Abstract
Background
Depression, one of the most significant mental disorders, is still poorly understood in terms of its pathogenetic mechanisms despite its well-recognized association with stress.
Objectives
The current study’s goal was to ascertain how the novel antidepressant drug vortioxetine (VOR) affected the BDNF (brain-derived neurotrophic factor), S100, amyloid β (Aβ), CREB (cAMP response element-binding protein), and NR2B, as well as its impact on depression-like behaviors, and tissue damage in an experimental rodent model of depression caused by chronic unpredictable stress.
Methods
We employed twenty-eight Wistar albino male rats, and we randomly divided them into four groups, each consisting of 7 rats: control, CUMS (chronic unpredictable mild stress), CUMS+vortioxetine (CUMS+VOR), and CUMS+fluoxetine (CUMS+FLU). Sucrose preference and forced swimming tests (SPT and FST, respectively), PCR, ELISA, and histopathological and immunohistochemical evaluation were made on brains.
Results
The behaviors of reduced immobility in the FST and increased sucrose preference were observed in the CUMS group and they improved in the groups treated with VOR and FLU. Compared with the control group, the group exposed to CUMS showed increased Aβ and decreased BDNF, CREB, and S-100 expressions, as well as neuronal degeneration (p<0.001). VOR and FLU treatment ameliorate the findings.
Conclusions
This study demonstrated significant ameliorative effects of VOR in an experimental model of chronic unpredictable depression to reduce brain tissue damage and depression-like behaviors in rats.
Graphical abstract

Effects of CUMS on the brain and possible effects of VOR
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most significant psychiatric illnesses, major depression, is a common occurrence worldwide (Malhi and Mann 2018). In addition to its enormous incidence, depression is viewed as an important public health issue and the importance of research in the topic is increased by its high risk of suicide, negative economic effects, and disability. It is a diverse type of mental illness that occurs as a consequence of the interaction of many different elements, including negative life events, neurological effects, and hereditary implications. Awareness of the origins of depression requires an understanding of early life stress (LeMoult et al. 2020). It is unknown exactly how early childhood stress relates to sensitivity to stress, depressive behavior, the emergence of clinical symptoms, and symptom recurrence. It is theorized that complex interactions between genetic factors and early life experiences during the phases of development influence future susceptibility to depression and vulnerability to environmental stressors (Torres-Berrio et al. 2019).
Numerous preclinical and clinical researches have shown that the neurochemical changes brought on by stress lead to neuroinflammatory processes, affect neurotrophic factors, and impair neuroplasticity, all of which have a negative impact on mental health as well as cognitive abilities like memory and learning. In the hippocampus, BDNF is necessary for neurogenesis, synaptic plasticity, and neuronal survival (Rauti et al. 2020). Glutamatergic synapses, in particular glial cells like astrocytes and microglia separated from the prefrontal cortex and hippocampus, express BDNF (Poyhonen et al. 2019). It has been shown that stress, both acute and chronic, lowers BDNF expression. Both the etiology of depression and the mechanism of action of antidepressants depend critically on BDNF (Colucci-D'Amato et al. 2020). In the prefrontal cortex and hippocampus of the lipopolysaccharide-induced neuroinflammation model, BDNF levels were shown to be lower (Zhang et al. 2014). It has been reported that mice under restraint stress had less BDNF mRNA expression in the hippocampal region (Ieraci et al. 2015). It was discovered that the platelet BDNF levels were lower in patients with drug-naive severe depression. Additionally, a link between BDNF levels and the severity of the condition has been found to be unfavorable (Karege et al. 2002). It was believed that long-term BDNF expression improved neuronal survival and protected neurons from the harmful effects of chronic stress (Bathina and Das 2015). The etiology of depression and the mechanism of action of antidepressants were both associated with the BDNF/phospho-cAMP response element-binding protein (CREB) signaling pathway (Xue et al. 2016).
Another significant neurotrophic factor made by oligodendrocytes and astrocytes is the S100 protein. It is thought to be a biomarker for depression and may play a part in the etiopathogenesis of the condition (Schroeter et al. 2013). It controls calcium homeostasis, cell proliferation, differentiation, and survival (Donato et al. 2009). It works by promoting neurons’ proliferation, maintaining their survival, and fostering their development (Ambree et al. 2015).
Although the negative effects of chronic stress on cognitive functions are widely documented, the mechanisms underlying these effects are still not fully understood. Under normal physiological circumstances, amyloid β (Aβ) peptides are produced, but excessive production results in neurotoxic effects and cognitive deficiencies (Xie et al. 2016). Although several potential pathways for the negative effects of chronic stress on cognitive functions have been proposed, the role of Aβ peptides in the pathophysiology of depression has not been fully investigated yet. Both preclinical and clinical investigations linked the etiology of depression to glutamatergic system dysfunction (Henter et al. 2018). N-methyl-D-aspartate (NMDA), one of the glutamate receptor subtypes, is a ligand-gated ion channel that opens when an agonist binds to it. Functional NMDA receptors (NMDAR) are heterotetrametric complexes mainly composed of GluN1 and GluN2 subunits (von Engelhardt et al. 2009). GluN2A (NR2A) and GluN2B (NR2B), NMDA receptors with the highest affinity for glutamate, have different roles in synaptic plasticity, including long-term potentiation (LTP), learning and memory activities, and emotional processes (Monaco et al. 2015). Neuropsychiatric conditions like Parkinson’s or Alzheimer’s disease, epilepsy, depression, schizophrenia, and ischemia injury have been associated with hyper- or hypoactivation of NMDA receptors (Paoletti et al. 2013).
Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) commonly used to treat human depression, anxiety, compulsive behavior, and eating disorders. It acts in the central nervous system by blocking the transport of serotonin, leading to its accumulation in the synaptic cleft, which in turn results in an attenuation of symptoms related to anxiety and depression (i.e., anxiolytic effect) (Kaye et al. 2001).
A fresh hope for treating depression and cognitive deficits in depression is vortioxetine (VOR), an antidepressant with multimodal activity (Sanchez et al. 2015). To fully comprehend the neurobiological mechanisms behind the antidepressant and cognitive effects of VOR, more study is required. We propose that neurotrophic factors, glutamatergic neurotransmission, and Aβ may all have a role in how VOR improves the symptoms of depression.
The current study’s objective was to ascertain how VOR affected BDNF, CREB, S100, Aβ, and NR2B in order to reduce tissue damage, anxiety, depression-like behaviors, and depression in rats using an experimental depression model that was developed using a chronic unpredictable stress model.
Materials and methods
Wistar Albino male 4-week-old rats (125±25 g) were used in this investigation. They were purchased from the Suleyman Demirel University’s Research Center of Experimental Animals and Medical Research Application in Isparta, Turkey. The study population was determined as 28 with G-power program by taking impact size 0.5, α=0.05, power (1-β) =0.90 at a confidence level of 90% and a substitute group composed of 7 individuals was added. Rats in the study and control groups were maintained in separate rooms, unless otherwise stated. Throughout the experiment, the animals were maintained in rooms with controlled humidity and temperature (humidity level of 55 ± 5%, room temperature of 22±2 °C; 12-h dark/light cycle).
Throughout the experiment, rats had unrestricted access to water and food, except during the CUMS application periods in the CUMS groups. The Suleyman Demirel University’s Ethical Committee on Care and Use of Experimental Animal Resources (Date: 7/1/2021 Number: 01/09) gave its approval to all experimental methods. At every stage of the trial, ARRIVE (the Animal Research: Reporting in Vivo Experiments) 2.0 principles were carefully followed.
Experimental protocol
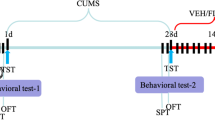
All of the animals were given a week to adjust before the experiment began. After the adaptation phase, the rats were randomly divided into four experimental groups, each with 7 animals. Group I (control) was not subjected to any stress; group II (chronic unpredictable mild stress, or CUMS) was subjected to the CUMS procedure; group III (CUMS+VOR) underwent the CUMS procedure and was treated with VOR at a dose of 10 mg/kg intraperitoneally in the last 3 weeks of the study; and group IV (CUMS+FLU) was used as positive control and subjected to the CUMS procedure and treated with FLU at a dose of 10 mg/kg intraperitoneally in the final three weeks of the study (Fig. 1). The CUMS-derived model used in previous studies was also employed in this study in a similar manner, with only minor modifications made to the order of stressor applications (Willner et al. 1987). Rats were individually housed in cages for 6 weeks and subjected to CUMS with the following stressors: cage tilted by 45° for 4 h, water restriction for 1 day, fasting for 1 day, continuous illumination, wet pad for 4 h, behavioral restrictions for 4 h by limiting their movement in a specific area, social stress by placing rats in soiled cages of other rats for 4 h, water stress induced by placing rats in an empty cage with 1 cm of water in the bottom for 4 h, and isolation (kept alone in the cage) for 4 h. A 2-day break was taken between water restriction and food deprivation. No stressor was applied for two or more consecutive days. Only one stress was induced at a time. VOR (synthesized by H. Lundbeck A/S, Istanbul, Turkey) and fluoxetine (FLU) (synthesized by Mustafa Nevzat A/S, Istanbul, Turkey) were dissolved in distilled water and administered at a dose of 10 mg/kg as previously described (Eskelund et al. 2017). The CUMS and control groups received 0.9% sterile saline injections. For a period of 3 weeks, all solutions were freshly prepared each day at the same time and administered intraperitoneally at a volume of 1 ml/kg.
Schematic diagram of the timeline for CUMS, drug treatment and behavioral testing schedule. CUMS, chronic unpredictable mild stress; ip, intraperitoneal. After environmental adaption for 1 week, rats were randomly divided into four groups on the last day of the week. Groups other than the control group were subjected to CUMS for 6 weeks. Drug treatments were administered in the last 3 weeks of the experiment. Behavioral tests were conducted between 42 and 45 days
Behavior tests
The sucrose preference and forced swimming tests were conducted 1 day apart during the daytime phase (9 am to 3 pm) under low light conditions (15 lux).
For the sucrose preference test (SPT), the animals were given two bottles of 1% w/v sucrose solution and 100 g ad libitum on the first day to acclimate them to the taste of sucrose (Zhang et al. 2019). On the second day, one of the bottles was replaced with water. The rats were deprived of food and water for 23 h on the third day. One bottle contained 100 ml of a 1% sucrose solution, while the other bottle held the same volume of pure water. The rats were given 1 h to choose between the two bottles of liquids. To prevent potential preference effects, the positions of the bottles were switched after 30 min. The sucrose preference percentage was calculated using the formula: sucrose preference percentage = sucrose consumption / (sucrose consumption + water consumption). Sucrose preference is used as an index of anhedonia, a key symptom of depression (Liu et al. 2018). The link between reduced sucrose consumption and anhedonia was an interpretation rather than a direct measure of behavior results supported by the other methods of brain tissue evaluation.
The FST was conducted on rats following the method with a few modifications to identify whether they displayed depressive or hopeless behaviors (immobility and active swimming activity) (Porsolt et al. 1978). Rats were individually kept in plastic cylinders (80 × 40 cm) filled with 50 cm of water maintained at 24± 1°C. Each rat floated in the water for a total of 6 min, and its immobility time was recorded. The test was recorded using a Sony SSC-DC398P video camera, and the behavioral patterns of rats in the FST were examined using the Smart Version 2.0 application.
The rats were humanely euthanized under the influence of ketamine (90 mg/kg, 10%) and xylazine (10 mg/kg, 2%) anesthesia after completing all behavioral tests. Although anesthesia can alter some biochemical markers, in this study, neuroprotective ketamine was used, and efforts were made to prevent artifacts since brain markers were studied. Ketamine was preferred as an anesthesia method not to affect the histopathological and immunohistochemical findings (Braeuninger and Kleinschnitz 2009). Since the same method was applied to all groups and group averages were taken, it is believed that the average of the biochemical parameters will not change. In order to avoid damaging brain tissue, customized equipment such as toothless forceps, curved-tip scissors, and brain knife was used to harvest hippocampus, hypothalamus, and prefrontal cortex from both hemispheres after washing in cold phosphate buffer solution. The routine process was followed for preparing and storing the tissue; the samples were fixed in 10% buffered formalin solution for histopathological and immunohistochemical examinations, and stored at −80°C for other analyses.
Reverse transcriptase-polymerase chain reaction
Hippocampal mRNA expression levels of NR2B and BDNF genes were determined by RT-PCR. RT-PCR was performed with Roche LightCycler 480 II system. The primary sequences used for amplification of ACTB (internal control), NR2B, and BDNF genes are shown in Table 1. For this, approximately 40–50 mg of thawed hippocampus tissue was homogenized in 1 ml of triazole. Using the High Pure RNA Tissue Kit (Roche Diagnostic), total RNA was extracted from tissue samples. The A260:A280 ratios of the obtained RNA samples ranged from 1.6 to 1.8. Following the manufacturer’s instructions, complementary DNA (cDNA) was created from the total RNA samples obtained using the RevertAid RT Reverse Transcription Kit (Thermo ScientificTM). The generated cDNAs were used as templates for RT-PCR amplification using LightCycler® 480 SYBR Green I Master. The threshold cycle (CT) of the genes was calculated using the acquired data, and normalization was performed using the internal control. After computing the relative expression levels using the 2−∆∆CT technique, the results were compared to the control group.
Biochemical evaluations
A glass Teflon homogenizer was used to first homogenize the samples, and then a homogenization buffer containing a protease inhibitor was used to sonicate the mixture. The homogenates were centrifuged at 13,000 g for 10 min in a refrigerated centrifuge to produce the supernatants. NR2B was analyzed by the ELISA method using My BioSource (CA, San Diego, USA) and BDNF E-Lab Science (Houston, TX, USA) commercial kit. There are seven standards in the NR2B and BDNF kit with different concentrations between 40–640 pg/ml and 31.25–2000 pg/ml, respectively. Standards were pipetted in duplicate, and a standard optical density–concentration graph was drawn based on the optical density values obtained, and the concentrations of the working samples were calculated using this graph. The results were then reported as the concentration per microgram of protein. The sensitivity limit of the kit for NR2B was 1.0 pg/ml, and the sensitivity limit of the kit for BDNF was 18.75 pg/ml.
Histopathological method
The tissue samples from the prefontal cortex were collected during necropsy and fixed in a 10% neutral formalin solution. After a 2-day fixation period, the brain samples were routinely processed and paraffin-embedded using an automated tissue processor. Then, 5-μm-thick sections were cut from the paraffin blocks using a fully automatic rotary microtome. The sections were stained with hematoxylin-eosin (H&E) and covered with a glass coverslip before being evaluated under a light microscope.
Immunohistochemical examinations
Sections taken onto polylysined slides were immunostained with BDNF [anti-BDNF Picoband antibody (PB9075), Boster Bio, CA, USA], CREB [anti-CREB/CREB1 Picoband antibody (PB9100), Boster Bio, CA, USA], Aβ (β amyloid 1-42 antibody (bs-0076R) Bioss Antibodies Inc., MA, USA), and S100 (anti-S 100 beta antibody, Astrocyte Marker, ab868, Abcam, Cambridge, UK) using the streptavidin biotin technique. We utilized 1/100 dilutions of all primary antibodies. After 60 min of incubation with primary antibodies, sections were immunohistochemically stained with biotinylated secondary antibodies and streptavidin-alkaline phosphatase conjugate. A ready to use commercial kit (EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC kit (ab80436) (Abcam, Cambridge, UK) was utilized as secondary antibody and diaminobenzidine (DAB) as chromogen. The antibody dilution solution was used for negative controls in place of the initial antiserum phase. All tests were carried out using blinded samples. Immunohistochemical staining was used to assess the expression of each antigen individually. To determine the number of positive cells, 100 cells (20 cells from each) were counted in five randomly selected areas of the prefrontal cortex, using a magnification of 40×. The immunohistochemical scores were calculated and analyzed using ImageJ software (version 1.48, National Institutes of Health, Bethesda, MD). Prior to counting, the images were cropped, separated by color channel, and any artifacts were removed. A selection tool was used to highlight the regions of interest, and cells located within these regions were counted using the counter tool in the software. Positive staining was identified by a brown color, and only cells that stained bright brown were considered positive. Counts were performed by a pathologist who was blinded to the group assignment. The same areas of the prefrontal cortex were examined for all rats, and the results were statistically evaluated. A semi-quantitative immunohistochemical scoring analysis was used only to evaluate Aβ because this marker was localized in the extracellular matrix. The following scoring system was used: (0) negative, (1) slight and focal staining, (2) slight and diffuse staining, and (3) marked and diffuse staining. Five separate areas were examined under a 40× objective magnification in each section. Microphotography was performed using the Database Manual Cell Sens Life Science Imaging Software System (Olympus Co., Tokyo, Japan).
Statistical methods
The data were analyzed using mean±standard deviation. One-way ANOVA was used to examine the differences between the groups. The Mann-Whitney U test was utilized to compare the immunohistochemical expression values between the groups. To assess the consistency of the distribution of the number of positive cells across the experimental groups, a chi-squared goodness of fit test was performed. The Duncan test was used for the post hoc comparison of means. For statistical analyses, SPSS 22.0 program pack was used (SPSS Inc., Chicago, IL, USA). The significance level was chosen as p<0.05. The “fold change” (FC) denoting and F values levels the ratio of the average expression of a particular gene or protein in one group compared to another group was given together with the p-value for immunohistochemical parameters.
Results
Vortioxetine improved behavior changes associated with CUMS in rats
In this study, the FST and SPT were utilized to assess anhedonia-like behaviors, such as immobility, and to evaluate the core depressive symptom of reduced interest in pleasurable stimuli, which is considered the gold standard for measurement. As shown in Fig. 2A, the rats in the control group (83.4 ± 7.3%, p=0.002 vs CUMS), CUMS+VOR group (84.2 ± 7.5%, p=0.001 vs CUMS), and CUMS+FLU group (86.7 ± 5.4%, p<0.001 vs CUMS) all exhibited significantly higher sucrose preference rates compared to the rats in the CUMS group (69.4 ± 3.2%). The sucrose preference rates of rats in the control, CUMS+VOR, and CUMS+FLU groups did not differ significantly from each other. As depicted in Fig. 2B, the rats in the control (208.1 ± 14.5 s, p=0.030 vs CUMS), CUMS+VOR (237.5 ± 13.4 s, p=0.001 vs CUMS), and CUMS+FLU groups (217.4 ± 22.7 s, p=0.010 vs CUMS) demonstrated significantly longer mobility periods compared to the rats in the CUMS group (141.20 ± 9.8 s). There was no statistically significant difference in mobility time between the rats in the control, CUMS+VOR, and CUMS+FLU groups in the FST (F=11.26 for SPT and 6.80 for FST, DF=27 for both).
Vortioxetine ameliorates depression-like behaviors in CUMS rat. A Sucrose preference in the SPT for rats in each group B Mobility and immobility time in the FST for rats in each group. The values represent mean ± SD (n = 7). *p < 0.05, **p < 0.001 compared with the CUMS. CUMS, chronic unpredictable mild stress; VOR, vortioxetine; FLU, fluoxetine
BDNF and NR2B expressions in the hippocampus of rats suffered from CUMS
Based on our findings, rats exposed to CUMS exhibited significantly lower expression of NR2B compared to rats in the control group (FC=0.25, p=0.017). Following treatment with VOR and FLU, the expression of NR2B was upregulated. The immunoreactivity of NR2B in the hippocampus of rats in the CUMS+VOR and CUMS+FLU groups was higher than that in the CUMS group (FC=10.21, p<0.001, FC=5.62, p=0.003; respectively) (Fig. 3).
When comparing CUMS rats to controls, BDNF expression was significantly downregulated (FC=0.11, p=0.031). However, BDNF expression was upregulated after VOR and FLU administration. The CUMS, CUMS+VOR, and CUMS+FLU groups showed significantly increased expression of NR2B and BDNF in the hippocampus compared to the control group (FC=12.32, p=0.026, FC=9.86, p=0.025; respectively) (Fig. 4).
Vortioxetine increased BDNF level but not NR2B in the hypothalamus of CUMS rats
In this study, ELISA was utilized to measure the levels of BDNF and NR2B in the hypothalamus. Although the NR2B level in the hypothalamus appeared lower in the CUMS group compared to the control group, as depicted in Fig. 3A, the difference was not statistically significant. However, rats exposed to CUMS exhibited significantly lower levels of BDNF in their hypothalamus compared to rats in the control group, as shown in Fig. 3B (p < 0.05). Treatment with VOR and FLU effectively reversed these alterations by increasing BDNF levels relative to the CUMS group (p < 0.05).
Histopathological and immunohistochemical findings
At the microscopic analysis of the prefrontal cortex, while the control group exhibited normal tissue histology, the cortex of the CUMS group showed a significant increase in the number of degenerative neurons prefrontal characterized by shrinkage and dark-stained neurons (Fig. 5). Immunohistochemical analysis also showed a small increase in extracellular Aβ immunoreactivity in the CUMS group (P<0.01 and F=4.5), while the other groups did not show any immunoreaction (Fig. 6). Moreover, the CUMS group had lower levels of intracytoplasmic BDNF expressions in the prefrontal cortex than the other groups (p<0.001 and F=378.24), which were restored by treatment with VOR and FLU (Fig. 7). Similarly, the CUMS group exhibited a significant decrease in intracytoplasmic CREB expression compared to the other groups (P<0.001 and F=275.43) (Fig. 8). Finally, the CUMS group exhibited a significant reduction in intracytoplasmic S100 immunoexpression compared to the other groups (p<0.001 and F=255.24) (Fig. 9).
Representative histopathological appearance of prefrontal cortex between the groups. A Normal tissue architecture in control group. B Numerous degenerated neurons (arrows) characterized by alterations in their shape and staining, shrinkage, and dark-stained neurons in CUMS group. C Decreased degenerative neurons (arrow) in CUMS+VOR group. D Normal tissue histology in CUMS+FLU group, HE, scale bars= 20 μm
β amyloid immunohistochemistry findings of prefrontal cortex among the groups. A Negative expression in control group. B Slight β amyloid accumulation (arrows) in CUMS group. C Negative immunoreaction in CUMS+VOR group. D No expression in CUMS+FLU group, streptavidin biotin peroxidase method, scale bars= 20 μm
Representative images of BDNF expressions of prefrontal cortex between the groups. A Marked expression in control groups. B Decreased immune reaction in neurons in prefrontal cortex (arrows) in CUMS group. C Increased expression in CUMS+VOR group. D Increased immunoreaction in CUMS+FLU, streptavidin biotin peroxidase method, scale bars= 20 μm
CREB immunochemistry findings of prefrontal cortex between the groups. A Normal expression in control group. B Markedly decreased expression in neurons in CUMS group. C Increased immunoreaction in CUMS+VOR group. D Increased expression in CUMS+FLU group, streptavidin biotin peroxidase method, scale bars= 20 μm
S-100 immunohistochemistry of prefrontal cortex among the groups. A Marked immunoreaction in control group. B Decreased expression in neurons (arrows) in CUMS group. C Increased expression in CUMS+VOR group. D Increased immunoreaction in CUMS+FLU group, streptavidin biotin peroxidase method, scale bars= 20 μm
According to the study findings, chronic stress can potentially elevate the susceptibility to neural disorders like Alzheimer’s disease and neuronal damage. Consequently, the administration of antidepressant medication may serve as a viable approach to mitigate stress-related risk factors. Additionally, the study demonstrated the protective effects of VOR and FLU against CUMS-induced brain damage. The statistical analysis results of the immunohistochemistry data are presented in Table 2.
Discussion
VOR is a new antidepressant with multimodal effects as 5-HT3, 5-HT7, and 5-HT1-D receptor antagonists, 5-HT transporter inhibitor, 5HT1-A agonist, and 5-HT1-B partial agonist. It is currently unknown if this is the only mechanism by which VOR exerts its antidepressant effects. There has not been much molecular study done on the pharmacological effects of VOR. New treatment targets for depression could be created using other pathways. Our study induced depression-like behaviors in rats subjected to various types of chronic stress, and these animals displayed core symptoms similar to those seen in human depression. In order to understand the possible mechanisms of CUMS-mediated brain structural and molecular changes and to reveal the effect of VOR on these changes, we analyzed BDNF, CREB, S100, Aβ, and NR2B through biochemical, genetic analyses, histopathological and immunohistochemical methods. The results of the current investigation demonstrated that hippocampus-specific NR2B and BDNF expressions were downregulated in CUMS rats, but were restored by VOR injection. In the prefrontal cortices of the CUMS group, we found that VOR therapy significantly increased S100 expression and raised levels of Aβ and CREB expressions, while decreasing neuronal degeneration. These results suggest a novel antidepressant mechanism for VOR and a different direction for future study into depression therapy targets.
The hippocampus is a crucial brain region involved in learning and memory processes. BDNF is a neurotrophic factor that affects hippocampal plasticity and plays a crucial role in learning and memory functions. NR2, on the other hand, is a subunit of the glutamate receptor that affects the function of hippocampal neurons. Stress can lead to learning disorders, and the hippocampus is also involved in modulating the stress response (Maletic-Savatic et al. 1999). Therefore, in this study, NR2 and BDNF analyses were conducted to investigate the effects of stress on the hippocampus. Histopathological and immunohistochemical analyses of the hippocampus could not be performed due to insufficient tissue. To simulate the challenges faced by people in daily life, we employed CUMS, a widely accepted method for animal modeling of depression. To determine if rats exhibited depressive-like behavior, we used two commonly used tests in behavioral research: the sucrose preference test (SPT) and the forced swimming test (FST). Compared to the control group, the CUMS rats displayed a decrease in sucrose preference, which is indicative of anhedonia-like behavior, a core depressive symptom (Liu et al. 2018). Reduced efforts to escape and physical inactivity during the FST are considered markers of behavioral despair (Ramos-Hryb et al. 2019). Our findings show that CUMS induces depressive-like behavior while VOR and FLU treatments alleviate this behavior. According to the behavioral tests, the CUMS + VOR and CUMS + FLU groups exhibited significantly higher sucrose preference rates and shorter immobility times during the FST compared to the CUMS group. This result demonstrates the antidepressant effects of VOR and FLU. Our results on VOR’s behavioral effects in rats are consistent with those of previous studies (Lu et al. 2018; Yu et al. 2017). Neurotoxicity can result from excessive stimulation or dysfunction of the neuroendocrine system through the hypothalamic-pituitary-adrenal (HPA) axis, which is activated in response to stress (Milligan Armstrong et al. 2021). It is known that stress exposure can increase the risk of neurodegeneration. Several neurodegenerative disorders, ranging from mild cognitive impairment to dementia and Alzheimer’s disease, have been associated with chronic stress and early life stress (Desplats et al. 2020). Stress can either cause or accelerate neurodegeneration. Stress-reduction strategies in treatment may be effective against neurodegeneration. This study examined the prefrontal cortex histopathologically and found that chronic stress exposure had caused degenerative changes in the brain. We also observed that these changes were reversed by VOR and FLU treatments. This research suggests that both VOR and FLU can protect the brain against stress-induced neurodegeneration. Additionally, we examined the cortical level of Aβ in rats subjected to the CUMS protocol in this study. Our findings corroborate earlier research indicating that stress increases Aβ formation. A recent research reported that increased Aβ production and/or aggregation in the brain results in neuronal death, oxidative stress, and memory impairment (Leong et al. 2020). It was reported that VOR treatment enhances not just mood symptoms and functionality in patients with major depressive disorder, but also cognitive functions (Sanchez et al. 2015). According to our findings, VOR treatment prevented Aβ accumulation caused by chronic stress exposure. The mechanism underlying the improvement in cognitive performance with VOR administration in patients with depression may be the reduction in Aβ production. These findings are also consistent with a preclinical investigation conducted by Caruso et al. (2021), which showed that both FLU and VOR are capable of reversing the memory deficits and depressive-like phenotype caused by intracerebroventricular injection of Aβ oligomers in mice.
A deficiency of neurotrophic support appears to be a major factor in the marked atrophy and death of neurons linked to depressive behavior (Levy et al. 2018). Numerous preclinical, clinical, and postmortem studies have revealed a reduction in BDNF levels in depressive patients (Molendijk et al. 2014; Sheldrick et al. 2017). Additionally, chronic antidepressant therapy has been shown to increase BDNF levels in vivo, and BDNF delivered intrahippocampally or peripherally has antidepressant-like effects in experimentally induced murine model depression (Schmidt and Duman 2010; Bjorkholm and Monteggia 2016). BDNF is a neurotrophic factor that plays critical roles in neuron survival, axonal growth, and synaptic plasticity (Bathina and Das 2015).
According to the findings of the current study, prolonged stress exposure decreased BDNF expression in the prefrontal cortex, hippocampus, and hypothalamus of rats, but administration of VOR and FLU treatments significantly increased BDNF levels compared to the CUMS group. These study results are consistent with previous studies and support the view that VOR may exert a neuroprotective effect through BDNF in the brains of rats exposed to CUMS (Riga et al. 2016; Lu et al. 2018).
Numerous studies have reported that the expression and function of CREB, a transcription factor involved in the regulation of neurotrophic factors such as BDNF, vary in depression (Wang and Mao 2019). A recent study reported that CREB deletion is related to resistance in developing depression-like behavior in mice (Manners et al. 2019). Additionally, some antidepressants were reported to contribute to the improvement of symptoms by reversing the downregulated CREB/BDNF pathway in depression (Blendy 2006). The results of this research revealed that chronic stress exposure decreased CREB expression in the brain cortices of rats and VOR and FLU administration restored these levels. According to our findings, VOR can inhibit anxiety and depression-like behavioral changes through modification of the CREB/BDNF signaling pathway.
S100 proteins are calcium-binding proteins that regulate intracellular processes including neuron development and synaptogenesis, cell cycle regulation, transcription, DNA repair, and differentiation (Arora et al. 2019). It has been shown that S100 proteins contribute to the pathogenesis of various neuropsychiatric diseases such as Alzheimer’s disease, depression, or anxiety (Arora et al. 2019). Numerous earlier studies suggest that S100 proteins can be used as a depression biomarker, response to antidepressant treatment, and blood-brain barrier permeability (Ambree et al. 2015; Pearlman et al. 2014). Our results are in agreement with the available literature and indicate that S100 expressions are decreased in the prefrontal cortex of rats, and that VOR and FLU treatments lead to an increase in S100 expression.
NMDA receptors are implicated in the development of neural circuits, the formation of the central nervous system, synaptic plasticity, learning, and memory (Henter et al. 2018). Additionally, glutamate-induced excitotoxicity caused by prolonged stress is mediated by NMDA receptors (Autry et al. 2011). One of the NMDA receptors, NR2B, has been shown to contribute to the pathophysiology of psychiatric disorders such as bipolar and schizoaffective diseases, major depressive disorder, and schizophrenia (Pearlman and Najjar 2014).
In this study, CUMS exposed rats exhibited downregulated NR2B expression in the hippocampus, which could be reversed with treatment by VOR and FLU. But when we used the ELISA method for evaluation of the hypothalamus, we found no variation in NR2B levels between the groups. As shown in the present study, similar to previous studies, NR2B expression was lower in the hippocampus of mice with depression-like behaviors (Dong et al. 2010; Yang et al. 2018; Zhou et al. 2021).
According to the study by Feyissa et al. (2009), there was a marked loss in NR2B immunoexpression in the prefrontal cortex compared to healthy controls in the postmortem brain tissue examination of subjects diagnosed with major depressive disorder (Feyissa et al. 2009). Treatment with fluoxetine was demonstrated to restore NR2B loss in the nucleus accumbens in rats subjected to chronic stress (Jiang et al. 2013).
The effect of vortioxetine, a multimodal serotonergic compound, on glutamate neurotransmission is not clear. Vortioxetine’s antidepressant and procognitive effects are hypothesized to be mediated by regulating glutamate neurotransmission and enhancing neuroplasticity (Dale et al. 2015). The results of the present study indicate that the antidepressant effects of VOR in rats exposed to CUMS are mediated by NR2B. The possible mechanisms underlying the effects of VOR in ameliorating the effects of CUMS may be related to the modulation of the serotonin system. VOR may contribute to the regulation of imbalances in the serotonin system by inhibiting serotonin reuptake and interacting with various serotonin receptors. This modulation may play a role in regulating mood and alleviating symptoms of depression. Additionally, VOR has the potential to affect the transmission of chemical signals involved in cognitive function and mood regulation by enhancing the release of neurotransmitters such as glutamate and acetylcholine. Furthermore, VOR is believed to contribute to the improvement of CUMS symptoms through its neuroprotective effects, including the promotion of neurogenesis, enhancement of synaptic plasticity, reduction of oxidative stress, and prevention of inflammation in the brain. While VOR and FLU are both used to treat depression, they differ in their mechanisms of action and effects on anxiety. VOR is an antidepressant with multimodal effects that modulates various neurotransmitter systems, including serotonin, dopamine, and glutamate. Although VOR has been shown to have some anxiolytic effects, they are generally not as potent as those of FLU, a selective serotonin reuptake inhibitor that is frequently used to treat anxiety disorders. In this study, FLU was used as a positive control for its antidepressant effects, and our results suggest that VOR is just as effective as FLU in treating depression in this rodent model of CUMS. To reduce the number of animals used in this study, we did not include groups receiving VOR and FLU in non-stressed animals.
In this study, we used a variety of techniques including ELISA, PCR, histopathology, and immunohistochemistry to assess neurodegeneration. Histopathological examination allowed us to visualize morphological changes in neurons (such as shrinkage and discoloration) and detect proteins associated with neurodegeneration via immunohistochemical staining. These changes were then compared between experimental groups.
This study has some limitations, with the most significant one being the inability to assess blood hormone levels in animals. One other limitation of our study was that we were unable to analyze serum samples, which would have allowed for diagnosis of neurological damage. Due to financial constraints, the study did not include a group of control animals administered VOR and FLU and the number of groups could not be increased.
Our results clearly demonstrated the negative effects of chronic unpredictable mild stress on the brain. There is a need for more comprehensive future studies to better demonstrate the effects of CUMS and treatment choices.
Conclusions
Our study found that VOR reduced neuronal deterioration and depressive-like behaviors as much as FLU. Additionally, according to our research, VOR achieved this effect by enhancing the BDNF/CREB pathway and increasing levels of S100 and NR2B expression. More research is needed to fully understand the effects and molecular mechanisms of vortioxetine in CUMS.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ambree O, Bergink V, Grosse L, Alferink J, Drexhage HA, Rothermundt M, Arolt V, Birkenhager TK (2015) S100B serum levels predict treatment response in patients with melancholic depression. Int J Neuropsychopharmacol 19(3):pyv103. https://doi.org/10.1093/ijnp/pyv103
Arora P, Sagar R, Mehta M, Pallavi P, Sharma S, Mukhopadhyay AK (2019) Serum S100B levels in patients with depression. Indian J Psychiatry 61(1):70–76. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_391_16
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354):91–95. https://doi.org/10.1038/nature10130
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 11(6):1164–1178. https://doi.org/10.5114/aoms.2015.56342
Bjorkholm C, Monteggia LM (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79. https://doi.org/10.1016/j.neuropharm.2015.10.034
Blendy JA (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59(12):1144–1150. https://doi.org/10.1016/j.biopsych.2005.11.003
Braeuninger S, Kleinschnitz C (2009) Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Trans Stroke Med. 1:8. https://doi.org/10.1186/2040-7378-1-8
Caruso G, Grasso M, Fidilio A, Torrisi SA, Musso N, Geraci F, Tropea MR, Privitera A, Tascedda F, Puzzo D, Salomone S, Drago F, Leggio GM, Caraci F (2021) Antioxidant activity of fluoxetine and vortioxetine in a non-transgenic animal model of Alzheimer’s disease. Front Pharmacol 12:809541. https://doi.org/10.3389/fphar.2021.809541
Colucci-D'Amato L, Speranza L, Volpicelli F (2020) Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci 21(20):7777. https://doi.org/10.3390/ijms21207777
Dale E, Bang-Andersen B, Sanchez C (2015) Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol 95(2):81–97. https://doi.org/10.1016/j.bcp.2015.03.011
Desplats P, Gutierrez AM, Antonelli MC, Frasch MG (2020) Microglial memory of early life stress and inflammation: susceptibility to neurodegeneration in adulthood. Neurosci Biobehav Rev 117:232–242. https://doi.org/10.1016/j.neubiorev.2019.10.013
Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793(6):1008–1022. https://doi.org/10.1016/j.bbamcr.2008.11.009
Dong J, Min S, Wei K, Li P, Cao J, Li Y (2010) Effects of electroconvulsive therapy and propofol on spatial memory and glutamatergic system in hippocampus of depressed rats. J ECT 26(2):126–130. https://doi.org/10.1097/yct.0b013e3181a9947a
Eskelund A, Li Y, Budac DP, Muller HK, Gulinello M, Sanchez C, Wegener G (2017) Drugs with antidepressant properties affect tryptophan metabolites differently in rodent models with depression-like behavior. J Neurochem 142(1):118–131. https://doi.org/10.1111/jnc.14043
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B (2009) Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33(1):70–75. https://doi.org/10.1016/j.pnpbp.2008.10.005
Henter ID, de Sousa RT, Zarate CA Jr (2018) Glutamatergic modulators in depression. Harv Rev Psychiatry 26(6):307–319. https://doi.org/10.1097/HRP.0000000000000183
Ieraci A, Mallei A, Musazzi L, Popoli M (2015) Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 25(11):1380–1392. https://doi.org/10.1002/hipo.22458
Jiang B, Wang W, Wang F, Hu ZL, Xiao JL, Yang S, Zhang J, Peng XZ, Wang JH, Chen JG (2013) The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol Psychiatry 74(2):145–155. https://doi.org/10.1016/j.biopsych.2012.10.031
Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM (2002) Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 109(2):143–148. https://doi.org/10.1016/s0165-1781(02)00005-7
Kaye WH, Nagata T, Weltzin TE, Hsu LK, Sokol MS, McConaha C, Plotnicov KH, Weise J, Deep D (2001) Double-blind placebo-controlled administration of fluoxetine in restricting- and restricting-purging-type anorexia nervosa. Biol Psychiatry 49:644–652. https://doi.org/10.1016/s0006-3223(00)01013-1
LeMoult J, Humphreys KL, Tracy A, Hoffmeister JA, Ip E, Gotlib IH (2020) Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J Am Acad Child Adolesc Psychiatry 59(7):842–855. https://doi.org/10.1016/j.jaac.2019.10.011
Leong YQ, Ng KY, Chye SM, Ling APK, Koh RY (2020) Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab Brain Dis 35(1):11–30. https://doi.org/10.1007/s11011-019-00516-y
Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl) 235(8):2195–2220. https://doi.org/10.1007/s00213-018-4950-4
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13(7):1686–1698. https://doi.org/10.1038/s41596-018-0011-z
Lu Y, Ho CS, McIntyre RS, Wang W, Ho RC (2018) Effects of vortioxetine and fluoxetine on the level of Brain Derived Neurotrophic Factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res Bull 142:1–7. https://doi.org/10.1016/j.brainresbull.2018.06.007
Maletic-Savatic M, Malinow R, Svoboda K (1999) Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283(5409):1923–1927. https://doi.org/10.1126/science.283.5409.1923
Malhi GS, Mann JJ (2018) Depression. Lancet 392(10161):2299–2312. https://doi.org/10.1016/S0140-6736(18)31948-2
Manners MT, Brynildsen JK, Schechter M, Liu X, Eacret D, Blendy JA (2019) CREB deletion increases resilience to stress and downregulates inflammatory gene expression in the hippocampus. Brain Behav Immun 81:388–398. https://doi.org/10.1016/j.bbi.2019.06.035
Milligan Armstrong A, Porter T, Quek H, White A, Haynes J, Jackaman C, Villemagne V, Munyard K, Laws SM, Verdile G, Groth D (2021) Chronic stress and Alzheimer’s disease: the interplay between the hypothalamic-pituitary-adrenal axis, genetics and microglia. Biol Rev Camb Philos Soc 96(5):2209–2228. https://doi.org/10.1111/brv.12750
Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM (2014) Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry 19(7):791–800. https://doi.org/10.1038/mp.2013.105
Monaco SA, Gulchina Y, Gao WJ (2015) NR2B subunit in the prefrontal cortex: a double-edged sword for working memory function and psychiatric disorders. Neurosci Biobehav Rev 56:127–138. https://doi.org/10.1016/j.neubiorev.2015.06.022
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400. https://doi.org/10.1038/nrn3504
Pearlman DM, Brown JR, MacKenzie TA, Hernandez F Jr, Najjar S (2014) Blood levels of S-100 calcium-binding protein B, high-sensitivity C-reactive protein, and interleukin-6 for changes in depressive symptom severity after coronary artery bypass grafting: prospective cohort nested within a randomized, controlled trial. PLoS One 9(10):e111110. https://doi.org/10.1371/journal.pone.0111110
Pearlman DM, Najjar S (2014) Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res 157(1-3):249–258. https://doi.org/10.1016/j.schres.2014.05.001
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47(4):379–391. https://doi.org/10.1016/0014-2999(78)90118-8
Poyhonen S, Er S, Domanskyi A, Airavaara M (2019) Effects of neurotrophic factors in glial cells in the central nervous system: expression and properties in neurodegeneration and injury. Front Physiol 10:486. https://doi.org/10.3389/fphys.2019.00486
Ramos-Hryb AB, Bahor Z, McCann S, Sena E, MacLeod MR, Lino de Oliveira C (2019) Protocol for a systematic review and meta-analysis of data from preclinical studies employing forced swimming test: an update. BMJ Open Sci 3(1):e000043. https://doi.org/10.1136/bmjos-2017-000043
Rauti R, Cellot G, D'Andrea P, Colliva A, Scaini D, Tongiorgi E, Ballerini L (2020) BDNF impact on synaptic dynamics: extra or intracellular long-term release differently regulates cultured hippocampal synapses. Mol Brain 13(1):43. https://doi.org/10.1186/s13041-020-00582-9
Riga MS, Sanchez C, Celada P, Artigas F (2016) Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology 108:73–81. https://doi.org/10.1016/j.neuropharm.2016.04.023
Sanchez C, Asin KE, Artigas F (2015) Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. https://doi.org/10.1016/j.pharmthera.2014.07.001
Schmidt HD, Duman RS (2010) Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35(12):2378–2391. https://doi.org/10.1038/npp.2010.114
Schroeter ML, Sacher J, Steiner J, Schoenknecht P, Mueller K (2013) Serum S100B represents a new biomarker for mood disorders. Curr Drug Targets 14(11):1237–1248. https://doi.org/10.2174/13894501113149990014
Sheldrick A, Camara S, Ilieva M, Riederer P, Michel TM (2017) Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) levels in post-mortem brain tissue from patients with depression compared to healthy individuals - a proof of concept study. Eur Psychiatry 46:65–71. https://doi.org/10.1016/j.eurpsy.2017.06.009
Torres-Berrio A, Issler O, Parise EM, Nestler EJ (2019) Unraveling the epigenetic landscape of depression: focus on early life stress. Dialogues Clin Neurosci 21(4):341–357. https://doi.org/10.31887/DCNS.2019.21.4/enestler
von Engelhardt J, Doganci B, Seeburg PH, Monyer H (2009) Synaptic NR2A- but not NR2B-containing NMDA receptors increase with blockade of ionotropic glutamate receptors. Front Mol Neurosci 2:19. https://doi.org/10.3389/neuro.02.019.2009
Wang JQ, Mao L (2019) The ERK pathway: molecular mechanisms and treatment of depression. Mol Neurobiol 56(9):6197–6205. https://doi.org/10.1007/s12035-019-1524-3
Willner P, Towell A, Sampson D, Sophokleous S, Muskat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93(3):358–364. https://doi.org/10.1007/BF00187257
Xie F, Zhao Y, Ma J, Gong JB, Wang SD, Zhang L, Gao XJ, Qian LJ (2016) The involvement of homocysteine in stress-induced Abeta precursor protein misprocessing and related cognitive decline in rats. Cell Stress Chaperones 21(5):915–926. https://doi.org/10.1007/s12192-016-0718-0
Xue W, Wang W, Gong T, Zhang H, Tao W, Xue L, Sun Y, Wang F, Chen G (2016) PKA-CREB-BDNF signaling regulated long lasting antidepressant activities of Yueju but not ketamine. Sci Rep 6:26331. https://doi.org/10.1038/srep26331
Yang Y, Ju W, Zhang H, Sun L (2018) Effect of ketamine on LTP and NMDAR EPSC in hippocampus of the chronic social defeat stress mice model of depression. Front Behav Neurosci 12:229. https://doi.org/10.3389/fnbeh.2018.00229
Yu H, Chen JJ, Zeng BQ, Zhong QP, Xu JP, Liu YG (2017) Role of cAMP/CREB/BDNF signaling pathway in anti-depressive effect of vortioxetine in mice. Nan Fang Yi Ke Da Xue Xue Bao 37(1):107–112. https://doi.org/10.3969/j.issn.1673-4254.2017.01.20
Zhang H, He W, Huang Y, Zeng Z, Yang X, Huang H, Wen J, Cao Y, Sun H (2019) Hippocampal metabolic alteration in rat exhibited susceptibility to prenatal stress. J Affect Disord 259:458–467. https://doi.org/10.1016/j.jad.2019.08.002
Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K (2014) Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 18(4):pyu077. https://doi.org/10.1093/ijnp/pyu077
Zhou XM, Liu CY, Liu YY, Ma QY, Zhao X, Jiang YM, Li XJ, Chen JX (2021) Xiaoyaosan alleviates hippocampal glutamate-induced toxicity in the CUMS rats via NR2B and PI3K/Akt signaling pathway. Front Pharmacol 12:586788. https://doi.org/10.3389/fphar.2021.586788
Funding
This research received the Project Encouragement Award at the 24th Clinical Education Symposium of the Turkish Psychiatric Association (2-6 June 2021, Turkey). We are grateful to the Turkish Psychiatric Association for financial support.
Author information
Authors and Affiliations
Contributions
GÖÜ: project administration, conceptualization, methodology, investigation, writing—original draft, software. GE: investigation, visualization. KHÖ: investigation, data curation, resources. DKD: formal analysis, writing—review and editing. ÖÖ: conceptualization, methodology, investigation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ünal, G.Ö., Erkılınç, G., Öztürk, K.H. et al. The beneficial effects of vortioxetine on BDNF, CREB, S100B, β amyloid, and glutamate NR2b receptors in chronic unpredictable mild stress model of depression. Psychopharmacology 240, 2499–2513 (2023). https://doi.org/10.1007/s00213-023-06445-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06445-0