch G, Höllt V (2015) Tolerance to LSD and DOB induced shaking behaviour: differential adaptations of frontocortical 5-HT(2A) and glutamate receptor binding sites. Behav Brain Res 281:62–68. https://doi.org/10.1016/j.bbr.2014.12.014
Buchborn T, Lyons T, Knöpfel T (2018) Tolerance and tachyphylaxis to head twitches induced by the 5-HT2A agonist 25CN-NBOH in mice. Front Pharmacol 9:17. https://doi.org/10.3389/fphar.2018.00017
Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ (2016) In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A 113(10):2726–2731. https://doi.org/10.1073/pnas.1521238113
Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8:13877. https://doi.org/10.1038/ncomms13877
Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4(7–8):556–576. https://doi.org/10.1002/dta.1333
Cervo L, Samanin R (1995) Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res 673(2):242–250. https://doi.org/10.1016/0006-8993(94)01420-m
Chatwin C (2017) Assessing the ‘added value’ of European policy on new psychoactive substances. Int J Drug Policy 40:111–116. https://doi.org/10.1016/j.drugpo.2016.11.002
Corkery JM, Orsolini L, Papanti D, Schifano F (2017) From concept(ion) to life after death/the grave: The “natural” history and life cycle(s) of novel psychoactive substances (NPS). Hum Psychopharmacol. https://doi.org/10.1002/hup.2566
Custodio RJP, Sayson LV, Botanas CJ, Abiero A, You KY, Kim M, Lee HJ, Yoo SY, Lee KW, Lee YS, Seo JW, Ryu IS, Kim HJ, Cheong JH (2020) 25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: evidence of abuse potential. Addict Biol 25(6):e12850. https://doi.org/10.1111/adb.12850
de la Fuente Revenga M, Jaster AM, McGinn J, Silva G, Saha S, González-Maeso J (2022) Tolerance and cross-tolerance among psychedelic and nonpsychedelic 5-HT2A receptor agonists in mice. ACS Chem Neurosci 13(16):2436–2448. https://doi.org/10.1021/acschemneuro.2c00170
Dewey SL, Smith GS, Logan J, Alexoff D, Ding YS, King P, Pappas N, Brodie JD, Ashby CR Jr (1995) Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in vivo microdialysis. J Neurosci 15(1 Pt 2):821–829. https://doi.org/10.1523/JNEUROSCI.15-01-00821.1995
Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47:227–241. https://doi.org/10.1016/j.neuropharm.2004.06.032
Drug Enforcement Administration (2016) Schedules of controlled substances: placement of three synthetic phenethylamines into schedule I. final rule. Fed Regist 81(187):66181–66184. Federal Register Web. https://www.govinfo.gov/content/pkg/FR-2016-09-27/pdf/2016-23185.pdf
Ellenbroek B, Youn J (2016) Rodent models in neuroscience research: is it a rat race? Dis Model Mech 9(10):1079–1087. https://doi.org/10.1242/dmm.026120
Elmore JS, Decker AM, Sulima A, Rice KC, Partilla JS, Blough BE, Baumann MH (2018) Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2 C counterparts in male rats. Neuropharmacology 142:240–250. https://doi.org/10.1016/j.neuropharm.2018.02.033
Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A (2018) Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: high potency agonists at 5-HT2A receptors. Biochem Pharmacol 158:27–34. https://doi.org/10.1016/j.bcp.2018.09.024
Fantegrossi WE, Kiessel CL, Leach PT, Van Martin C, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH (2004) Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology 173(3–4):270–277. https://doi.org/10.1007/s00213-003-1741-2
Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O’Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P (1998) DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science 281(5378):838–842. https://doi.org/10.1126/science.281.5378.838
Foster JD, Vaughan RA (2017) Phosphorylation mechanisms in dopamine transporter regulation. J Chem Neuroanat 83–84:10–18. https://doi.org/10.1016/j.jchemneu.2016.10.004
Gong S, Fayette N, Heinsbroek JA, Ford CP (2021) Cocaine shifts dopamine D2 receptor sensitivity to gate conditioned behaviors. Neuron 109(21):3421–3435e5. https://doi.org/10.1016/j.neuron.2021.08.012
González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53(3):439–452. https://doi.org/10.1016/j.neuron.2007.01.008
Gould TD, Manji HK (2005) DARPP-32: a molecular switch at the nexus of reward pathway plasticity. Proc Natl Acad Sci U S A 102(2):253–254. https://doi.org/10.1073/pnas.0408700102
Green TA, Bardo MT (2020) Opposite regulation of conditioned place preference and intravenous drug self-administration in rodent models: motivational and non-motivational examples. Neurosci Biobehav Rev 116:89–98. https://doi.org/10.1016/j.neubiorev.2020.06.006
Gu SM, Cha HJ, Seo SW, Hong JT, Yun J (2020) Dopamine D1 receptor antagonist reduces stimulant-induced conditioned place preferences and dopamine receptor supersensitivity. Naunyn Schmiedebergs Arch Pharmacol 393(1):131–138. https://doi.org/10.1007/s00210-019-01694-3
Halberstadt AL (2017) Pharmacology and toxicology of N-benzylphenethylamine (NBOMe) hallucinogens. Curr Top Behav Neurosci 32:283–311. https://doi.org/10.1007/7854_2016_64
Halberstadt AL, Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61(3):364–381. https://doi.org/10.1016/j.neuropharm.2011.01.017
Halberstadt AL, Geyer MA (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2 C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77:200–207. https://doi.org/10.1016/j.neuropharm.2013.08.025
Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (2014) Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2 C agonists. ACS Chem Neurosci 5(3):243–249. https://doi.org/10.1021/cn400216u
Herian M, Skawski M, Wojtas A, Sobocińska MK, Noworyta K, Gołembiowska K (2021) Tolerance to neurochemical and behavioral effects of the hallucinogen 25I-NBOMe. Psychopharmacology 238(8):2349–2364. https://doi.org/10.1007/s00213-021-05860-5
Homberg JR, Wöhr M, Alenina N (2017) Comeback of the rat in biomedical research. ACS Chem Neurosci 8(5):900–903. https://doi.org/10.1021/acschemneuro.6b00415
Howell LL, Cunningham KA (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67(1):176–197. https://doi.org/10.1124/pr.114.009514
Jeon SY, Kim YH, Kim SJ, Suh SK, Cha HJ (2019) Abuse potential of 2-(4-iodo-2, 5-dimethoxyphenyl)N-(2-methoxybenzyl)ethanamine (25INBOMe); in vivo and ex vivo approaches. Neurochem Int 125:74–81. https://doi.org/10.1016/j.neuint.2019.02.007
Jo C, Joo H, Youn DH, Kim JM, Hong YK, Lim NY, Kim KS, Park SJ, Choi SO (2022) Rewarding and reinforcing effects of 25H-NBOMe in rodents. Brain Sci 12(11):1490. https://doi.org/10.3390/brainsci12111490
Kapur S, Remington G (1996) Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 153(4):466–476. https://doi.org/10.1176/ajp.153.4.466
Kim DS, Szczypka MS, Palmiter RD (2000a) Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J Neurosci 20(12):4405–4413. https://doi.org/10.1523/JNEUROSCI.20-12-04405.2000
Kim HS, Park IS, Lim HK, Choi HS, Oh S, Park WK, Jang CG, Kim SH, Chang MJ (2000b) N-Methyl-D-aspartate receptor antagonists enhance the head-twitch response, a 5-hydroxytryptamine2 receptor-mediated behaviour, in reserpine-treated mice. J Pharm Pharmacol 52(6):717–722. https://doi.org/10.1211/0022357001774390
Kim M, Lee JG, Yang CH, Lee S (2016) Silica stationary phase-based on-line sample enrichment coupled with LC-MS/MS for the quantification of dopamine, serotonin and their metabolites in rat brain microdialysates. Anal Chim Acta 923:55–65. https://doi.org/10.1016/j.aca.2016.03.021
Kim M, Kim DH, Lee YS, Jang CG, Yang CH, Lee S (2017) Changes in dopamine, serotonin and their metabolites in brain microdialysates from rats following exposure to new psychoactive drugs. Forensic Toxicol 35:66–76. https://doi.org/10.1007/s11419-016-0335-8
Kim M, Yang CH, Lee YS, Jang CG, Oh S, Lee S (2019) Effects of aromatic ring-substituted phenethylamines on the release of dopamine and serotonin. Forensic Toxicol 37:104–112. https://doi.org/10.1007/s11419-018-0440-y
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35(1):217–238. https://doi.org/10.1038/npp.2009.110
Korean Law Information Center (2019) Enforcement decree of the narcotics control act (Presidential Decree 30244). Korean. Korean Law Information Center Web. https://www.law.go.kr/LSW/nwRvsLsInfoR.do?lsiSeq=212321. Assessed 10 Dec 2019
Lee JG, Hur KH, Hwang SB, Lee S, Lee SY, Jang CG (2023) Designer drug, 25D-NBOMe, has reinforcing and rewarding effects through change of a dopaminergic neurochemical system. ACS Chem Neurosci 14(15):2658–2666. https://doi.org/10.1021/acschemneuro.3c00196
Lobo MK, Nestler EJ (2011) The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5:41. https://doi.org/10.3389/fnana.2011.00041
Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330(6002):385–390. https://doi.org/10.1126/science.1188472
Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ (2013) ∆FosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci 33(47):18381–18395. https://doi.org/10.1523/JNEUROSCI.1875-13.2013
McKinley JW, Shi Z, Kawikova I, Hur M, Bamford IJ, Sudarsana Devi SP, Vahedipour A, Darvas M, Bamford NS (2019) Dopamine deficiency reduces striatal cholinergic interneuron function in models of Parkinson’s disease. Neuron 103(6):1056–1072. https://doi.org/10.1016/j.neuron.2019.06.013
Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism. Cell Commun Signal 11(1):34. https://doi.org/10.1186/1478-811X-11-34
Miliano C, Serpelloni G, Rimondo C, Mereu M, Marti M, De Luca MA (2016) Neuropharmacology of new psychoactive substances (NPS): focus on the rewarding and reinforcing properties of cannabimimetics and amphetamine-like stimulants. Front Neurosci 10:153. https://doi.org/10.3389/fnins.2016.00153
Miliano C, Marti M, Pintori N, Castelli MP, Tirri M, Arfè R, De Luca MA (2019) Neurochemical and behavioral profiling in male and female rats of the psychedelic agent 25I-NBOMe. Front Pharmacol 10:1406. https://doi.org/10.3389/fphar.2019.01406
Muramatsu M, Tamaki-Ohashi J, Usuki C, Araki H, Chaki S, Aihara H (1988) 5-HT2 antagonists and minaprine block the 5-HT-induced inhibition of dopamine release from rat brain striatal slices. Eur J Pharmacol 153(1):89–95. https://doi.org/10.1016/0014-2999(88)90591-2
Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P (2004) The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology 47:14–23. https://doi.org/10.1016/j.neuropharm.2004.05.010
Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V (2004) The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull 63(4):295–299. https://doi.org/10.1016/j.brainresbull.2004.03.004
Nichols DE (2016) Psychedelics Pharmacol Rev 68(2):264–355. https://doi.org/10.1124/pr.115.011478
Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA (2015) Serotonin-2 C and – 2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience 297:22–37. https://doi.org/10.1016/j.neuroscience.2015.03.050
Passie T, Benzenhöfer U (2018) MDA, MDMA, and other mescaline-like substances in the US military’s search for a truth drug (1940s to 1960s). Drug Test Anal 10(1):72–80. https://doi.org/10.1002/dta.2292
Pessia M, Jiang ZG, North RA, Johnson SW (1994) Actions of 5-hydroxytryptamine on ventral tegmental area neurons of the rat in vitro. Brain Res 654(2):324–330. https://doi.org/10.1016/0006-8993(94)90495-2
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422(6932):614–618. https://doi.org/10.1038/nature01476
Pruitt DL, Bolanos CA, McDougall SA (1995) Effects of dopamine D1 and D2 receptor antagonists on cocaine-induced place preference conditioning in preweanling rats. Eur J Pharmacol 283(1–3):125–131. https://doi.org/10.1016/0014-2999(95)00309-9
Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (2015) Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2 C drugs). Neuropharmacology 99:546–553. https://doi.org/10.1016/j.neuropharm.2015.08.034
Ru Q, Xiong Q, Tian X, Xu C, Li C, Chen L, Wu Y (2022) Candidate chinese herbal medicine alleviates methamphetamine addiction via regulating dopaminergic and serotonergic pathways. Front Mol Neurosci 15:874080. https://doi.org/10.3389/fnmol.2022.874080
Santana N, Artigas F (2017) Expression of serotonin2C receptors in pyramidal and GABAergic neurons of rat prefrontal cortex: a comparison with striatum. Cereb Cortex 27(6):3125–3139. https://doi.org/10.1093/cercor/bhw148
Self DW (2010) Dopamine receptor subtypes in reward and relapse. In: Neve K (ed) The dopamine receptors. Humana, New York, pp 479–524. https://doi.org/10.1007/978-1-60327-333-6_17
Seo JY, Hur KH, Ko YH, Kim K, Lee BR, Kim YJ, Kim SK, Kim SE, Lee YS, Kim HC, Lee SY, Jang CG (2019) A novel designer drug, 25 N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents. Brain Res Bull 152:19–26. https://doi.org/10.1016/j.brainresbull.2019.07.002
Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, Gaspar R, Sotiropoulos I, Sousa N, Rodrigues AJ (2020) Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry 25(12):3241–3255. https://doi.org/10.1038/s41380-019-0484-3
Spanagel R (2017) Animal models of addiction. Dialogues Clin Neurosci 19(3):247–258. https://doi.org/10.31887/DCNS.2017.19.3/rspanagel
Suzuki J, Dekker MA, Valenti ES, Arbelo Cruz FA, Correa AM, Poklis JL, Poklis A (2015) Toxicities associated with NBOMe ingestion-a novel class of potent hallucinogens: a review of the literature. Psychosomatics 56(2):129–139. https://doi.org/10.1016/j.psym.2014.11.002
Swedish Code of Statutes (2018) Ordinance amending the ordinance (1992:1554) on the control of narcotics, SFS2018:2057. Swedish. Sveriges Riksdag Web. https://svenskforfattningssamling.se/sites/default/files/sfs/2018-12/SFS2018-2057.pdf. Assessed 20 Dec 2018
United States Code (2022) Controlled Substance Analogue, 21 U.S.C § 802(32)(A). UNITED STATES CODE Web. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title21-section802&num=0&edition=prelim
UNODC (2022) World Drug Report 2022. Booklet 4. United Nations publication, pp 87–103. United Nations Office on Drugs and Crime Web. https://www.unodc.org/res/wdr2022/MS/WDR22_Booklet_4.pdf
UK Statutory Instruments (2014) The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014, 2014 No. 1106. UK Legislation Web. https://www.legislation.gov.uk/ukdsi/2014/9780111110904. Assessed 28 April 2014
Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA (2001) Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2 C) receptor agonists. Pharmacol Biochem Behav 69(3–4):643–652. https://doi.org/10.1016/s0091-3057(01)00552-4
Volkow ND, Fowler JS, Wang GJ (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13(5–6):355–366. https://doi.org/10.1097/00008877-200209000-00008
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9(6):557–569. https://doi.org/10.1038/sj.mp.4001507
Volkow ND, Wise RA, Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18(12):741–752. https://doi.org/10.1038/nrn.2017.130
Wojtas A, Herian M, Maćkowiak M, Solarz A, Wawrzczak-Bargiela A, Bysiek A, Noworyta K, Gołembiowska K (2023) Hallucinogenic activity, neurotransmitters release, anxiolytic and neurotoxic effects in Rat’s brain following repeated administration of novel psychoactive compound 25B-NBOMe. Neuropharmacology 240:109713. https://doi.org/10.1016/j.neuropharm.2023.109713
Wood DM, Sedefov R, Cunningham A, Dargan PI (2015) Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin Toxicol (Phila) 53(2):85–92. https://doi.org/10.3109/15563650.2015.1004179
Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR (2002) Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP 32 or inhibitor 1. Biol Psychiatry 51(8):612–620. https://doi.org/10.1016/s0006-3223(01)01318-x
Zawilska JB, Kacela M, Adamowicz P (2020) NBOMes-Highly potent and toxic alternatives of LSD. Front Neurosci 14:78. https://doi.org/10.3389/fnins.2020.00078
Acknowledgements
This work was supported by the National Research Foundation of Korea and funded by the Ministry of Education (2022R1A6A1A03054419) and the Korea Food and Drug Administration (22214MFDS251).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, YJ., Kook, WA., Ma, SX. et al. The novel psychoactive substance 25E-NBOMe induces reward-related behaviors via dopamine D1 receptor signaling in male rodents. Arch. Pharm. Res. 47, 360–376 (2024). https://doi.org/10.1007/s12272-024-01491-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-024-01491-4