Abstract
Foraging behavior is a species-specific behavior which is considered to involve the decision making and higher cognitive functions. We previously established a novel method to detect the foraging behavior in chronic unpredictable mild stress (CUMS)-induced depression mice, in which the food foraging activity of mice was significantly reduced. Furthermore, it is generally assumed that the bilateral anterior cingulate cortex (ACC) is related to foraging activity in rat. Brain-derived neurotrophic factor (BDNF) is widely expressed in many regions of the brain and is down-regulated in depressive patients. However, the relationship between the precursor of brain-derived neurotrophic factor (proBDNF) and depression has not been fully elucidated. The results showed that CUMS in mice induced anxiety- and depression-like behaviors and significant reduction in BDNF messenger RNA (mRNA) in the brain. In this study, we evaluated the effect of anti-BDNF and anti-proBDNF in the ACC on the CUMS-induced depression mice. In contrast to the normal IgG group (normal IgG microinjection into the ACC), bilateral ACC treatment with anti-proBDNF microinjection not only reversed depressive activity but also significantly increased the amount of foraged food and BDNF mRNA in the brain. There was no significant alteration in the group of anti-BDNF microinjection into the ACC. Our data indicate that the proBDNF signaling pathway might down-regulate the foraging activity in CUMS rodents and be involved in the depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is becoming an increasingly prevalent health problem and has consequential morbidity and mortality. Summary of all previous studies has indicated that depression is linked with cognitive decline and even with an increased risk of dementia in later life (Richard et al. 2013; Panza et al. 2010). Depressed patients exhibit extensively multiple dysfunctions in attention, spatial recognition memory, visual memory, verbal memory, planning, and executive functioning (Elliott et al. 1996; O’Brien et al. 2004). Studies have shown that food foraging behavior is associated with high cognitive functions, such as struggle in decision making (Li et al. 2012b). Optimal decision making requires that organisms correctly evaluate both the costs and benefits of potential choices (Day et al. 2010). We previously established a novel method to detect the foraging behavior in depression mice-induced chronic unpredictable mild stress (CUMS), in which the food foraging activity of mice was significantly reduced (Yang et al. 2014b), but the molecular mechanism underlying this behavior is not clear.
Previous articles indicate that the foraging behavior is related with coordination of multiple brain areas and neurochemical pathway (Bartness et al. 2011; Dailey and Bartness 2010). The anterior cingulate cortex (ACC), which is on the medial subregion of the frontal lobe, is part of a neural system that involves motivating or ‘energizing’ behavior and hierarchical reinforcement learning (Holroyd and Yeung 2012). The object-based and action-based reinforce devaluation tasks in rhesus monkeys suggest that ACC is essential for optimal decision making or reward to effective choice behavior and learning the value of actions (Kennerley et al. 2006; Chudasama et al. 2013). Our previous studies have also suggested that ACC lesion attenuates food foraging activity in rats (Li et al. 2012a). Early detection and treatment suggest that the thalamus and ACC may comprise neural markers for detecting depression. From structural neuroimaging research, gray matter volume in the ACC in depressive patients is smaller in comparison to healthy controls (Hagan et al. 2015). It is revealed that depression may be associated with structural and functional alterations in the ACC.
Mature brain-derived neurotrophic factor (BDNF) is an important member of the neurotophin family produced by glia and neurons (Murakami et al. 2005; Juric et al. 2006), which plays a positively significant role in brain function and development, such as neuronal survival, differentiation, and synaptic actions with distinct behavioral effects on memory, food intake and energy balance, and mood (Xu et al. 2003; Pang et al. 2004; Hariri et al. 2003). BDNF is synthesized as precursor of BDNF (proBDNF), which dimerize after translation. Mature BDNF is released from proBDNF, which is cleaved intracellularly by furin or/and prohormone convertases or extracellularly by plasmin and matrix metalloproteases (MMPs) (Mowla et al. 2001; Barker 2009). ProBDNF exists in numerous regions in the peripheral and central nervous system (Zhou et al. 2004), which induces apoptosis (Fan et al. 2008), synaptic retraction (Yang et al. 2014a), neurite collapse (Sun et al. 2012), and possibly depression (Jiang and Salton 2013) and inhibits proliferation and migration of cerebellar granule cells (Xu et al. 2011). We previously found that the injection of anti-proBDNF antibody via intracerebroventricular (i.c.v.) and intraperitoneal (i.p.) approaches into the CUMS-induced depression rats significantly reversed the stress-induced depression-like behavior and restored the exploratory activity and spine growth (Bai et al. 2016). Multiple studies have widely reported that mature BDNF is reduced in depression (Karege et al. 2002; Lee et al. 2007; Zhou et al. 2013; Zhang et al. 2016). Furthermore, its precursor proBDNF signaling is up-regulated in sera and plasma from depressed patients (Yoshida et al. 2012; Zhou et al. 2013).
Foraging activity is attenuated in CUMS-induced depression mice. As ACC is required for the foraging activities, we have investigated whether proBDNF in ACC is involved in depression-like behaviors including foraging activities. We examined whether depressive symptoms could be relieved by neutralization of proBDNF by proBDNF antibodies injected in ACC.
Materials and Methods
Animals
The study was carried out on 2-month-old male C57BL/6J mice (23–27 g) obtained from the Institute of Molecular and Clinical Medicine (IMCM) of Kun Ming Medical University (KMU), Kunming, China. Before experiments, all the animals were housed in groups of three to five per standard cage with food and water for at least 2 weeks before the test. The animals were maintained under standard laboratory conditions (12-h light/dark cycle, 22 ± 1 °C, 52 ± 2% humidity). All animal ethics protocols were approved by KMU and met the standards of the Guide for Care and Use of Laboratory Animals established by the National Institutes of Health, USA. All efforts were made to minimize the number of mice used and their suffering. For each treatment group in these series of experiments, 10–12 mice were used.
Surgical Procedure
Mice were anesthetized with an intraperitoneal injection of 2% chloral hydrate (40 mg/kg). A mouse was placed on a Mouse Stereotaxic Instrument and the head was fixed (Leica, Germany). Anti-BDNF, anti-proBDNF, and normal IgG (1 mg/ml) (Fan et al. 2008) were dissolved in sterile saline and adjusted to pH 7.2–7.4 by using 0.1 M NaOH. The solution was injected into the ACC of the rodents as reported previously (Li et al. 2012a). For lesion experiments, a 28-g injection cannula (Plastics One, Roanoke, VA) was introduced stereotaxically and directed towards the ACC using the coordinates obtained from the atlas Paxinos and Watson (fourth edition) from the bregma, AP+ 0.4 mm, ML ±0.5–1 mm, DV 1 mm and AP+ 0.4 mm, ML ±0.5–1 mm, and DV 1 mm from the dura. Bilateral infusions of 1-μl antibody or normal IgG were microinjected over a 1–2-min period. The cannula was left in place for another 2 min to allow for complete diffusion after which the wound was sutured. Immediately after the surgery was completed, mice were placed in a warm environment until they regained consciousness. Mice were then allowed to recover in their home cages.
CUMS Procedure
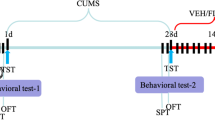
The CUMS procedure was performed as previously described, with a slight modification. Initially, animals were socially housed (less than five animals per cage) for 1 week to familiarize with the circumstances. After that, the animals were randomly grouped and singly housed. Twelve mild stressors were applied in the CUMS procedure (Fig. 1), which included cage shaking (1 time/s, 5 min) (CS), cage tilt 45 °C (8 h) (CT), cold swim (13 ± 1 °C, 5 min) (C), food and water deprivation (24 h) (F), tail pinch (for 60 s, 1 cm from the end of the tail) (T), moist bedding (8 h) (M), warm swim (37 ± 2 °C, 5 min) (W), overnight illumination (12 h) (O), tail pinch (for 90 s) (T 1), no stress (N), reverse day and night (24 h) (R), and tail pinch (for 120 s) (T 2). One of the stressors was randomly applied daily for the CUMS group(s), while the control mice were uninterrupted unless some necessary procedures such as routine cage cleaning. The stressing period changed according to different purpose in this paper. It took 4 weeks to develop a CUMS mouse depression model.
The time schedule of experimental design. a A timeline of CUMS protocol. CS cage shaking, CT cage tilt (45°), C cold swim, F food and water deprivations, T tail pinch (for 60 s), M moist bedding, W warm swim, O overnight illumination, T 1 tail pinch (for 90 s), N no stress, R reverse day and night, T 2 tail pinch (for 120 s). b A timeline of brain injection and behavioral testing. ACC anterior cingulate cortex
Behavioral Tests
CUMS-induced animals were subjected to behavioral tests before surgery and commenced 2 weeks after ACC lesion surgery (Murakami et al. 2005). The test room was illuminated with incandescent lamp (40 W) when the curtain was closed. Testing apparatuses were cleaned with ethanol between each test.
Sucrose Preference Test
According to our previous study (Yang et al. 2014b), sucrose preference test is used to assess the declining responses to incentive stimuli, which is one of the key symptoms of clinical depressive disorder. Sucrose preference test (SPT) was used to test the depression-like behavior in this paper. Before testing, two bottles of 1% (v/v) sucrose solution were placed on the cage for 48 h, in order to train the animals habituating to sucrose solution. In the next 24 h, food and water were deprived from the animals. After that, the right bottles were replaced with tap water and allow freely drink for 10 h, starting from 8:30 AM. The consumed liquids were weight by an electronic balance. The sucrose intake (%) was calculated by the formula (sucrose intake / (sucrose intake + water intake) × 100).
Open-Field Test
The apparatus and testing procedures were similar to those used previously reported method (Walsh and Cummins 1976; Blokland et al. 2002). Briefly, motor activity was quantified in a white square cage with 50 × 50 × 50 cm3. The apparatus was divided into 5 × 5-cm equal squares on the floor of the arena. Each experimental animal was placed in the center of the cage; the number of crossings (with the four paws), the number of rearings (posture sustained with hind paws on the floor), and the immobility time were recorded manually for 5 min according to recent studies (Shen and Xu 2007; Sartori Oliveira et al. 2012; Swiergiel and Dunn 2007). All behaviors were recorded with a video camera located 100–120 cm above the arena. After each test, the arena was cleaned with 75% alcohol solution.
Elevated Zero Maze Test
Elevated zero maze (Shanghai Bio-will, China; height 72.4 cm; inner diameter 40 cm) was used to evaluate the anxiety-like behavior, which is a modification of classic elevated plus maze. There are two elevated closed and open runways without a center position. The animal was placed just inside a closed arm, with nose pointing and all four paws inside. Five-minute spontaneous activity was allowed for each animal. Time in open arm was recorded by an overhead video camera and analyzed by ANY-maze software. After each test, the device was cleaned with 75% ethanol to remove the animal excretions and odor.
Tail Suspension Test
The tail suspension test reflects behavioral despair, which is used as a measured behavioral variable (Pollak et al. 2010). Mice were tested in an individual cubicle while suspended from a tail hanger with adhesive tape wrapped around the tail (1.5–2 cm from the tip), 35 cm above the floor (Svenningsson et al. 2007; Kutiyanawalla et al. 2011). The trial was performed for a period of 6 min, and the duration of immobility was manually measured. Mice were considered immobile when they hung passively and motionless.
Food Foraging Test
The food foraging test was performed as described by Li (Li et al. 2012b). Food foraging behavior involves searching for food as well as transporting and hoarding food. Many studies suggest that foraging behavior involves higher cognitive function such as effort-based decision making. Briefly, the test was performed in an open field, which was constructed with a white square cage 50 × 50 × 50 cm. Before the test, the mice were deprived of food for 12 h from 7:00 AM to 7:00 PM. The mice were individually allowed to navigate in the white square cage and forage for food freely from 7:00 PM to 7:00 AM on the same day. The food container was placed in the central edge of the arena and there was no illumination. There was also no nest box in the arena. Since food pellets are too heavy for a mouse to carry, 60-g sunflower seeds were used. The following day, the amount of sunflower seeds moved to the open field and the amount left in the food containers were separately calculated throw off the peel at 7:00 AM.
RNA Isolation and qRT-PCR
Total RNA of the entire cortex and the hippocampus isolated from the animals were extracted by TRIzol® reagent (Sigma), following the manufacturer’s recommendation. The RNA samples were dissolved in 30 μl of DEPC-H2O and subsequently stored at −80 °C. The quantification and purity of the RNA were measured by using a NanoDrop 2000 Spectrophotometer (Thermo-Fisher Scientific, CA, USA). The quality of RNA was assessed by the absorbance at 260 and 280 nm, and a ratio of A260/A280 ranging from 1.9 to 2.1 was considered acceptable. Total RNA was transcribed by a High-Capacity cDNA Reverse Transcription Kit (DRR037A; TaKaRa, Japan) according to the manufacturer’s protocol. Real-time PCR was performed on the ABI7300 platform (Applied Biosystems, Life Technologies, USA) by using FastStart Universal SYBR Green Master (ROX) (Roche). Primers utilized were as follows: BDNF forward primer 5′-GCAGCCTTCTTTTGTGTAACC-3′ and reverse primer 5′-AGAGTG ATGACCATCCTTTTC-3′ and β-actin forward primer 5′-AGCCATGTACGTAGCCATCCA-3′ and reverse primer 5′-TCTCCGGAGTCCATCACAATG-3′; primer specificity was confirmed to be a single band of the predicted size by melting curve analysis and electrophoresis of PCR products on a 2% agarose gel. The relative expression of target gene messenger RNA (mRNA) was normalized to the amount of housekeeping gene β-actin in the same cDNA of animals from the different treatment groups compared with control group or CUMS group by using the relative quantification method (2−△△CT method) described by the manufacturer.
Statistical Analysis
Data were presented as mean ± SEM and analyzed by SPSS 11.0. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by post hoc Dunnett testing or Tukey post hoc multiple comparison test which were appropriate for detecting intergroup difference. Unpaired two-tailed Student’s t test was used if only two groups were applied. P < 0.05 was considered statistically significant.
Results
Anti-proBDNF Treatment in ACC Reverses CUMS-Induced Decrease of Locomotor Activities in Mice
Anti-BDNF, anti-proBDNF, and normal IgG antibody (1 mg/ml) were dissolved in sterile saline and adjusted to pH 7.2–7.4 by using 0.1 M NaOH. The solution was microinjected into the bilateral ACC region of the rodents under 4-week CUMS treatment. CUMS-induced depression model treated with normal IgG was used as a vehicle control group. The CUMS-induced mice before surgery showed no significant differences in behavioral tests.
CUMS-induced depression model that is a crucial research tool to investigate depression, which is regarded as being close to the unexpected stressor of everyday life in humans, can be reproduced in this study. A 4-week period of CUMS exposure resulted in a typical reduction in the number of crossings (***p < 0.001; Fig. 2a), rearings (***p < 0.001; Fig. 2b), and the mobility time (***p < 0.001; Fig. 2c) in mice, as compared to the controls. Blocking BDNF and proBDNF with antibodies in ACC region were tested for open-field test (OFT) in CUMS model. Bilateral microinjections of anti-proBDNF into the ACC partly improved the development of locomotor activity and exploratory in the OFT. As shown in Fig. 2d–f, the number of rearings in OFT significantly decreased in anti-BDNF-treated CUMS mice compared with normal IgG group (*p < 0.05; Fig. 2e). However, no significant difference was found in the number of crossings (Fig. 2d) and rearings (Fig. 2e) in OFT when anti-proBDNF was compared to normal IgG group. Anti-proBDNF treatment obviously increased the average mobility time in OFT (*p < 0.05; Fig. 2f) and exercise capacity which is similar to normal rodents. Perhaps the exploratory activity was mediated partly through BDNF in the ACC region of CUMS-induced mice.
The effects of anti-proBDNF and anti-BDNF treatments by ACC injection in CUMS-induced decreases of mouse locomotor activities. CUMS decreased locomotor activities in mice as shown by decreased crossing numbers (a), rearing numbers (b), and percentage of mobile time (c) in OFT. Treatment of anti-proBDNF in ACC of CUMS mice significantly increased mobile time (f) but not crossing and rearing activities (d, e), while treatment of anti-BDNF significantly decreased rearing activity (e) but not crossings (d) and mobility (f). ACC anterior cingulate cortex, CUMS chronic unpredictable mild stress, OFT open-field test; *p < 0.05, ***p < 0.001 (unpaired Student’s t test or one-way ANOVA). All data are presented as mean ± SEM
Anti-proBDNF Treatment in ACC Reverses CUMS-Induced Anxiety-Like Behavior in Mice
The percentage of the time spent in the open/closed arms in elevated zero maze test (EOM) (***p < 0.001; Fig. 3a) decreased significantly. This demonstrated that anxiety-like behavior was increased after 4-week CUMS stimuli. And the change was significantly reversed in anti-proBDNF-treated CUMS mice (*p < 0.05; Fig. 3b).
The effects of anti-proBDNF and anti-BDNF treatments by ACC injection in CUMS-induced mouse anxiety-like behavior. CUMS increased anxiety-like behavior in mice as shown by decrease in percentage of time spent in open vs closed zone in EOM (a). Treatment of anti-proBDNF in ACC of CUMS mice significantly decreased anxiety-like behavior as displayed by increase in percentage of time spent in open vs closed zone (b), whereas no significantly different effect was found by anti-BDNF treatment (b). ACC anterior cingulate cortex, CUMS chronic unpredictable mild stress, EOM elevated zero maze test; *p < 0.05, ***p < 0.001 (unpaired Student’s t test or one-way ANOVA). All data are presented as mean ± SEM
Anti-proBDNF Treatment in ACC Reverses CUMS-Induced Depression-Like Behavior in Mice
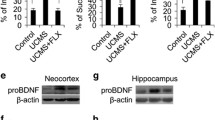
There was a time-dependent change in depression-like behaviors, such as anhedonia and increased despair. The depression-like behaviors increased with a time-dependent manner under CUMS exposure. As shown in Fig. 4, the percentage of sucrose consumption (***p < 0.001; Fig. 4a) gradually decreased and immobility in tail suspension test (TST) (***p < 0.001; Fig. 4c) significantly increased at week 3 and week 4, while the changes seem to decelerate at week 5. So, the series of results suggest that the model induction with the 4-week CUMS protocol was successful. The percentage of sucrose intake in SPT decreased significantly in CUMS mice (***p < 0.001; Fig. 4b), while the time of immobility in TST (***p < 0.001; Fig. 4d) significantly increased after 4-week CUMS treatment. The depression-like behaviors were significantly increased in the CUMS mouse model. As shown in Fig. 4e, f, the percentage of sucrose intake significantly increased in anti-proBDNF-microinjected CUMS mice (*p < 0.05; Fig. 4e), while no statistical effect was observed in anti-BDNF-treated CUMS mice (slightly decreased by 10%) compared with normal IgG group. The results suggest that bilateral microinjections of anti-proBDNF into the ACC relieve CUMS-induced anhedonia. The percentage of immobility in TST significantly increased in CUMS mice (***p < 0.001; Fig. 4d), and the change was also significantly reversed in anti-proBDNF-treated CUMS mice (*p < 0.05; Fig. 4f).
The effects of anti-proBDNF and anti-BDNF treatments by ACC injection in CUMS-induced mouse depression-like behaviors. CUMS decreased depression-like behaviors in mice as shown by time-dependent decrease in percentage of sucrose preference in SPT (a, b) and increase in immobile time in TST (c, d). Treatment of anti-proBDNF in ACC of CUMS mice significantly decreased depression-like behaviors as shown in increase in percentage of sucrose preference in SPT (e) and decrease in immobile time in TST (f), whereas no significantly different effect was observed by anti-BDNF treatment (e, f). ACC anterior cingulate cortex, CUMS chronic unpredictable mild stress, SPT sucrose preference test, TST tail suspension test; *p < 0.05, ***p < 0.001 (unpaired Student’s t test or one-way ANOVA). All data are presented as mean ± SEM
Anti-proBDNF Treatment in ACC Reverses CUMS-Induced Decrease of Foraging Behavior in Mice
The foraging activity is based on the natural foraging and hoarding behavior by rodents. It includes searching for food as well as transporting, hoarding, and competitively snatching food from other animals, which involves high cognitive functions such as effort-based decision making. We examined food foraging activity in depressed mice and the controls. The amount of transported food (**p < 0.01; Fig. 5a) and consumed food (**p < 0.01; Fig. 5b) were significantly reduced after 4-week CUMS stimuli, compared with the controls. After blocking, BDNF and proBDNF with antibodies in ACC region were tested for a food foraging behavior in CUMS model. The normal IgG group carried sunflower seeds from the food container to the field and gathered sunflower seeds into piles in the open field (Fig. 5c). The amount of foraged food was slightly decreased 0.6 g in normal IgG group when compared to CUMS group. No difference was found in the foraging test between anti-BDNF treatment and normal IgG group (Fig. 5c). Interestingly, the foraging activities were recorded, increased more than double in anti-proBDNF treatment compared with normal IgG group (*p < 0.05; Fig. 5c). The amount of food consumed was decreased in CUMS group (**p < 0.01; Fig. 5b). There was a significant difference in the amount of food consumed over the testing period between anti-proBDNF treatment compared with normal IgG group (**p < 0.01; Fig. 5d).
The effects of anti-proBDNF and anti-BDNF treatments by ACC injection in CUMS-induced decrease of mouse foraging behavior. CUMS decreased foraging behaviors in mice as displayed by decreases in foraged food (a) and amount of consumed food in FFT (b); brain injection of anti-proBDNF in ACC of CUMS mice significantly increased mouse foraging behaviors as shown by increases in foraged food (c) and amount of consumed food (d), whereas no significantly different effect was observed with AAC treatment of anti-BDNF in CUMS mice (c, d). ACC anterior cingulate cortex, CUMS chronic unpredictable mild stress, FFT foraging food test; *p < 0.05, **p < 0.01 (unpaired Student’s t test or one-way ANOVA). All data are presented as mean ± SEM
Anti-proBDNF Treatment in ACC Restores CUMS-Induced Decrease of BDNF mRNA Levels in Cortex and Hippocampus
To investigate the expression of BDNF in depression, 4-week CUMS mouse model was used to collect brain samples for real-time PCR. The statistical results demonstrate that the relative expression of BDNF mRNA in cortex (**p < 0.01; Fig. 6a) and hippocampus (*p < 0.05; Fig. 6b) both did significantly change between CUMS mice and control mice. To confirm the role of BDNF and proBDNF in the development of depression, blocking BDNF and proBDNF antibody in ACC region was microinjected in cortex of 4-week CUMS mice. As shown in Fig. 6, BDNF mRNA slightly down-regulated in the blocking BDNF antibody-treated CUMS mice compared with the normal IgG-treated CUMS mice (p > 0.05; Fig. 6a, b). However, BDNF mRNA was relatively down-regulated in the cortex and hippocampus of CUMS mice, while this down-regulation effect was prevented and even obviously up-regulated in the blocking proBDNF antibody-treated CUMS mice (**p < 0.01; Fig. 6a, b). Especially, BDNF mRNA increased nearly fourfold in the hippocampus of anti-proBDNF treatment compared with normal IgG group. It seems that the down-regulation of BDNF in the cortex and hippocampus of CUMS mice could be reversed by treating with blocking proBDNF antibody in ACC region.
The mRNA levels of BDNF in cortex and hippocampus of CUMS mice treated with anti-proBDNF and anti-BDNF in ACC. CUMS in mice decreased the mRNA levels of BDNF in cortex (a) and hippocampus (b), while anti-proBDNF treatment in ACC restored the CUMS-induced decreases in BDNF levels in cortex (a) and hippocampus (b); no significant difference was found after anti-BDNF treatment. mRNA levels were measured by real-time PCR; β-actin was measured as loading control. ACC anterior cingulate cortex, CUMS chronic unpredictable mild stress; **p < 0.01 (one-way ANOVA). All data are presented as mean ± SEM
Discussion
In the present study, using a CUMS-induced mouse model, we successfully tested our hypothesis that proBDNF in ACC affects the foraging activity and depression-like behaviors. Foraging activity is reduced in a mouse model of depression, confirming our previous finding (Yang et al. 2014b). Moreover, in our earlier study, the foraging behavior can also be reversed by clozapine treatment, an anti-depressant, which mainly blocks receptors in the brain for several neurotransmitters including 5-HT, D2, D4, and NMDA receptors (Yang et al. 2014b). ACC plays a critical role in the food foraging paradigm related with an effort-related event under natural environment (Li et al. 2012a). A series of previous studies have demonstrated that surgical incision induces an anxiety-like behavior that is tightly linked to the ACC (Dai et al. 2011; Zhong et al. 2012). In our experiment, we speculate that ACC region is involved in the depression and anti-proBDNF microinjection into the ACC may be important in depression as well as the natural food foraging behavior. In addition, we also found that down-regulation of BDNF mRNA under CUMS can also be reversed by anti-proBDNF, which can improve the decreased adult hippocampal neurogenesis under stress.
Chronic stress to animals was reported to closely mimic the unexpected stressors that humans experience on a daily basis (Bambico and Belzung 2013; Patricio et al. 2013). CUMS-induced depression model is a crucial research tool to investigate depression, which can be reproduced in our study. In the present study, CUMS model mice exhibited a time-dependent change in depression- and anxiety-like behaviors, such as anhedonia, reduced motor activity, increased anxiety, and despair. And expression of BDNF mRNA is down-regulated in cortex and hippocampus by CUMS. The reduction in BDNF mRNA expression in the brain in our study further supports that our model development is successful. These findings are consistent with those analogous rodent depression model (Ruan et al. 2014, 2015; Su et al. 2016). Besides the development of depressive- and anxiety-like behaviors including anhedonia and reduction in locomotion (Kong et al. 2014), rodents also enhanced aggressive behavior (Yang et al. 2015) and reduction in higher cognitive function such as foraging (Cheng et al. 2013). Recently, a simple method, food foraging test, has been developed to detect higher cognitive functions and decision making (Li et al. 2012a, b). The foraging activities of CUMS-exposed mice were dramatically reduced, and food consumption also was decreased. Based on the preceding studies (Holec et al. 2014; Li et al. 2012a), ACC damage caused significant decrease in the food foraging behavior, suggesting that ACC is implicated in guiding effort-reward decision making. So, we further explored ACC and examined how it plays an important role in the foraging behavior and depression of CUMS-exposed mice.
Despite ACC-mediated, effort-based decision making is reported, the role of the ACC in guiding emotion is controversial (Rudebeck et al. 2008). The rostral anterior cingulate cortex influenced depression but not anxiety-related behavior in the rat (Bissiere et al. 2006). Our results demonstrated that, in a CUMS mouse depression model, we found that in contrast to the normal IgG group (normal IgG microinjection into the ACC), bilateral ACC treatment with anti-proBDNF microinjection not only reversed the depressive activity but also significantly increased the amount of foraged food. There is no obvious change in these behaviors in the group of anti-BDNF microinjection into the ACC. Anti-proBDNF can improve depression-like behavior of mice as displayed by increase sucrose intake, the decreased immobility time, and the increased climbing numbers in TST. Anti-proBDNF also changed the anxiety-like behavior of mice as displayed by increased mobility time in OFT and in the open arm of EOM. These results suggest that proBDNF in ACC enhances depression-like and anxiety-like behaviors in mice under CUMS. These results also indicate that proBDNF may be important for the maintenance of normal mood, in particular for the balancing animals from euphoretic mood. This result is consistent with our previous report that proBDNF and its receptors p75 and sortilin are up-regulated in patients with major depression (Zhou et al. 2013). It is well known that proBDNF plays opposing roles to that of mature BDNF. ProBDNF suppresses the proliferation and migration of developing cerebellar granule cells (Xu et al. 2011), inhibits neurite growth of sensory and cortical neurons (Sun et al. 2012), and triggers long-term depression (Woo et al. 2005). In addition, our results showed that ACC modulated not only depression but also anxiety, which is inconsistent with the study of Bissiere et al. (2006). Furthermore, in our experiment, anti-proBDNF caused significantly increased foraging food and consumption of food, suggesting that proBDNF normally inhibits the effort-related behavior and possibly suppresses the food intake. Our data also suggests that the proBDNF signaling pathway in the ACC suppresses the foraging activity in CUMS rodents. Furthermore, our data suggests that proBDNF in the ACC promotes the depression-like behaviors.
In humans, multiple studies have widely reported that mature BDNF mRNA and protein are reduced in the cortex and hippocampus in depression (Karege et al. 2002; Lee et al. 2007). Our data show that BDNF mRNA was relatively down-regulated in the cortex and hippocampus of CUMS mice, while this down-regulation effect was prevented and even obviously up-regulated in the blocking proBDNF antibody-treated CUMS mice. In animals, decreased BDNF serum levels result in memory deficit and suppressed the food intake; thus, increasing BDNF availability in the brain is a viable strategy to counteract cognitive decline and food consumption with depression-like behaviors (Macedo et al. 2015). Consistent with the results from anti-BDNF treatment, we found that depression- and anxiety-like behaviors are even more serious than before injection. Furthermore, our result is consistent with the report showing that decreased BDNF suppress food consumption (Beilharz et al. 2015; Macedo et al. 2015). Nevertheless, its precursor proBDNF signaling is up-regulated in sera and plasma from depressed patients (Yoshida et al. 2012; Zhou et al. 2013). More recent studies have suggested that levels of proBDNF were higher in the medial prefrontal cortex in a learned helplessness model of depression (Shirayama et al. 2015). An imbalance between levels of BDNF and proBDNF in ACC might contribute to neurocircuitry of depression. Thus, modification of the proBDNF signaling pathway or the inhibition of proBDNF might have a therapeutic significance in depression.
In conclusion, our results indicate that the proBDNF signaling pathway in ACC inhibits the foraging activity in CUMS rodents and promotes the depression-like behaviors. Anti-proBDNF treatment may have the therapeutic significance for major depression. In light of the complexity of ACC functions, the mechanism of the foraging behavior and the relation with depression should be further studied.
References
Bai YY, Ruan CS, Yang CR, Li JY, Kang ZL, Zhou L, Liu D, Zeng YQ, Wang TH, Tian CF, Liao H, Bobrovskaya L, Zhou XF (2016) ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology
Bambico FR, Belzung C (2013) Novel insights into depression and antidepressants: a synergy between synaptogenesis and neurogenesis? Curr Top Behav Neurosci 15:243–291
Barker PA (2009) Whither proBDNF? Nat Neurosci 12(2):105–106
Bartness TJ, Keen-Rhinehart E, Dailey MJ, Teubner BJ (2011) Neural and hormonal control of food hoarding. Am J Physiol Regul Integr Comp Physiol 301(3):R641–R655
Beilharz JE, Maniam J, Morris MJ (2015) Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients 7(8):6719–6738
Bissiere S, McAllister KH, Olpe HR, Cryan JF (2006) The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav Brain Res 175(1):195–199
Blokland A, Lieben C, Deutz NE (2002) Anxiogenic and depressive-like effects, but no cognitive deficits, after repeated moderate tryptophan depletion in the rat. J Psychopharmacol 16(1):39–49
Cheng Y, Su Q, Shao B, Cheng J, Wang H, Wang L, Lin Z, Ruan L, ZhuGe Q, Jin K (2013) 17 beta-estradiol attenuates poststroke depression and increases neurogenesis in female ovariectomized rats. Biomed Res Int 2013:392434
Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH, Murray EA (2013) The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cereb Cortex 23(12):2884–2898
Dai RP, Li CQ, Zhang JW, Li F, Shi XD, Zhang JY, Zhou XF (2011) Biphasic activation of extracellular signal-regulated kinase in anterior cingulate cortex distinctly regulates the development of pain-related anxiety and mechanical hypersensitivity in rats after incision. Anesthesiology 115(3):604–613
Dailey MJ, Bartness TJ (2010) Arcuate nucleus destruction does not block food deprivation-induced increases in food foraging and hoarding. Brain Res 1323:94–108
Day JJ, Jones JL, Wightman RM, Carelli RM (2010) Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry 68(3):306–309
Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES (1996) Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med 26(5):975–989
Fan YJ, Wu LL, Li HY, Wang YJ, Zhou XF (2008) Differential effects of pro-BDNF on sensory neurons after sciatic nerve transection in neonatal rats. Eur J Neurosci 27(9):2380–2390
Hagan CC, Graham JM, Tait R, Widmer B, van Nieuwenhuizen AO, Ooi C, Whitaker KJ, Simas T, Bullmore ET, Lennox BR, Sahakian BJ, Goodyer IM, Suckling J (2015) Adolescents with current major depressive disorder show dissimilar patterns of age-related differences in ACC and thalamus. Neuroimage Clin 7:391–399
Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23(17):6690–6694
Holec V, Pirot HL, Euston DR (2014) Not all effort is equal: the role of the anterior cingulate cortex in different forms of effort-reward decisions. Front Behav Neurosci 8:12
Holroyd CB, Yeung N (2012) Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci 16(2):122–128
Jiang C, Salton SR (2013) The role of neurotrophins in major depressive disorder. Transl Neurosci 4(1):46–58
Juric DM, Miklic S, Carman-Krzan M (2006) Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res 1108(1):54–62
Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM (2002) Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 109(2):143–148
Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF (2006) Optimal decision making and the anterior cingulate cortex. Nat Neurosci 9(7):940–947
Kong H, Zeng XN, Fan Y, Yuan ST, Ge S, Xie WP, Wang H, Hu G (2014) Aquaporin-4 knockout exacerbates corticosterone-induced depression by inhibiting astrocyte function and hippocampal neurogenesis. CNS Neurosci Ther 20(5):391–402
Kutiyanawalla A, Terry AV Jr, Pillai A (2011) Cysteamine attenuates the decreases in TrkB protein levels and the anxiety/depression-like behaviors in mice induced by corticosterone treatment. PLoS One 6(10):e26153
Lee BH, Kim H, Park SH, Kim YK (2007) Decreased plasma BDNF level in depressive patients. J Affect Disord 101(1–3):239–244
Li F, Li M, Cao W, Xu Y, Luo Y, Zhong X, Zhang J, Dai R, Zhou XF, Li Z, Li C (2012a) Anterior cingulate cortical lesion attenuates food foraging in rats. Brain Res Bull 88(6):602–608
Li F, Cao WY, Li MB, Xu Y, Zhang JW, Zhang JY, Luo XG, Dai RP, Zhou XF, Li CQ (2012b) A simple method for detection of food foraging behavior in the rat: involvement of NMDA and dopamine receptors in the behavior. Neuroscience 205:73–80
Macedo IC, Rozisky JR, Oliveira C, Oliveira CM, Laste G, Nonose Y, Santos VS, Marques PR, Ribeiro MF, Caumo W, Torres IL (2015) Chronic stress associated with hypercaloric diet changes the hippocampal BDNF levels in male Wistar rats. Neuropeptides 51:75–81
Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276(16):12660–12666
Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E (2005) Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res 53(2):129–139
O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N (2004) A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 161(11):2081–2090
Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306(5695):487–491
Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V (2010) Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry 18(2):98–116
Patricio P, Mateus-Pinheiro A, Sousa N, Pinto L (2013) Re-cycling paradigms: cell cycle regulation in adult hippocampal neurogenesis and implications for depression. Mol Neurobiol 48(1):84–96
Pollak DD, Rey CE, Monje FJ (2010) Rodent models in depression research: classical strategies and new directions. Ann Med 42(4):252–264
Richard E, Reitz C, Honig LH, Schupf N, Tang MX, Manly JJ, Mayeux R, Devanand D, Luchsinger JA (2013) Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol 70(3):374–382
Ruan CS, Wang SF, Shen YJ, Guo Y, Yang CR, Zhou FH, Tan LT, Zhou L, Liu JJ, Wang WY, Xiao ZC, Zhou XF (2014) Deletion of TRIM32 protects mice from anxiety- and depression-like behaviors under mild stress. Eur J Neurosci 40(4):2680–2690
Ruan CS, Zhou FH, He ZY, Wang SF, Yang CR, Shen YJ, Guo Y, Zhao HB, Chen L, Liu D, Liu J, Baune BT, Xiao ZC, Zhou XF (2015) Mice deficient for wild-type p53-induced phosphatase 1 display elevated anxiety- and depression-like behaviors. Neuroscience 293:12–22
Rudebeck PH, Bannerman DM, Rushworth MF (2008) The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci 8(4):485–497
Sartori Oliveira CE, Gai BM, Godoi B, Zeni G, Nogueira CW (2012) The antidepressant-like action of a simple selenium-containing molecule, methyl phenyl selenide, in mice. Eur J Pharmacol 690(1–3):119–123
Shen X, Xu GZ (2007) Effect of interleukin-1 beta on glutamine synthetase in rat retinal Müller cell under high glucose conditions. Zhonghua Yan Ke Za Zhi 43(8):744–749
Shirayama Y, Yang C, Zhang JC, Ren Q, Yao W, Hashimoto K (2015) Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur Neuropsychopharmacol
Su CL, Su CW, Hsiao YH, Gean PW (2016) Epigenetic regulation of BDNF in the learned helplessness-induced animal model of depression. J Psychiatr Res 76:101–110
Sun Y, Lim Y, Li F, Liu S, Lu JJ, Haberberger R, Zhong JH, Zhou XF (2012) ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One 7(4):e35883
Svenningsson P, Tzavara ET, Qi H, Carruthers R, Witkin JM, Nomikos GG, Greengard P (2007) Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci 27(15):4201–4209
Swiergiel AH, Dunn AJ (2007) Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav 86(4):651–659
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83(3):482–504
Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B (2005) Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci 8(8):1069–1077
Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF (2003) Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6(7):736–742
Xu ZQ, Sun Y, Li HY, Lim Y, Zhong JH, Zhou XF (2011) Endogenous proBDNF is a negative regulator of migration of cerebellar granule cells in neonatal mice. Eur J Neurosci 33(8):1376–1384
Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL (2014a) proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep 7(3):796–806
Yang CR, Zhang ZG, Bai YY, Zhou HF, Zhou L, Ruan CS, Li F, Li CQ, Zheng HY, Shen LJ, Zhou XF (2014b) Foraging activity is reduced in a mouse model of depression. Neurotox Res 25(3):235–247
Yang CR, Bai YY, Ruan CS, Zhou HF, Liu D, Wang XF, Shen LJ, Zheng HY, Zhou XF (2015) Enhanced aggressive behaviour in a mouse model of depression. Neurotox Res 27(2):129–142
Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K (2012) Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One 7(8):e42676
Zhang F, Luo J, Min S, Ren L, Qin P (2016) Propofol alleviates electroconvulsive shock-induced memory impairment by modulating proBDNF/mBDNF ratio in depressive rats. Brain Res
Zhong XL, Wei R, Zhou P, Luo YW, Wang XQ, Duan J, Bi FF, Zhang JY, Li CQ, Dai RP, Li F (2012) Activation of anterior cingulate cortex extracellular signal-regulated kinase-1 and -2 (ERK1/2) regulates acetic acid-induced, pain-related anxiety in adult female mice. Acta Histochem Cytochem 45(4):219–225
Zhou XF, Song XY, Zhong JH, Barati S, Zhou FH, Johnson SM (2004) Distribution and localization of pro-brain-derived neurotrophic factor-like immunoreactivity in the peripheral and central nervous system of the adult rat. J Neurochem 91(3):704–715
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF (2013) Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord 150(3):776–784
Acknowledgements
The animal experimental work was done in Kunming Medical University (KMU) and supported by a MOST grant 2011CB944200 and NHMRC grants APP1021408 and APP1021409. X.F.Z. is a visiting professor of KMU. F.H. Zhou was supported by NHMRC overseas bio-medical training fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal ethics protocols were approved by KMU and met the standards of the Guide for Care and Use of Laboratory Animals established by the National Institutes of Health, USA.
Rights and permissions
About this article
Cite this article
Yang, C.R., Bai, Y.Y., Ruan, C.S. et al. Injection of Anti-proBDNF in Anterior Cingulate Cortex (ACC) Reverses Chronic Stress-Induced Adverse Mood Behaviors in Mice. Neurotox Res 31, 298–308 (2017). https://doi.org/10.1007/s12640-016-9687-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9687-4