Abstract
Depression is a worldwide problem with a great social and economic burden in many countries. In our previous research, we found that the expression of proBDNF/p75NTR/sortilin is upregulated in patients with major depressive disorder. In addition, the treatment of proBDNF antibodies reversed both the depressive behaviors and the reduced BDNF mRNA detected in our rodent chronic stress models. Antidepressant drugs are usually only effective in a subpopulation of patients with major depression with a delayed time window of 2–4 weeks to exert their efficacy. The mechanism underlying such delayed response is not known. In this study, we hypothesize that antidepressant drugs exert their therapeutic effect by modulating proBDNF/p75NTR and mature BDNF/TrkB signaling pathways. To test the hypothesis, C57 mice were randomly divided into normal control, chronic unpredictable mild stress (CUMS), vehicle (VEH), fluoxetine (FLU), and clozapine (CLO) groups. Behavioral tests (sucrose preference, open field, and tail suspension tests) were performed before and after 4 weeks of CUMS. The gene and protein expression of proBDNF, the neurotrophin receptor (p75NTR), sortilin, and TrkB in the cortex and hippocampus were examined. At the protein level, CUMS induced a significant increase in proBDNF, p75NTR, and sortilin production while the TrkB protein level was found to be lower in the cortex and hippocampus compared with the control group. Consistently, at the mRNA level, p75NTR expression increased with reduced BDNF/TrkB mRNA in both cortex and hippocampus, while sortilin increased only in the hippocampus after CUMS. FLU and CLO treatments of CUMS mice reversed all protein and mRNA expression of the biomarkers in both cortex and hippocampus, except for sortilin mRNA in the cortex and proBDNF in the hippocampus, respectively. This study further confirms that the imbalance between proBDNF/p75NTR/sortilin and mBDNF/TrkB production is important in the pathogenesis of depression. It is likely that antidepressant FLU and antipsychotic CLO exert their antidepressant-like effect correcting the imbalance between proBDNF/p75NTR/sortilin and mBDNF/TrkB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a recurrent common mental disorder, which is not only costly but also difficult to understand. According to the World Health Organization, depression is the single largest contributor to non-fatal health loss worldwide, and it accounts for 7.5% of global years lived with disability (YLD) (Bert et al. 2017). One of the major risks of depression is suicide, which has been reportedly linked to psychiatric disorders in general and to inadequate drug treatment (Oved et al. 2017). Presently, the selective serotonin uptake inhibitor (SSRI) anti-depressant drugs, which block 5-HT reuptake via binding directly to the serotonin transporter (SERT), are the most commonly employed first line of treatment for depression (Thaler et al. 2012). Although several antidepressant drugs are currently available, approximately, only 50–60% of depressed patients can reach sufficient remission after an adequate treatment of 3 weeks (Souery et al. 2007). The remission usually occurs only 2–4 weeks after the initiation of treatment and is not consistent with the increase in serotonin in the brain which occurs immediately after SSRI (Bymaster et al. 2002). The mechanism for how these drugs work may not be entirely due to the inhibition of neurotransmitter reuptake. There must be another mechanism which is causing this inconsistency between the delayed efficacy and the level of neurotransmitters in the brain. Previous studies have shown that these antidepressants can increase neurogenesis of depressive-like animals under chronic stress (Segi-Nishida 2017; Gundersen et al. 2013). Thus, exploring the mechanisms of antidepressants is essential for developing novel and more effective intervention strategies.
A number of studies have demonstrated a role of brain-derived neurotrophic factor (BDNF) and its precursor molecule (proBDNF) in the pathogenesis of depression (Furuse et al. 2019; Jin et al. 2017; Luo et al. 2019; Bai et al. 2016). BDNF, a member of the neurotrophin family, is synthesized as proBDNF, which is enzymatically processed to mature BDNF (mBDNF) and BDNF prodomain. ProBDNF and mBDNF play opposite roles in neuronal remodeling, synaptic transmission, and synaptic plasticity (Fan et al. 2008; Shen et al. 2018; Xu et al. 2011; Sun et al. 2012). ProBDNF elicits opposing effects to that of mBDNF via binding p75NTR and co-receptor sortilin. There is accumulating evidence showing that the balance between proBDNF and mBDNF plays pivotal roles in several physiological functions (Yoshida et al. 2012; Teng et al. 2005; Xu et al. 2011; Sun et al. 2012). The BDNF/TrkB signaling pathway mediates neuronal survival and growth, while the proBDNF/p75NTR/sortilin signaling pathway can induce neuronal apoptosis (Fan et al. 2008), neurite collapse (Sun et al. 2012), and inhibition of neurogenesis (Xu et al. 2011; Li et al. 2017). Since BDNF is reported to cross the blood-brain barrier in both directions, the positive correlation between blood BDNF levels and brain-tissue BDNF levels has been demonstrated; peripheral BDNF is a promising candidate biomarker associated with depression and antidepressant responses (Pan et al. 1998; Klein et al. 2011). However, well-documented evidences have accumulated that not all antidepressant treatments increase serum levels of BDNF, and antidepressant-induced increases in BDNF levels are more prominent in responders than in non-responders (Molendijk et al. 2011; Kurita et al. 2012). ProBDNF/p75NTR/sortilin plays a pathological role during depression, whether antidepressant drugs can exert their functions by alleviating the upregulation of these genes remains the question.
Our previous clinical studies have reported that proBDNF/p75NTR/sortilin proteins were upregulated in the serum of female depressed patient and positively correlated with their depression scores and in alcoholic patients (Zhou et al. 2018). Furthermore, our experimental studies have shown that proBDNF/p75NTR/sortilin expression was increased with spine loss in the hippocampus and cortex in rats with depressive-like disorders, and anti-proBDNF antibody injection via intracerebroventricular and intraperitoneal approaches reversed the depressive behavior (Bai et al. 2016). We propose that antidepressants and antipsychotics exert their antidepressant efficacy by restoring the imbalance of proBDNF/p75NTR/sortilin and mBDNF/TrkB production.
In the present study, we conducted experiments on a chronic unpredictable mild stress (CUMS) mouse model, detected the depression by behavior tests, and determined the expression of BDNF/TrkB and proBDNF/p75NTR/sortilin by western blot assays and quantitative (qRT-PCR). In addition, we further quantified the antidepressant-like effect of fluoxetine (FLU) and clozapine (CLO) in CUMS mice. We identified that antidepressants and antipsychotics reversed the depressive behavior and reduced the expression of proBDNF/p75NTR/sortilin in the hippocampus and cortex. We postulate proBDNF/p75NTR/sortilin signaling can be one of the key measures for the management of major depression.
Materials and Methods
Animals and Ethical Statements
The study was carried out on 2-month-old male C57BL/6J mice (23–27 g). Before the experiments, all the animals were housed in groups of three to five per standard cage with food and water for at least 2 weeks before the test. The animals were maintained under standard laboratory conditions (12 h light/dark cycle, 22 ± 1 °C, 52 ± 2% humidity). All animal ethics protocols were approved by Institutional Animal Ethics Committees and met the standards of the Guide for Care and Use of Laboratory Animals established by the National Institutes of Health, USA. All efforts were made to minimize the number of mice used and their suffering. For each treatment group in these series of experiments, 14 mice were used.
Experimental Design and Antidepressant Drugs Treatment
Fluoxetine hydrochloride (FLU) was purchased from Lilly S. A. Spain. Clozapine (CLO) was made by the Jiangsu Nhwa Pharmaceutical Group. Both FLU and CLO were dissolved separately in sterile saline.
Modeling of Depression by Chronic Unpredictable Mild Stress and the Administration of Vehicle, Fluoxetine, and Clozapine
The detailed CUMS procedures were described in our previous paper (Yang et al. 2015; Yang et al. 2017). The animals were randomly divided: (Group I) unstressed mice with free access to standard food and water, used as normal control; (Group II) mice after CUMS exposure for 28 days; (Group III) CUMS-induced depression models treated with daily intraperitoneal (I.P.) injections of sterile saline (0.01 ml/g) for 14 days, used as a vehicle (VEH) group; (Group IV) CUMS-induced depression models treated with daily I.P. injections of FLU (18 mg/kg; 0.01 ml/g) for 14 days; (Group V) CUMS-induced depression models treated with daily I.P. injections of CLO (20 mg/kg; 0.01 ml/g) for 14 days. Both Groups IV and V were used as the positive controls for antidepressant action.
Sucrose Preference Test
According to our previous study, sucrose preference test was used to assess anhedonia, which is one of the key symptoms of clinical depressive disorder. Before testing, two bottles of 1% (v/v) sucrose solution were placed on the cage for 48 h, in order to train the animals habituating to sucrose solution. In the next 24 h, food and water were deprived from the animals. After that, the right bottles were replaced with tap water and allow freely drink for 10 h, starting from 8:30 AM. The consumed liquids were weighted by an electronic balance. The sucrose intake (%) was calculated by the formula (sucrose intake/(sucrose intake + water intake) × 100).
Open Field Test
The apparatus and testing procedures were similar to those used by previously reported method. Briefly, motor activity was quantified in a white square cage 50 × 50 × 50 cm3. The apparatus was divided into 5 × 5 cm equal squares on the floor of the arena. Each experimental animal was placed in the center of the cage; the number of crossings (with the four paws), the number of rearings (posture sustained with hind paws on the floor), and the immobility time were recorded manually for 5 min according to recent studies. All behaviors were recorded with a video camera located 100–120 cm above the arena. After each test, the arena was cleaned with 75% alcohol solution.
Tail Suspending Test
The tail suspension test reflects behavioral despair, which is used as a measured behavioral variable. Mice were tested in an individual cubicle while suspended from a tail hanger with an adhesive tape wrapped around the tail (1.5–2 cm from the tip), 35 cm above the floor. The trial was performed for a period of 6 min, and the duration of immobility was manually measured. Mice were considered immobile when they hung passively and motionless. Animals were humanely killed when all the behavioral tests were completed.
Western Blot
Western blot was done as described in our previous paper. Briefly, animals were sacrificed by overdosing ether and rapid cervical dislocation. The whole brain of each mouse was collected and rinsed in cold PBS. Then, the cortex and hippocampus were isolated on a cold plate. The tissue samples were frozen in liquid nitrogen and then stored at − 80 °C for protein extraction, followed by incubation of primary antibodies (sheep anti-human proBDNF 1:200, from professor Xin-Fu Zhou’s laboratory; mouse p75NTR 1:200, cat.14–9400-82, Invitrogen, USA; rabbit sortilin 1:1000, cat.#ab16640, Abcam, USA; rabbit TrkB 1:1000, cat.#07–225, Millipore, USA; mouse beta-actin 1:1000, cat.#CW0097, CoWin Biosciences, China) at 4 °C, overnight. After washing with TBST, the membranes were incubated at room temperature for 1 h with horseradish peroxidase-conjugated anti-rabbit/mouse/sheep/goat species-specific secondary antibodies (cat.#sc-2020, Santa Cruz, USA; cat.#CW0103, Cwbiotech, China). After washing again in TBST, enhanced chemiluminescent (ECL) substrate (cat.#cw0049, Cwbiotech, China) was applied on the membrane before imaging using ChemiDoc™ XRS+ System (Bio-Rad, USA). To quantify the protein level in the brain, beta actin was blotted as loading control. The blots were analyzed using ImageJ software (NIH, USA).
Quantitative Real-Time PCR
Total RNA of the entire cortex and the hippocampus isolated from the animals were extracted by TRIzol® reagent (Sigma), following the manufacturer’s recommendation. The RNA samples were dissolved in 30 μl of DEPC-H2O and subsequently stored at − 80 °C. The quantification and purity of the RNA were measured by using a NanoDrop 2000 Spectrophotometer (Thermo-Fisher Scientific, CA, USA). The quality of RNA was assessed by the absorbance at 260 and 280 nm, and a ratio of A260/A280 ranging from 1.9 to 2.1 was considered acceptable. Total RNA was transcribed by a High-Capacity cDNA Reverse Transcription Kit (DRR037A; TaKaRa, Japan) according to the manufacturer’s protocol. Gene expression levels of BDNF, TrkB, p75NTR, and sortilin were detected by running quantitative real-time PCR on the ABI7300 platform (Applied Biosystems, Life Technologies, USA) by using FastStart Universal SYBR Green Master (ROX) (Roche). The gene expression of β-actin was detected as internal control. The following primers were used:
BDNF forward primer: 5′-GCAGCCTTCTTTTGTGTAACC-3′
reverse primer: 5′-AGAGTGATGACCATCCTTTTC-3′
TrkB forward primer: 5′-GTTTCATAAGATCCCACTGGATGG-3′
reverse primer: 5′-TGCTGCTTAGCTGCCTGAGAGTTA-3′
p75NTR forward primer: 5′-TGGACAGCGTGACGTTCTCC-3′
reverse primer: 5′-GATCTCCTCGCACTCGGCGT-3′
sortilin forward primer: 5′-TGACCTCACAGTTGGCTCTG-3′
reverse primer: 5′-GTATGCGGTGGGAGTTGAGT-3′
and β-actin forward primer: 5′-AGCCATGTACGTAGCCATCCA-3′
reverse primer: 5′-TCTCCGGAGTCCATCACAATG-3′
The primer specificity was confirmed to be a single band of the predicted size by melting curve analysis and electrophoresis of PCR products on a 2% agarose gel. The relative expression of target gene (BDNF, TrkB, p75NTR, and sortilin) messenger RNA (mRNA) was normalized to the amount of housekeeping gene β-actin in the same cDNA of animals from the different treatment groups compared with the control group or CUMS group by using the relative quantification method (2−△△CTmethod) described by the manufacturer.
Statistical Analyses
Data were presented as mean ± SEM and analyzed by SPSS 19.0 version. For difference comparison within groups, Student’s t test was used for the two groups, one-way analysis of variance (ANOVA) for more than two groups, and two-way ANOVA for two groups with two factors. The p < 0.05 was considered statistically significant.
Results
Effects of Antidepressant Drugs on Depressive and Anxiety-Like Behavior
To examine the effects of antidepressant drugs on CUMS-induced depression, mice were exposed to CUMS protocol for 28 days, and antidepressant drugs or vehicle sterile saline was i.p. injected daily during the last 14 days (Fig. 1). At the end of treatment protocol, behavioral assessments were conducted in all five groups of animals (control, CUMS, CUMS + VEH, CUMS + FLU, and CUMS+CLO; n = 14 each). The percentage of sucrose consumption in the sucrose preference test was significantly decreased in the CUMS mice group (n = 14) compare with the control group (n = 14) (Student’s t test, ***p < 0.001, t = 7.286, Fig. 2a), and antidepressant (FLU) and antipsychotic drugs (CLO) treatment reversed the decrease (###p < 0.001, ###p < 0.001, F(2,39) = 46.047, Fig. 2a). In the OFT, a 4-week period of CUMS exposure resulted in a typical reduction in the number of crossings (***p < 0.001, t = 5.578, Fig. 2b) and rearings (**p = 0.006, t = 3.129, Fig. 2c) and increased the immobility time (**p = 0.002, t = − 3.363, Fig. 2d) in mice, as compared with the controls. We have found that FLU significantly reversed these behavioral impairments in CUMS mice. Our results showed that the vehicle-treated CUMS group exhibited the lowest crossings and rearings and the highest immobility time and FLU treatment reversed these behaviors (one-way ANOVA, #p = 0.016, F(2,39) = 3.22, Fig. 2b; ##p = 0.001, F(2,39) = 8.723, Fig. 2c; #p = 0.011, F(2,39) = 3.953, Fig. 2d). Whereas CLO treatment was able to reverse rearings (##p = 0.002, F(2,39) = 8.723, Fig. 2c) and immobility (#p = 0.046, F(2,39) = 3.953, Fig. 2d) compared with the VEH group, there was no significance in the number of crossings (p = 0.281, F(2,39) = 3.22, Fig. 2b). In addition, the number of climbings (***p < 0.001, t = 13.273, Fig. 2e) in the TST was declined in CUMS mice vs the control group, and antidepressant drugs’ (FLU, CLO) treatment significantly increased the numbers in these mice (##p = 0.001, F(2,39) = 9.159, Fig. 2e; ##p = 0.001, F(2,39) = 9.159, Fig. 2e). At the same time, CUMS exposure caused the increased immobility time (***p < 0.001, t = 13.273, Fig. 2f), and antidepressant drugs’ (FLU, CLO) treatment significantly decreased the immobility time in these mice (###p < 0.001, F(2,39) = 18.105, Fig. 2f; ###p < 0.001, F(2,39) = 18.105, Fig. 2f).
Influence of fluoxetine and clozapine administration on depressive-like behaviors in chronic unpredictable mild stress (CUMS) mouse model. Timeline of CUMS paradigm (28d), antidepressant administration, and behavioral test. VEH: vehicle; FLU: fluoxetine; CLO: clozapine; SPT: sucrose preference test; OFT: open field test; TST: tail suspending test
Chronic treatment with antidepressants abolished CUMS-induced depressive state. (a) CUMS mice showed less sucrose consumption than the control mice. The treatment of FLU and CLO for 14 days obviously reversed the decrease of sucrose preference in CUMS mice. (b, c, d) FLU treatment increased the number of crossings and rearings and decreased immobility time in the CUMS group in OFT. CLO treatment played the same effect, but except to raise the number of crossings. (e, f) FLU and CLO exposure significantly increased the number of climbings, and induced immobility time in TST compared with VEH. OFT: open field test; TST: tail suspending test; n = 14 in each group; *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001; All data are presented as mean ± SEM
ProBDNF/p75NTR/Sortilin Signaling Was Activated in CUMS Mice
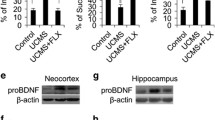
The protein level of proBDNF was significantly higher in the CUMS group than that in the control group both in the cortex (***p < 0.001, t = − 7.04, Fig. 3a) and hippocampus (*p = 0.027, t = − 2.583, Fig. 3b). Furthermore, the protein and mRNA levels of p75NTR were increased in the CUMS group compared with the control group both in the cortex (*p = 0.014, t = − 2.963, Fig. 4a; *p = 0.041, t = − 2.433, Fig. 4b) and hippocampus (*p = 0.18, t = − 3.457, Fig. 4c; **p = 0.001, t = − 5.108, Fig. 4d). However, the mRNA level of sortilin was not statistically different in the cortex between the two groups (p = 0.727, t = − 0.359, Fig. 5b) but significantly increased in the hippocampus of the CUMS group compared with the control (*p = 0.043, t = − 2.394, Fig. 5d). At the protein level, sortilin in the CUMS group was significantly higher in the cortex (**p = 0.006, t = − 4.467, Fig. 5a) and hippocampus (**p = 0.001, t = − 5.438, Fig. 5c) of the CUMS group than that in the control group. The above results suggest that proBDNF/p75NTR/sortilin signaling was activated in CUMS mice.
ProBDNF protein expression in the different groups. (a) ProBDNF protein expression was increased after CUMS in the cortex. A significant downregulation of proBDNF protein expression was detected in treatment with FLU and CLO compared with the VEH group. (b) ProBDNF protein expression was prominent increased after CUMS in the hippocampus. FLU and CLO treatment decreased proBDNF protein levels when compared with the VEH group. *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001; All data are presented as mean ± SEM
The protein and mRNA level of p75NTR in the different groups. (a, b) P75NTR protein and mRNA expression were increased after CUMS in the cortex. A significant downregulation of p75NTR protein and mRNA expression was detected in treatment with FLU and CLO compared with the VEH group. (c, d) P75NTR protein and mRNA expression were prominent increased after CUMS in the hippocampus. FLU and CLO treatment decreased p75NTR protein and mRNA levels when compared with the VEH group. *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001; All data are presented as mean ± SEM
The protein and mRNA level of sortilin in the different groups. (a) Sortilin protein expression was increased after CUMS in the cortex. A significant downregulation of sortilin protein expression was detected in treatment with FLU and CLO compared witht the VEH group. (b) No significant difference was found in sortilin mRNA expression. (c, d) Sortilin protein and mRNA expression were prominent increased after CUMS in the hippocampus. FLU and CLO treatment decreased the sortilin protein level when compared with the VEH group, while no difference in the mRNA level. *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001; All data are presented as mean ± SEM
mBDNF/TrkB Signaling Was Suppressed in CUMS Mice
A clinical study in 2004 has shown that serum BDNF levels negatively correlated with the depression-related personality traits (Lang et al. 2004). mBDNF binding to TrkB receptor acts a beneficial role in neuronal survival, which is opposing to the proBDNF/p75NTR/sortilin signaling pathway. The mRNA levels of BDNF and TrkB were reduced in the CUMS group compared with the control group both in the cortex (**p = 0.004, t = 3.71, Fig. 6a; *p = 0.019, t = 2.809, Fig. 7b) and hippocampus (*p = 0.019, t = 2.809, Fig. 6b; *p = 0.027, t = 2.595, Fig. 7d). The protein level of TrkB was significantly reduced in the CUMS group when compared with the control group in the cortex (***p < 0.001, t = 8.508, Fig. 7a) and hippocampus (***p < 0.001, t = 10.277, Fig. 7c). These results suggested that mBDNF/TrkB signaling was suppressed in CUMS mice.
Effect of CUMS and antidepressents on mRNA expression of BDNF in mice. RT-PCR was performed to detect the mRNA expression of the BDNF. (a) There was a significant difference between treated groups and the VEH group in the cortex. (b) BDNF mRNA expression was prominent increases after CUMS in the hippocampus. FLU and CLO treatment increased BDNF mRNA level when compared with the VEH group as described in the hippocampus. *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001; All data are presented as mean ± SEM
The protein and mRNA level of TrkB in the different groups. (a, b) TrkB protein and mRNA expression were decreased after CUMS in the cortex. A significant upregulation of TrkB protein and mRNA expression was detected in treatment with FLU and CLO compared with the VEH group. (c, d) TrkB protein and mRNA expression were prominent decreased after CUMS in the hippocampus. FLU and CLO treatment increased TrkB protein and mRNA levels when compared with the VEH group. *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.05, ##p < 0.01, ###p < 0.001; All data are presented as mean ± SEM
Effects of Antidepressant and Antipsychotic Drugs on proBDNF/p75NTR/Sortilin Signaling in the Brain of CUMS Mice
To investigate whether antidepressant and antipsychotic drugs could against impaired balance between proBDNF and mBDNF, the two signaling pathways were detected after i.p. injected daily during last 14 days. The results showed that FLU injection obviously reversed the increase in proBDNF protein compared with the VEH group in the cortex (one-way ANOVA, ###p < 0.001, F(2,39) = 91.252, Fig. 3a) and hippocampus (###p < 0.001, F(2,39) = 76.938, Fig. 3b). Furthermore, CLO treatment decreased the proBDNF protein level when compared with the VEH group in the cortex (###p < 0.001, F(2,39) = 91.252, Fig. 3a), and it was the same change in the hippocampus (###p < 0.001, F(2,39) = 76.938, Fig. 3b). As for p75NTR, a significant downregulation of P75NTR protein and mRNA expression in the cortex was detected by the treatment with FLU (###p < 0.001, F(2,39) = 27.853, Fig. 4a; ###p < 0.001, F(2,39) = 20.216, Fig. 4b) and CLO (###p < 0.001, F(2,39) = 27.853, Fig. 4a; ###p < 0.001, F(2,39) = 20.216, Fig. 4b) compared with the VEH group. FLU (###p < 0.001, F(2,39) = 31.018, Fig. 4c; #p = 0.012, F(2,39) = 21.665, Fig. 4d) and CLO (###p < 0.001, F(2,39) = 31.018, Fig. 4c; ###p < 0.001, F(2,39) = 21.665, Fig. 4d) treatment decreased P75NTR protein and mRNA levels in the hippocampus when compared with the VEH group. The mRNA level of sortilin from the treated group was not statistically different compared with the VEH group. A significant downregulation of sortilin protein expression in the cortex and hippocampus was detected in the CLO group compared with the VEH group (#p = 0.035, F(2,39) = 4.481, Fig. 5a; ##p = 0.001, F(2,39) = 33.239, Fig. 5c), and FLU treatment also increased the protein level of sortilin in the cortex (#p = 0.013, F(2,39) = 4.481, Fig. 5a) and hippocampus (###p < 0.001, F(2,39) = 33.239, Fig. 5c). These results indicated that antidepressant and antipsychotic drugs play an important role in the proBDNF/p75NTR/sortilin signaling pathway in the brain of CUMS mice.
Effects of Antidepressant and Antipsychotic Drugs on mBDNF/TrkB Signaling in the Brain of CUMS Mice
As it is well known that the mBDNF/TrkB signaling is inhibited in the brains of stressed rodents and depressed patients, we then asked a question of whether antidepressant and antipsychotic drugs could abolish the imbalance of proBDNF/mBDNF. In this study, we found that chronic treatment with FLU (#p = 0.47, F(2,39) = 5.872, Fig. 6a; ##p = 0.008, F(2,39) = 24.545, Fig. 6b) and CLO (##p = 0.005, F(2,39) = 5.872, Fig. 6a; ###p < 0.001, F(2,39) = 24.545, Fig. 6b) could upregulate the BDNF mRNA level when compared with the VEH group in the cortex and hippocampus. The protein level of TrkB was significantly increased in the treatment with FLU (##p = 0.001, F(2,39) = 9.504, Fig. 7a; ###p < 0.001, F(2,39) = 53.038, Fig. 7c) and CLO (#p = 0.029, F(2,39) = 9.504, Fig. 7a; ###p < 0.001, F(2,39) = 53.038, Fig. 7c) compared with the VEH group in the cortex and hippocampus. The mRNA level of TrkB in the cortex and hippocampus was also significantly increased in the FLU group (###p < 0.001, F(2,39) = 12.258, Fig. 7b; #p = 0.029, F(2,39) = 5.546, Fig. 7d) and CLO group (##p = 0.009, F(2,39) = 12.258, Fig. 7b; ##p = 0.006, F(2,39) = 5.546, Fig. 7d) compared with the VEH group. These results indicate that antidepressant and antipsychotic drugs play an important role in the mBDNF/TrkB signaling pathway in the brain of CUMS mice.
Discussion
In the present study, we successfully established a depression model in mice as reflected by reduction in sucrose consumption and increase in immobility in TST with a chronic stress protocol. Consistent with the depressive behaviors, the protein and mRNA levels of BDNF and TrkB were downregulated whereas the expression of proBDNF/p75NTR/sortilin was evidently enhanced, suggesting that neurotrophic balance was broken after stress. The treatment with FLU and CLO restored all depression-like behaviors in CUMS model mice. Furthermore, FLU treatment obviously decreased proBDNF, p75NTR, sortilin, and increased TrkB protein in the cortex and hippocampus, and CLO treatment reversed all the biomarkers in both cortex and hippocampus. Consistent with the changes of the proteins, FLU and CLO treatment reversed the mRNA expression of all markers except sortilin. Our study suggests that antidepressant FLU and antipsychotic CLO can normalize the imbalance of proBDNF/mBDNF pathways. These observations also suggest that intervention of the neurotrophic pathway can be one of the key measures for the management of major depression.
Chronic Stress Caused the Imbalance Between the proBDNF/p75NTR/Sortilin Signaling and mBDNF/TrkB Signaling Pathways
In our present experiment, we confirmed that proBDNF, p75NTR, and sortilin are increased but TrkB is decreased after modeling of mild chronic stress. These results indicate that chronic stress not only elicits depressive phenotype but also causes an abnormal proBDNF signaling, which is consistent with previous studies (Zhou et al. 2013; Bai et al. 2016). In contrast, mature BDNF and its receptor TrkB are downregulated in both patients with major depression and animals under chronic stress (Zhou et al. 2013; Bai et al. 2016; Yang et al. 2019; Peng et al. 2018; Serra et al. 2018). The depressive behaviors are closely associated with the upregulation of proBDNF and p75NTR in the brain, as the neutralization of proBDNF in both the peripheral or in the brain can alleviate depressive behaviors (Bai et al. 2016). Our further study found that proBDNF in the anterior cingulate cortex regulates the depressive behaviors as blocking proBDNF in this brain region can reverse the phenotype (Yang et al. 2017). Consistent with previous findings, in the present study, we found that proBDNF was upregulated and mBDNF levels and its receptors TrkB were downregulated in the hippocampus and cortex in the CUMS mice model. These results suggested that under CUMS or pathological conditions (Luo et al. 2019), the proBDNF/mBDNF balance was broken down or chronic stress caused the imbalance between the proBDNF/p75NTR and mBDNF/TrkB signaling pathways.
Antidepressant FLU and Antipsychotic CLO Restore the Imbalance Between proBDNF/p75NTR/Sortilin Signaling and mBDNF/TrkB Signaling
Both SSRIs (FLU) and tricyclic (CLO) drugs were employed in initial studies of CUMS treatment and both were effective. FLU, as one of the most commonly prescribed SSRI antidepressants, has been thought to be effective and safe for patients with depression in light of randomized controlled trials (Castren and Rantamaki 2010). CLO, as an atypical antipsychotic drug (which is often used to treat the psychotic symptoms of treatment-resistant schizophrenic patients or the augmentation of antidepressant-like effect in case of treatment resistant depression although there are many side effects), has also been shown to reverse ketamine-induced psychotic symptoms in healthy human and to reduce the ketamine-induced exacerbation of positive symptoms in patients (Malhotra et al. 1997; Rame et al. 2017). In this experiment, we found that the treatment with FLU reversed depressive-like behavior resulted in an increased sucrose preference, distance traveled (crossing), rearing frequency and velocity, and a decreased immobility time in the OFT and TST. However, the treatment with CLO reversed mostly depressive-like behavior, but not distance traveled and velocity in the OFT. Some reports showed that atypical antipsychotics, especially CLO, are able to change the motivational state even inducing a higher reward response to sugar, food, and abuse drugs like cocaine (Galistu et al. 2011; Zhang et al. 2005); therefore, we should be cautious about the interpretation of the results on SPT. However, the reversal of sucrose consumption observed in CLO-exposed mice is likely due to the recovery from the depression as we also see the decreased immobility time in TST.
In this study, we also found that chronic treatment with FLU and CLO also could restore the imbalance between proBDNF/p75NTR/sortilin and mBDNF/TrkB signaling. We demonstrated that the treatment of FLU or CLO alters the proBDNF/mBDNF level in the hippocampus and cortex. FLU treatment obviously downregulates the expression of proBDNF protein in the cortex and hippocampus and upregulates the expression of BDNF mRNA. It is known that the BDNF gene is regulated by the cyclic AMP-responsive element-binding protein (CREB) pathway or via the activation of PKA disassociate MeCP2-CREB-Bdnf promoter IV complex by epigenetic modification (Lee et al. 2011; Jin et al. 2017). CLO treatment reversed the expression of BDNF mRNA and proBDNF protein in both cortex and hippocampus. Increased proBDNF may be associated with the pathophysiology of depression, and decreased proBDNF is associated with the mechanisms of actions of antidepressant drugs. The injection of anti-proBDNF antibody via intracerebroventricular (i.c.v.) and intraperitoneal (i.p.) approaches in the CUMS rats significantly attenuate the depressive behavior (Bai et al. 2016). Consistent with the changes of proBDNF and mature BDNF after FLU or CLO, we observed chronic treatment with FLU restored the CUMS-induced changes in both mRNA and protein levels of p75NTR and TrkB protein in both cortex and hippocampus. These results suggest that FLU and CLO may exert their antidepressant-like effect by restoring the imbalance between proBDNF/p75NTR/sortilin and mBDNF/TrkB signaling.
Therapeutic Mechanism of Antidepressants and Antipsychotics and Potential Relationship Between Neurotrophic Imbalance and Other Mechanisms
In as early as 1960, scientists found that serotonin levels in cerebrospinal fluid of depressive patients are three times lower than those of normal people (Ashcroft and Sharman 1960). By 1987, FLU was approved by the FDA and became the first serotonin drug to treat depression. However, it appears that this hypothesis is wrong, as serotonin levels in the brain can rise several times within hours of taking FLU, but the symptoms of patients need 2–4 weeks of taking FLU to relieve. In addition, some drugs such as tricyclic antidepressants do not act on serotonin at all (Peng et al. 2018). Therefore, the mechanism of action by FLU remains a topic of investigations.
Some studies have found that FLU can promote the neurogenesis in the hippocampus of mice (David et al. 2009; Santarelli et al. 2003). In animal experiments, inhibition of neuron regeneration by experimental means can also prevent FLU from taking effect. This mechanism of action by FLU appears well explaining its delayed effect. Other studies showed that FLU can increase the gene expression of BDNF (Jin et al. 2017), which can clearly explain its delayed efficacy on depression symptom relieve. A recent study also shows that the acute relieve of depression by ketamine treatment is also via the upregulation of tPA which can convert proBDNF to mature BDNF (Zhang et al. 2018). These studies all point to the view that BDNF signaling is critical for the pathogenesis of depression and likely the drug target of SSRI and other antidepressants. Our current investigation adds an additional evidence that the treatment of FLU or CLO can restore the imbalance of proBDNF/p75NTR/sortilin and mBDNF/TrkB signaling pathways after chronic stress. Certainly, the neurotrophic imbalance hypothesis can also explain the neurogenesis hypothesis regulated by FLU as proBDNF is a strong negative regulator of neurogenesis as shown in our recent findings (Li et al. 2017). Restoration of the neurotrophic imbalance by FLU or CLO would also enhance neurogenesis.
Conclusion
To summarize, the balance between proBDNF/p75NTR/sortilin and mBDNF/TrkB signaling pathways in depression was disrupted. Our data indicate that antidepressant and antipsychotic drugs likely exert their therapeutic effects by correcting the abnormal proBDNF/mBDNF signaling. Furthermore, intervening the balance between proBDNF/p75NTR/sortilin and mBDNF/TrkB signaling pathways might provide a novel therapeutic target for clinical therapy.
References
Ashcroft GW, Sharman DF (1960) 5-Hydroxyindoles in human cerebrospinal fluids. Nature 186:1050–1051
Bai YY, Ruan CS, Yang CR, Li JY, Kang ZL, Zhou L, Liu D, Zeng YQ, Wang TH, Tian CF, Liao H, Bobrovskaya L, Zhou XF (2016) ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology 41(12):2882–2892
Bert F, Giacomelli S, Ceresetti D, Zotti CM (2017) World Health Organization framework: multimodal hand hygiene strategy in Piedmont (Italy) health care facilities. J Patient Saf
Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW (2002) Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology 160(4):353–361
Castren E, Rantamaki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70(5):289–297
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62(4):479–493
Fan YJ, Wu LL, Li HY, Wang YJ, Zhou XF (2008) Differential effects of pro-BDNF on sensory neurons after sciatic nerve transection in neonatal rats. Eur J Neurosci 27(9):2380–2390
Furuse K, Ukai W, Hashimoto E, Hashiguchi H, Kigawa Y, Ishii T, Tayama M, Deriha K, Shiraishi M, Kawanishi C (2019) Antidepressant activities of escitalopram and blonanserin on prenatal and adolescent combined stress-induced depression model: possible role of neurotrophic mechanism change in serum and nucleus accumbens. J Affect Disord 247:97–104
Galistu A, Modde C, Pireddu MC, Franconi F, Serra G, D'Aquila PS (2011) Clozapine increases reward evaluation but not overall ingestive behaviour in rats licking for sucrose. Psychopharmacology 216(3):411–420
Gundersen BB, Briand LA, Onksen JL, Lelay J, Kaestner KH, Blendy JA (2013) Increased hippocampal neurogenesis and accelerated response to antidepressants in mice with specific deletion of CREB in the hippocampus: role of cAMP response-element modulator tau. J Neurosci 33(34):13673–13685
Jin HJ, Pei L, Li YN, Zheng H, Yang S, Wan Y, Mao L, Xia YP, He QW, Li M, Yue ZY, Hu B (2017) Alleviative effects of fluoxetine on depressive-like behaviors by epigenetic regulation of BDNF gene transcription in mouse model of post-stroke depression. Sci Rep 7(1):14926
Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14(3):347–353
Kurita M, Nishino S, Kato M, Numata Y, Sato T (2012) Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLoS One 7(6):e39212
Lang UE, Hellweg R, Gallinat J (2004) BDNF serum concentrations in healthy volunteers are associated with depression-related personality traits. Neuropsychopharmacology 29(4):795–798
Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, Kim DH, Kwon YG, Kim YM, Won MH (2011) Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol 229(2):450–459
Li JY, Liu J, Manaph NPA, Bobrovskaya L, Zhou XF (2017) ProBDNF inhibits proliferation, migration and differentiation of mouse neural stem cells. Brain Res 1668:46–55
Luo L, Li C, Du X, Shi Q, Huang Q, Xu X, Wang Q (2019) Effect of aerobic exercise on BDNF/proBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav Brain Res 362:323–331
Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A (1997) Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry 42(8):664–668
Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RC, Elzinga BM (2011) Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 16(11):1088–1095
Oved K, Farberov L, Gilam A, Israel I, Haguel D, Gurwitz D, Shomron N (2017) MicroRNA-mediated regulation of ITGB3 and CHL1 is implicated in SSRI action. Front Mol Neurosci 10:355
Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ (1998) Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37(12):1553–1561
Peng S, Li W, Lv L, Zhang Z, Zhan X (2018) BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med 26(143):127–136
Rame M, Caudal D, Schenker E, Svenningsson P, Spedding M, Jay TM, Godsil BP (2017) Clozapine counteracts a ketamine-induced depression of hippocampal-prefrontal neuroplasticity and alters signaling pathway phosphorylation. PLoS One 12(5):e0177036
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301(5634):805–809
Segi-Nishida E (2017) The effect of serotonin-targeting antidepressants on neurogenesis and neuronal maturation of the hippocampus mediated via 5-HT1A and 5-HT4 receptors. Front Cell Neurosci 11:142
Serra MP, Poddighe L, Boi M, Sanna F, Piludu MA, Corda MG, Giorgi O, Quartu M (2018) Effect of acute stress on the expression of BDNF, trkB, and PSA-NCAM in the hippocampus of the Roman rats: a genetic model of vulnerability/resistance to stress-induced depression. Int J Mol Sci 19(12)
Shen LL, Manucat-Tan NB, Gao SH, Li WW, Zeng F, Zhu C, Wang J, Bu XL, Liu YH, Gao CY, Xu ZQ, Bobrovskaya L, Lei P, Yu JT, Song W, Zhou HD, Yao XQ, Zhou XF, Wang YJ (2018) The ProNGF/p75NTR pathway induces tau pathology and is a therapeutic target for FTLD-tau. Mol Psychiatry 23(8):1813–1824
Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Serretti A, Zohar J, Mendlewicz J (2007) Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry 68(7):1062–1070
Sun Y, Lim Y, Li F, Liu S, Lu JJ, Haberberger R, Zhong JH, Zhou XF (2012) ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One 7(4):e35883
Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25(22):5455–5463
Thaler KJ, Morgan LC, Van Noord M, Gaynes BN, Hansen RA, Lux LJ, Krebs EE, Lohr KN, Gartlehner G (2012) Comparative effectiveness of second-generation antidepressants for accompanying anxiety, insomnia, and pain in depressed patients: a systematic review. Depress Anxiety 29(6):495–505
Xu ZQ, Sun Y, Li HY, Lim Y, Zhong JH, Zhou XF (2011) Endogenous proBDNF is a negative regulator of migration of cerebellar granule cells in neonatal mice. Eur J Neurosci 33(8):1376–1384
Yang CR, Bai YY, Ruan CS, Zhou HF, Liu D, Wang XF, Shen LJ, Zheng HY, Zhou XF (2015) Enhanced aggressive behaviour in a mouse model of depression. Neurotox Res 27(2):129–142
Yang CR, Bai YY, Ruan CS, Zhou FH, Li F, Li CQ, Zhou XF (2017) Injection of anti-proBDNF in anterior cingulate cortex (ACC) reverses chronic stress-induced adverse mood behaviors in mice. Neurotox Res 31(2):298–308
Yang SJ, Song ZJ, Wang XC, Zhang ZR, Wu SB, Zhu GQ (2019) Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol Sin
Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, Shiina A, Hashimoto T, Kanahara N, Hasegawa T, Enohara M, Kimura A, Iyo M, Hashimoto K (2012) Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One 7(8):e42676
Zhang Z, Rickard JF, Asgari K, Body S, Bradshaw CM, Szabadi E (2005) Quantitative analysis of the effects of some “atypical” and “conventional” antipsychotics on progressive ratio schedule performance. Psychopharmacology 179(2):489–497
Zhang F, Luo J, Zhu X (2018) Ketamine ameliorates depressive-like behaviors by tPA-mediated conversion of proBDNF to mBDNF in the hippocampus of stressed rats. Psychiatry Res 269:646–651
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF (2013) Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord 150(3):776–784
Zhou L, Xiong J, Ruan CS, Ruan Y, Liu D, Bao JJ, Zhou XF (2018) ProBDNF/p75NTR/sortilin pathway is activated in peripheral blood of patients with alcohol dependence. Transl Psychiatry 7(11):2
Funding
This research was financially supported by grant from The Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2018KJ086).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C.R., Zhang, X.Y., Liu, Y. et al. Antidepressant Drugs Correct the Imbalance Between proBDNF/p75NTR/Sortilin and Mature BDNF/TrkB in the Brain of Mice with Chronic Stress. Neurotox Res 37, 171–182 (2020). https://doi.org/10.1007/s12640-019-00101-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00101-2