Abstract
A2A adenosine receptor antagonists have been proposed as a new therapy for Parkinson’s disease (PD). Since oxidative stress plays an important role in the pathogenesis of PD, we studied the effect of the selective A2A adenosine receptor antagonists 8-(3-chlorostyryl)caffeine (CSC) and 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385) on l-3,4-dihydroxyphenylalanine (l-DOPA)-induced hydroxyl radical generation using in vivo microdialysis in the striatum of freely moving rats. l-DOPA (100 mg/kg; in the presence of benserazide, 50 mg/kg) given acutely or repeatedly for 14 days generated a high level of hydroxyl radicals, measured by HPLC with electrochemical detection, as the product of their reaction with p-hydroxybenzoic acid (PBA). CSC (1 mg/kg) and ZM 241385 (3 mg/kg) decreased haloperidol (0.5 mg/kg)-induced catalepsy, while at low doses of 0.1 and 0.3 mg/kg, respectively, they did not display an effect. CSC (1 and 5 mg/kg) and ZM 241385 (3 and 9 mg/kg) given acutely, or CSC (1 mg/kg) and ZM 241385 (3 mg/kg) given repeatedly, increased the production of hydroxyl radicals in dialysates from rat striatum. Both acute and repeated administration of CSC (0.1 and 1 mg/kg) and ZM 241385 (3 mg/kg) decreased l-DOPA-induced generation of hydroxyl radicals. However, a high single dose of either CSC (5 mg/kg) and ZM 241385 (9 mg/kg) markedly potentiated the effect of l-DOPA on hydroxyl radical production. The increase in hydroxyl radical production by acute and chronic injection of CSC and ZM 241385 may be related to the increased release of dopamine (DA) and its metabolism in striatal dialysates. Similarly, increased DA release following a single high dose of CSC or ZM 241385 appears to be responsible for augmentation of l-DOPA-induced hydroxyl radical formation. Conversely, the inhibition of l-DOPA-induced production of hydroxyl radical by single and repeated low doses of CSC or repeated low doses of ZM 241385 may be related to reduced DA metabolism. Summing up, A2A antagonists, used as a supplement of l-DOPA therapy, depending on the dose used, may have a beneficial or adverse effect on ongoing neurodegenerative processes and accompanying oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a consequence of specific progressive neurodegeneration of substantia nigra (SN) pars compacta dopaminergic neurons, with the ensuing diminution of dopamine (DA) levels in the striatum and SN, accompanied by an increase in the number of glial cells, the disappearance of neuromelanin and appearance of intracytoplasmic eosinophilic inclusions—Lewy bodies which consist of aggregates of α-synuclein (Fahn and Sulzer 2004). Although the cause of neurodegeneration of SN neurons is unknown, several factors such as, e.g., environmental toxins, genetic factors, mitochondrial dysfunction and oxidative stress play an important role in the pathogenesis of PD (Fahn and Sulzer 2004). The role of oxidative stress, which is regarded as the main factor contributing to the etiology of PD, has been a matter of paramount interest (Halliwell 2006). SN cells appear to be particularly susceptible, due to presence of a high amount of DA and neuromelanin-bound iron, or a lower expression of vesicular monoamine transporter-2 (VMAT2) (Fahn and Sulzer 2004). DA is normally deaminated by monoamine oxidase (MAO), which results in the production of 3,4-dihydroxyphenylacetic acid (DOPAC) and hydrogen peroxide. DA is also metabolized by auto-oxidation, which leads to the formation of hydrogen peroxide in addition to very reactive DA quinones and semiquinones (Halliwell 2006). The excessive DA concentration in cytosol, e.g., after l-3,4-dihydroxyphenylalanine (l-DOPA) treatment (Chen et al. 2008), may suppress cellular defense processes and inhibit mitochondrial complex-I, as well as evoke proteosomal dysfunction, which indicates that DA is involved in oxidative stress (Przedborski et al. 1993; Saha et al. 2003; Gluck and Zeevalk 2004; Jana et al. 2007). Moreover, DA-associated oxidative stress may contribute to inflammatory reactions (Rowe et al. 1998) and microglia activation resulting in production of free radicals (Wu et al. 2003; Block et al. 2007).
Limitations of PD therapy with l-DOPA, e.g., dyskinesias, as well as reduced efficacy with long-term treatment (Cenci 2007) and suspected l-DOPA toxicity (Chen et al. 2008) are elements underlying the search for new antiparkinsonian drugs. Recently, therapy with adenosine A2A receptor antagonists has been proposed for treatment of PD (Schwarzschild et al. 2006).
A2A adenosine receptors are known to populate striatopallidal GABAergic neurons and to functionally oppose the actions of dopamine D2 receptors (Schwarzschild et al. 2006). A number of behavioral studies showed that A2A antagonists alone and in combination with l-DOPA relieved motor deficits in animal models of PD (Schwarzschild et al. 2006; Bishnoi et al. 2007; Morelli et al. 2007). Promising data obtained in non-human primates (Kanda et al. 1998) were instrumental in the launching of clinical trials with A2A antagonists in PD patients (Xu et al. 2005). The mechanism by which A2A antagonists improve parkinsonian motor dysfunctions is related to modulation of DA responses through existing A2A-D2 receptor interactions (Schwarzschild et al. 2006) and their ability to modulate GABA release and DA-dependent c-fos activation in the indirect striatopallidal pathway (Pollack and Fink 1995; Ochi et al. 2000). Presynaptically—by opposing D2 receptors—A2A receptors are able to regulate corticostriatal glutamatergic transmission (Tozzi et al. 2007). A2A receptor antagonists apparently increase DA release in an indirect manner, as A2A receptors have not been found in nigrostriatal DA neurons (Gołembiowska and Dziubina 2004a, b).
The converging epidemiological and experimental evidence has raised the possibility that A2A antagonists may exert neuroprotective properties in PD. Epidemiological studies have indicated an inverse relationship between the consumption of caffeine, a non-selective adenosine receptor antagonist, and the risk of developing PD (Ross et al. 2000; Ascherio et al. 2001). A protective effect of caffeine and more selective antagonists of A2A receptors, or genetic inactivation of A2A receptors, was observed in an animal MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity model (Xu et al. 2005; Chen et al. 2007) or in ischemia and excitotoxic brain injury models (Popoli et al. 2004; Chen et al. 2007). The mechanism by which A2A antagonists protect dopaminergic neurons has not been fully elucidated, but the extent of their effect on various types of neurons, e.g., glutamatergic nerve terminals and glial or immune cells, suggests its complex nature (Chen et al. 2007).
Because oxidative stress is regarded as the main factor contributing to the etiology of PD, and since SN cells appear to be highly vulnerable due to presence of a high level of DA, it seemed of crucial importance to determine whether A2A adenosine antagonists may regulate the release and metabolism of DA in nigrostriatal neurons. The effectiveness of a synergistic combination of l-DOPA and an A2A antagonist, shown in animal models (Wardas et al. 2001) and in parkinsonian patients (Xu et al. 2005) to counteract symptoms of PD, points the usefulness of A2A antagonists as a supplement to l-DOPA therapy. Therefore, a question arises as to whether the overproduction of DA and its auto-oxidation may be modulated by A2A antagonists. To this end, the present research was aimed at investigating the possibility of inducing oxidative stress by means of an elevated cytosolic DA level after administration of l-DOPA; and determining the action of A2A antagonists on the cellular production of hydroxyl radicals, as assessed with the use of in vivo microdialysis in freely moving rats. To determine the effective doses of A2A antagonist for biochemical experiments, the effect of 8-(3-chlorostyryl)caffeine (CSC) and 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385) on haloperidol-induced catalepsy was determined.
Materials and Methods
Animals
In vivo microdialysis studies were conducted on male Wistar rats (250–300 g), bred at the Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland. Rats were housed in temperature- and humidity-controlled rooms on a 12-h light/dark cycle, with free access to filtered tap water and standard pelleted laboratory chow throughout the study. The experimental procedures and housing conditions used were in strict accordance with the Polish legal regulations concerning experiments on animals (Dz. U. 05.33.289). All experimental protocols were approved by the Local Bioethics Commission for Animal Experiments.
Drugs
l-DOPA, CSC, benserazide, haloperidol and p-hydroxybenzoic acid (PBA) were obtained from Sigma–Aldrich (Poznań, Poland), while ZM 241385 came from TOCRIS (Warsaw, Poland). All chemicals used for HPLC were purchased from Merck (Warsaw, Poland). l-DOPA and benserazide were dissolved in saline. A solution of PBA was prepared in an artificial cerebrospinal fluid (aCSF) and was then adjusted to pH 7.4 with 0.1 M NaOH. CSC was initially dissolved in dimethyl sulphoxide (DMSO; Sigma–Aldrich, Poznań, Poland) and was then diluted in at least 20 volumes of the vehicle consisting of a 20:80 v/v mixture of Alkamulus EL-620 (Rhone-Poulenc, Cranbury, NJ) and a phosphate-buffered saline. ZM 241385 was dissolved in a small amount of DMSO and was then diluted in Cremophor EL (Sigma–Aldrich, Poznań, Poland) and 0.9% NaCl (final concentration: 15% DMSO and 15% Cremophor EL). All injections were made by an intraperitoneal route (i.p.). The adenosine A2A receptor antagonists CSC (0.1, 1 and 5 mg/kg) and ZM 241385 (0.3, 3 and 9 mg/kg) were administered 20 min prior to the injection of l-DOPA (100 mg/kg) with benserazide (50 mg/kg). In the chronic experiment, l-DOPA was given twice a day in a dose of 50 mg/kg, together with benserazide (25 mg/kg), for 14 days in order to eliminate diurnal fluctuations in brain DA level. CSC (1 mg/kg) and ZM 241385 (3 mg/kg) were given once a day for 14 days between the injections of l-DOPA. Control animals received respective vehicles in either acute or chronic experiments.
In Vivo Microdialysis
Rats were initially anaesthetized with ketamine (75 mg/kg i.m.) and xylazine (10 mg/kg i.m.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The skull was exposed and small burr holes were drilled for the insertion of microdialysis probes in the striatum, using the following coordinates: 1.8 mm anterior from the bregma; 2.8 mm lateral from the sagittal suture; −7.0 mm ventral from the dura (Paxinos and Watson 1998). Vertical microdialysis probes were constructed as described in detail elsewhere (Gołembiowska and Dziubina 2004a, b). Probe inlets were connected to a syringe pump (BAS, IN, USA) which delivered an aCSF composed of [mM]: NaCl 147, KCl 4.0, MgCl2 1.0, CaCl2 2.2; pH 7.4 at a flow rate of 1.5 μl/min. All metal parts of the aCSF delivery system were replaced with PEEK components or were passivated with 6 M HNO3. Baseline samples were collected every 20 min after the washout period to obtain a stable extracellular neurotransmitter level. Appropriate drugs were then administered 20 min before l-DOPA injection given at time 0, as shown in figures, and dialysate fractions were collected for 240 min. In the chronic experiment, fraction collection started immediately after the injection of a challenging dose of l-DOPA (100 mg/kg), together with benserazide (50 mg/kg). At the end of the experiment, rats were sacrificed and the brain was histologically examined to validate probe placement.
Analytical Procedure
DA, DOPAC and homovanillic acid (HVA) were analyzed by HPLC with electrochemical detection. The level of hydroxyl radical was estimated as 3,4-dihydroxybenzoic acid (3,4-DHBA), a product of the spin trap reagent PBA (1 mM) applied via microdialysis probe. DA and its metabolites were simultaneously determined in the same fractions of striatal dialysates. Chromatography was performed using an LC-10 AD pump (Shimadzu Europa GmbH, Warsaw, Poland), an LC-4B amperometric detector with a cross-flow detector cell (BAS, IN, USA), and a BDS-Hypersil C18 analytical column (3 × 100 mm, 3 μm; Thermo Electron Corp., UK). The mobile phase consisted of 0.1 M monochloroacetic acid adjusted to pH 3.7 with 3 M sodium hydroxide, 0.5 mM EDTA, 13 mg/l 1-octanesulfonic acid sodium salt, 5.7% methanol and 0.8% acetonitrile. The flow rate was 0.5 ml/min, and the applied potential of a 3-mm glassy carbon electrode was +600 mV at a sensitivity of 2 nA/V. All compounds were calculated by comparing their peak areas with respective standards and were processed by Chromax 2001 (Pol-Lab, Warsaw, Poland) software on a personal computer. The obtained values were not corrected for in vitro probe recovery, which was approximately 10–15%.
Catalepsy
Catalepsy induced by haloperidol (0.5 mg/kg) was determined according to a six-test method described by Simon et al. (1969). The degree of catalepsy was evaluated for 4 h starting at 30 min after haloperidol injection. CSC (0.1 and 1 mg/kg) and ZM 241385 (0.3 and 3 mg/kg, respectively) were administered simultaneous with haloperidol. A positive response in each test (when the animals maintained an abnormal position for at least 10 s) was regarded as 1 point (lower bar), or 2 points (higher bar) for forepaw, and thus each rat could score a maximum of 6 points.
Data Analysis
In acute experiments, an average concentration of three stable samples prior to drug administration was regarded as the control value and was considered to be 100%. In chronic experiments in which repeated drug treatment influenced the basal neurotransmitter level, all obtained data are given in absolute numbers. The statistical significance between experimental groups was calculated using a one-way ANOVA for repeated-measures, followed by Tukey’s post-hoc test. The results were considered statistically significant at P < 0.05.
Results
Effect of CSC and ZM 24135 on Haloperidol-Induced Catalepsy
Haloperidol (0.5 mg/kg)-induced catalepsy, with the mean score being 4.8, was assessed at 30–240 min after administration. The catalepsy produced by haloperidol was significantly reduced by CSC (1 mg/kg) [F 1,68 = 21.24; P < 0.001] and ZM 241385 (3 mg/kg) [F 1,54 = 44.99; P < 0.001], given mutually with the neuroleptic (Fig. 1). There was no effect of CSC and ZM 241385 at doses of 0.1 and 0.3 mg/kg, respectively, on haloperidol-induced catalepsy (results not shown).
Effect of Acute and Chronic Administration of l-DOPA on the Extracellular level of Hydroxyl Radical, DA, DOPAC and HVA in rat Striatum
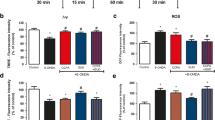
l-DOPA, administered as a single dose of 100 mg/kg, together with benserazide (50 mg/kg), or repeatedly in a dose of 50 mg/kg together with benserazide (25 mg/kg) for 14 days, twice daily, increased the level of hydroxyl radical in striatal dialysates. The effect of chronic l-DOPA treatment on the hydroxyl radical level was greater than that after single dose administration, and attained a value of ~4600 pg/10 μl, while that of a single l-DOPA treatment attained a value of ~2000 pg/10 μl after 240 min [F 1,22 = 91.25, P < 0.01; F 1,22 = 33.10, P < 0.01, respectively, in comparison with the control group] (Fig. 2a). At the same time, l-DOPA increased the extracellular levels of DA, DOPAC and HVA in striatal dialysates. The area under the curve (AUC) values, which represent an increase in DA, DOPAC and HVA levels versus time and are given in absolute numbers, are presented in Fig. 2b–d. l-DOPA administered in single or multiple doses significantly elevated the extracellular level of DA [F 1,18 = 18.33, P < 0.01; F 1,21 = 6.25, P < 0.05, respectively]. The levels of DOPAC and HVA were increased to a similar extent after single and repeated l-DOPA treatment [F 1,20 = 51.23, P < 0.01; F 1,22 = 79.94, P < 0.01, respectively, after a single dose; F 1,21 = 55.09, P < 0.01; F 1,21 = 77.68, P < 0.01, respectively, after multiple doses] (Fig. 2b–d).
The effect of l-DOPA administered as a single dose of 100 mg/kg, together with benserazide (50 mg/kg), or chronically in a dose of 50 mg/kg together with benserazide (25 mg/kg) for 14 days, twice daily, on the extracellular level of hydroxyl radical (a) and extraneuronal DA, DOPAC and HVA in rat striatum (b–d). Time of injection is indicated with an arrow. The values are the mean ± SEM (n = 10–15). The hydroxyl radical was expressed as 3,4-DHBA in pg/10 μl. Bars in lower panels of the figure show AUC values representing increase in DA, DOPAC and HVA levels versus time and are given as absolute numbers. *P < 0.05, **P < 0.01 vs control. Note differences in ordinate scaling among the panels
Effect of Acute Treatment with CSC and ZM 241385, Administered Alone or Mutually with l-DOPA, on the Extracellular Level of Hydroxyl Radical, DA, DOPAC and HVA in Rat Striatum
The striatal level of hydroxyl radical was increased following CSC (1 and 5 mg/kg) and ZM 241385 (3 and 9 mg/kg), administered as a single dose. CSC (1 mg/kg) and ZM 241385 (3 and 9 mg/kg) significantly elevated the level of hydroxyl radical to a similar degree, attaining a value of ~2000 pg/10 μl at 160–240 min of the collection period [F 1,22 = 93.22, P < 0.01; F 1,22 = 98.29, P < 0.01; F 1,22 = 46.28, P < 0.01, respectively, in comparison with the control group] (Fig. 3a, c). Conversely, CSC in a dose of 5 mg/kg markedly enhanced the production of hydroxyl radical, attaining a value of ~4700 pg/10 μl at 240 min after administration [F 1,22 = 46.32, P < 0.01] (Fig. 3a). CSC and ZM 241385 in doses of 0.1 and 0.3 mg/kg were not effective in altering production of hydroxyl radical (Fig. 3a, c).
The effect of acute treatment with CSC (0.1, 1 or 5 mg/kg) and ZM 241385 (0.3, 3 or 9 mg/kg; ZM), administered alone (a, c) or mutually (b, d) with l-DOPA (100 mg/kg) in the presence of benserazide (50 mg/kg), on the extracellular level of hydroxyl radical expressed as 3,4-DHBA, in rat striatum. The values are the mean ± SEM (n = 6–9). *P < 0.01 vs. control (a, c), or a group treated with l-DOPA (b, d)
The level of hydroxyl radical was significantly elevated following l-DOPA (100 mg/kg, in the presence of benserazide, 50 mg/kg) [F 1,22 = 33.10, P < 0.01] (Fig. 3b, d). A low CSC dose (0.1 or 1 mg/kg) attenuated l-DOPA-induced hydroxyl radical production to near control levels [F 1,22 = 13.02, P < 0.01, F 1,22 = 16.17, P < 0.01, respectively], while a high CSC dose (5 mg/kg) enhanced l-DOPA-induced hydroxyl radical production [F 1,22 = 23.62, P < 0.01] (Fig. 3b). A low dose of ZM 241385 (0.3 or 3 mg/kg) had no effect, but in high dose ZM 241385 (9 mg/kg) enhanced l-DOPA-induced hydroxyl radical production [F 1,22 = 28.04, P < 0.01] (Fig. 3d).
The levels of DA, DOPAC and HVA, shown as the AUC and representing the total increase in the level versus time, are expressed as percent of basal level following CSC and ZM 241385 (Fig. 4a–c). Extracellular striatal DA was substantially altered by a single dose of CSC (1 and 5 mg/kg) or ZM 241385 (3 and 9 mg/kg) [F 1,11 = 16.62, P < 0.01; F 1,11 = 21.20, P < 0.01; F 1,10 = 35.64, P < 0.01; F 1,13 = 116.22, P < 0.01, respectively, in comparison with the control group], but not by a low dose of either agent (CSC, 0.1 mg/kg; or ZM 241385, 0.3 mg/kg) (Fig. 4a). The extracellular level of DOPAC was significantly increased after a single dose of CSC (1 or 5 mg/kg) [F 1,11 = 7.83, P < 0.05; F 1,11 = 9.11, P < 0.01, respectively] or ZM 241385 (3 or 9 mg/kg) [F 1,10 = 22.76, P < 0.01; F 1,13 = 26.21, P < 0.01, respectively] (Fig. 4b). Low doses of CSC (0.1 mg/kg) and ZM 241385 (0.3 mg/kg) were without effect on extracellular DOPAC level. The level of HVA was significantly increased after CSC (1 or 5 mg/kg) [F1,11 = 6.09, P < 0.05; F 1,11 = 11,73 P < 0.01, respectively, in comparison with the control group] (Fig. 4c). A high dose of ZM 241385 (3 or 9 mg/kg) increased the extracellular level of HVA to a level similar to that of the control group [F 1,10 = 12.80, P < 0.01; F 1,13 = 176.70, P < 0.01, respectively] (Fig. 4c). Low dose CSC (0.1 mg/kg) and ZM 241385 (0.3 mg/kg) did not alter the extracellular level of HVA.
The effect of acute treatment with CSC (0.1, 1 and 5 mg/kg) and ZM 241385 (0.3, 3 and 9 mg/kg; ZM), administered alone or mutually with l-DOPA (100 mg/kg) in the presence of benserazide (50 mg/kg), on extraneuronal DA (a), DOPAC (b) and HVA (c) in rat striatum. The AUC values are the mean ± SEM (n = 6–14) and represent total effect expressed as a percent of the baseline. *P < 0.05, **P < 0.01 vs. control; + P < 0.05, ++ P < 0.01 vs. l-DOPA group. The basal extracellular levels of DA, DOPAC and HVA (pg/10 μl) were respectively 8.83 ± 0.62, 2478 ± 260, 1788 ± 150 in a control group; 9.00 ± 0.25, 2819 ± 169, 1680 ± 220 in a group treated with CSC, 0.1 mg/kg; 8.80 ± 0.54, 2714 ± 206, 2089 ± 189 in a group treated with CSC, 1 mg/kg; 7.39 ± 0.55, 3629 ± 349, 2538 ± 215 in a group treated with CSC, 5 mg/kg; 7,92 ± 077, 2783 ± 99, 1556 ± 145 in a group treated with ZM 241385, 0.3 mg/kg; 6.33 ± 0.29, 3408 ± 354, 1481 ± 122 in a group treated with ZM 241385, 3 mg/kg; 6.29 ± 0.37, 3518 ± 122, 2064 ± 96 in a group treated with ZM 241385, 9 mg/kg; 8.48 ± 0.37, 2326 ± 137, 1900 ± 91 in a group treated with l-DOPA, 100 mg/kg; 7.88 ± 0.36, 3250 ± 245, 1989 ± 140 in a group treated with l-DOPA, 100 mg/kg + CSC 0.1 mg/kg; 9.00 ± 0.63, 2359 ± 181, 1839 ± 175 in a group treated with l-DOPA, 100 mg/kg + CSC, 1 mg/kg; 8.10 ± 0.78, 2938 ± 185, 2327 ± 95 in a group treated with l-DOPA, 100 mg/kg + CSC, 5 mg/kg; 10.20 ± 0.87, 2900 ± 120, 1890 ± 99 in a group treated with l-DOPA, 100 mg/kg + ZM 241385, 0.3 mg/kg; 11.60 ± 0.86, 3187 ± 166, 2270 ± 165 in a group treated with l-DOPA, 100 mg/kg + ZM 241385, 3 mg/kg; 10.00 ± 0.88, 2569 ± 142, 1696 ± 85 in a group treated with l-DOPA, 100 mg/kg + ZM 241385, 9 mg/kg
Levels of DA, DOPAC and HVA were increased by acute l-DOPA [F 1,18 = 72.11, P < 0.01; F 1,22 = 49.95, P < 0.01; F 1,22 = 77.92, P < 0.01, respectively, in comparison with the control group] (Fig. 4a–c). Low dose CSC (0.1 or 1 mg/kg) administered together with l-DOPA was without effect, while high dose CSC (5 mg/kg) enhanced the l-DOPA-induced elevation of extraneuronal DA levels [F 1,16 = 11.74, P < 0.01] (Fig. 4a). Low dose ZM 241385 (0.3 or 3 mg/kg) administered together with l-DOPA did not effect a change, high dose ZM 241385 (9 mg/kg) enhanced the l-DOPA-induced elevation of extraneuronal DA levels [F 1,14 = 4.61, P < 0.05] (Fig. 4a). The l-DOPA elevation of extracellular DOPAC level was attenuated by low dose CSC (0.1 and 1 mg/kg) [F 1,16 = 10.22, P < 0.01; F 1,19 = 5.17, P < 0.05] but was not altered by ZM 241385 (0.3, 3 or 9 mg/kg) (Fig. 4b). The l-DOPA-induced elevation of extraneuronal HVA was attenuated by low dose CSC (0.1 or 1 mg/kg) [F 1,16 = 8.88, P < 0.01; F1,20 = 4.72, P < 0.05, respectively], but was not changed by ZM 241385 (0.3, 3 or 9 mg/kg) (Fig. 4c).
Effect of Chronic Treatment with CSC and ZM 241385, Alone or in Combination with l-DOPA, on the Extraneuronal Level of Hydroxyl Radical, DA, DOPAC and HVA in Rat Striatum
In rats treated chronically (14 days) with CSC (1 mg/kg), the last dose of that antagonist increased the production of hydroxyl radical to a lesser extent than that after a single dose, to a value of ~600 pg/10 μl at 240 min after administration [F 1,22 = 57.04, P < 0.01 in comparison with the control group] (Fig. 5a). In turn, in rats treated chronically (14 days) with ZM 241385 (3 mg/kg), the last dose of that antagonist elevated the level of hydroxyl radical to a similar extent as that observed after a single dose, i.e., to a value of 2700 pg/10 μl [F 1,22 = 74.15, P < 0.01 in comparison with the control group] (Fig. 5c).
The effect of chronic (14 days) treatment with CSC (1 mg/kg) and ZM 241385 (3 mg/kg; ZM), administered alone (a, c) or mutually (b, d) with l-DOPA (50 mg/kg) in the presence of benserazide (25 mg/kg) twice daily, on the extracellular level of hydroxyl radical expressed as 3,4-DHBA, in rat striatum. The values are the mean ± SEM (n = 6–9). *P < 0.01 vs. control. Note differences in ordinate scaling among the panels
The level of hydroxyl radical was substantially elevated by chronic l-DOPA administration [F 1,22 = 91.25, P < 0.01 in comparison with the control animals] (Fig. 5b, d). Repeated administration (14 days) of CSC (1 mg/kg) and ZM 241385 (3 mg/kg), together with l-DOPA, diminished the production of hydroxyl radical induced by l-DOPA [F 1,22 = 25.96, P < 0.01; F 1,22 = 36.42, P < 0.01; respectively] (Fig. 5b, d).
The AUC values showing increases in DA, DOPAC and HVA levels versus time (including three consecutive baseline fractions) are expressed in absolute numbers following chronic administration of CSC and ZM 241385 (Fig. 6a–c). CSC (1 mg/kg) and ZM 241385 (3 mg/kg), administered for 14 days, did not alter the extraneuronal level of DA (Fig. 6a). Conversely, the extracellular level of DOPAC was elevated following chronic administration of CSC (1 mg/kg) [F 1,10 = 22.91, P < 0.01] or ZM 241385 (3 mg/kg) [F 1,15 = 14.34, P < 0.01] (Fig. 6b). No change in extracellular HVA level was observed following chronic treatment with CSC (1 mg/kg) or ZM 241385 (3 mg/kg) (Fig. 6c).
The effect of chronic (14 days) treatment with CSC (1 mg/kg) and ZM 241385 (3 mg/kg; ZM), administered alone or mutually with l-DOPA (50 mg/kg) in the presence of benserazide (25 mg/kg) twice daily, on the extraneuronal DA (a), DOPAC (b) and HVA (c) in rat striatum. The AUC values are the mean ± SEM (n = 6–14) and represent total effect given as absolute values. *P < 0.05, **P < 0.01 vs. control; + P < 0.05, ++ P < 0.01 vs. l-DOPA group. Note differences in ordinate scaling among the panels
The extracellular levels of DA, DOPAC and HVA were elevated by chronic (14 day) l-DOPA treatments [F 1,21 = 6.25, P < 0.05; F 1,21 = 55.92; P < 0.01; F 1,21 = 77.68, P < 0.01, respectively] (Fig. 6a–c). CSC (1 mg/kg) and ZM 241385 (3 mg/kg), administered chronically and together with l-DOPA, were without effect on l-DOPA-induced DA release (Fig. 6a). CSC markedly diminished the l-DOPA-induced increase in extracellular DOPAC [F 1,18 = 17.50, P < 0.01] and HVA [F 1,18 = 29.91, P < 0.01], while ZM 241385 effectively attenuated the l-DOPA elevation of extraneuronal HVA [F 1,18 = 4.94, P < 0.05], but not extracellular level of DOPAC (Fig. 6b, c).
Discussion
Our study has shown that CSC and ZM 241385, A2A antagonists belonging to different chemical classes, given acutely or chronically, generate hydroxyl radical in the striatum of intact rats. This appears to be related to increase in DA release/metabolism induced by either agent. In low multiple doses, CSC and ZM 241385 administered together with l-DOPA, has a similar effect, resulting in reduced hydroxyl radical production. However, at a single high dose, the A2A antagonists increase hydroxyl radical production. Differences between CSC and ZM 241385 can be seen at low single doses, where CSC diminishes the l-DOPA-induced increase in hydroxyl radical level, while ZM 241385 has no effect.
DA is a readily oxidized molecule, particularly notable after high dose l-DOPA, as also shown in our study, in which acute or repeated l-DOPA treatment generated hydroxyl radical. We observed a higher level of hydroxyl radical after repeated versus single l-DOPA dose, despite the fact that both treatments were equally effective in elevating the extraneuronal DA and its metabolites. The noted difference may be related to the fact that l-DOPA, which is easily transported to the cytosolic compartment, may per se undergo auto-oxidation and additionally generate hydroxyl radicals when given repeatedly.
Moreover, the formation of free radicals following chronic l-DOPA administration may account for a decrease in the extracellular components of neuronal antioxidants (Serra et al. 2000), in addition to mitochondrial respiratory chain inhibition and cellular energy impairment (Gluck and Zeevalk 2004; Jana et al. 2007). Hence, oxidative stress induced by l-DOPA may be the cause of its toxicity observed in in vitro and in vivo experimental models following rotenone (Nakao et al. 1997), 6-hydroxydopamine (6-OHDA) (Heikkila and Cohen 1971; Cleren et al. 1999) or manganese (Migheli et al. 1999).
A2A antagonists are promising candidates for non-dopaminergic therapy of PD. A2A antagonists reportedly have a dual action: improvement in motor symptoms of PD, but also a neuroprotective potential, as shown in various animal models of neurotoxicity (Chen et al. 2007). The neuroprotection afforded by the xanthine-based compounds CSC and KW-6002 or the non-xanthine structure SCH 58261 was found in MPTP-treated mice (Chen et al. 2001; 2002; Ikeda et al. 2002; Pierri et al. 2005) and in rats following the 6-OHDA lesion of dopaminergic neurons (Ikeda et al. 2002, Bové et al. 2005; Aguiar et al. 2008). Similar results were obtained in global and focal cerebral ischemia models (Phillis 1995; Von Lubitz et al. 1995; Chen et al. 1999; Melani et al. 2003) and glutamate or quinolic acid-induced excitotoxicity (Popoli et al. 2002; Pintor et al. 2004). ZM 241385, SCH 58261 and CSC counteracted kainate and quinolic acid-induced neuronal damage (Jones et al. 1998; Behan and Stone 2002). Understanding the neuroprotective mechanism of action of A2A antagonists is of particular importance, as these drugs have already been under clinical trial in parkinsonian patients (LeWitt et al. 2008). Surprisingly, neuroprotective effects in excitotoxicity models were achieved by A2A antagonists at very low dose, while in PD models, both low and behaviorally active doses exerted protective effects (for review see Popoli et al. 2004).
Findings in our present study indicate that modulation of oxidative stress by A2A antagonists may be an important facet of the neuroprotective action of the tested drugs. We demonstrate that CSC and ZM 241385, at very low, behaviorally inactive doses, do not enhance DA release and metabolism and do not generate hydroxyl radical in striatal dialysates. However, CSC and ZM 241385, at pharmacologically relevant doses in which they counteract haloperidol-induced catalepsy, increased the synaptic level of DA in acute experiments. The augmented production of hydroxyl radical may relate to their effect on DA release. Similar increases in the extracellular levels of DOPAC and HVA indicate that A2A antagonists, at acute doses, appear to stimulate DA release and metabolism and to generate free radicals in the extraneuronal space. In contrast to CSC, the dose-response relationship was not observed for the ZM 241385 effect on both DA release and its metabolites DOPAC and HVA, as well as on production of hydroxyl radical. ZM 241385, belonging to a different non-xanthine class of A2A antagonists, had limited bioavailability, which may account for lack of a clear dose-dependent effect.
It was of interest to investigate whether prolonged exposure to CSC or ZM 241385 would lead to formation of free radical in the extraneuronal compartment of the striatum. CSC, given repeatedly, produced a very small level of hydroxyl radical in comparison with single-dose administration (30% of the single-dose effect); in contrast, ZM 241385 administered chronically was as effective as when given acutely. The increase in DA metabolism, but not DA release by those agents may contribute to the formation of free radicals. A distinctly weaker effect of CSC on hydroxyl radical generation in chronic administration may indicate scavenging activity or tolerance development toward this A2A antagonist action during the course of treatment.
Significant differences in the effect of low and high doses of the antagonists studied were observed when either drug was given together with l-DOPA. In that experiment, a low single dose of CSC and ZM 241385 either inhibited or did not alter, respectively, generation of hydroxyl radical by l-DOPA; on the other hand, in high dose they were equipotent toward the elevation of hydroxyl radical levels produced by l-DOPA. The suppressing effect of low single dose CSC on hydroxyl radical formation appears to be related to an inhibition of DA metabolism, as CSC has been shown to be a potent competitive monoamine oxidase-B (MAO-B) inhibitor, its Ki being 100 nM (Chen et al. 2002). Conversely, ZM 241385, which is devoid of MAO-B inhibitory properties, was ineffective in influencing either the l-DOPA-induced DA release or hydroxyl radical production when it was administered acutely in low dose. A2A antagonists given chronically in low, but pharmacologically effective doses, inhibited the oxidative effect of l-DOPA. The reduced generation of hydroxyl radical by CSC may be related to an inhibition of oxidative DA deamination by CSC and reduced production of hydrogen peroxide. A comparable inhibitory effect of ZM 241385 on formation of hydroxyl radical also appears to be related to weak suppression of DA metabolism. However, the mechanism by which repeated ZM 241385 inhibits DA metabolism following l-DOPA is still unclear. In turn, a rise in the level of hydroxyl radical, induced by high dose A2A antagonist, may relate to the marked increase in DA release stemming from exogenous l-DOPA. Furthermore, as shown in our previous study, high dose CSC enhanced the entry of l-DOPA into brain (Gołembiowska and Dziubina 2004a), thus influencing the availability of the DA precursor for monoamine and hydroxyl radical production.
The divergent effects of the two drugs under study, alone and in combination with l-DOPA, represent an intriguing finding—one deserving further exploration. Also, as indicated above for CSC, MAO-B inhibition may be the mechanism by which this methylxanthine reduces the l-DOPA-induced production of free radical. Nevertheless, it is not clear why the above-mentioned CSC activity is seen when CSC is given together with l-DOPA. In fact, in rat brain MAO-B contribution in DA metabolism is considerably low (Oreland 1991). Some activity of MAO-B in rat striatum has been found under high DA concentration after administration of l-DOPA (Buu and Angers 1987). Thus, it is quite likely that MAO-B inhibition by CSC might induce comparatively small changes in intact rats; but this is probably not the case under extreme conditions, e.g., after l-DOPA treatment. Moreover, l-DOPA may be partially taken-up by astrocytes, which contain high levels of MAO-B (Levitt et al. 1982) and thus resulting in metabolism in these cells as well as in DA neurons. It is possible that MAO-B inhibition by low CSC doses in non-DA cells may be responsible for a decrease in the extraneuronal level of DOPAC and HVA as well as diminished production of hydroxyl radical. Furthermore, the presence of adenosine A2A receptors has been ascertained in microglial cells (Daré et al. 2007; Pocock and Kettenmann 2007), known to rapidly react to chemical changes in their environment and to rapidly generate adenosine from extracellular nucleotides (Daré et al. 2007). The activation of microglial A2A receptors by adenosine released, e.g., in response to l-DOPA administration, may induce excessive release of nitrous oxide which is likely to contribute to the promotion of free radicals by microglial cells (Saura et al. 2005). Thus, modulation of glial cells by A2A antagonists may be attributed to their ability to reduce the oxidative stress induced by l-DOPA. At the same time, microglia activated by DA or DA oxidative products may also be associated with the generation of free radicals (Block et al. 2007), an effect that is observed in the presence of high doses of A2A antagonists which markedly increase DA release.
Because oxidative stress is a factor implicated in the etiology of PD, caution is urged on use of A2A antagonists in the early, presymptomatic phase of PD when DA terminals are still present in reasonable number. Adenosine A2A receptor antagonists, by stimulating DA release/metabolism, may have adverse instead of beneficial effects when used alone or as a supplement to l-DOPA therapy.
In conclusion, we have presented evidence that CSC and ZM 241385, two A2A adenosine receptor antagonists belonging to different chemical classes, can modulate the release and metabolism of DA when given in single or multiple doses. An apparent increase in DA release is the source of hydroxyl radicals in the extracellular compartment of rat striatum following A2A antagonist treatment. The generation of hydroxyl radicals by l-DOPA, after single dose or chronic administration, is attenuated by low dose CSC and low multiple doses of ZM 241385. Modulation of DA release and metabolism by A2A antagonists apparently underlies the above effect. Current findings were obtained with naïve animals which possess an intact cellular milieu and an intact system of defense against an oxidative insult. Our successive study will show the effect of A2A antagonists on the generation of free radicals in an animal model of PD when DA innervation is absent.
Abbreviations
- aCSF:
-
Artificial cerebrospinal fluid
- AUC:
-
Area under the curve
- CSC:
-
8-(3-Chlorostyryl)caffeine
- DA:
-
Dopamine
- 3,4-DHBA:
-
3,4-Dihydroxybenzoic acid
- DMSO:
-
Dimethyl sulphoxide
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- i.m.:
-
Intramuscular
- l-DOPA:
-
l-3,4-Dihydroxyphenylalanine
- HVA:
-
Homovanillic acid
- MAO:
-
Monoamine oxidase
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PBA:
-
p-Hydroxybenzoic acid
- PD:
-
Parkinson’s disease
- SN:
-
Substantia nigra
- VMAT2:
-
Vesicular monoamine transporter-2
- ZM 241385:
-
4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
References
Aguiar LMV, Macêdo DS, Vasconcelos SMM, Oliveira AA, de Sousa FCF, Viana GSB (2008) CSC, an adenosine A2A receptor antagonist and MAO B inhibitor, reverses behavior, monoamine neurotransmission, and amino acid alterations in the 6-OHDA-lesioned rats. Brain Res 1191:192–199
Ascherio A, Zhang SH, Hernán MA, Kawachi I, Colditz GA, Speizer FE, Willett WC (2001) Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol 50:56–63
Behan WMH, Stone TW (2002) Enhanced neuronal damage by co-administration of quinolic acid and free radicals, and protection by adenosine A2A receptor antagonists. Br J Pharmacol 135:1435–1442
Bishnoi M, Chopra K, Kulkarni SK (2007) Theophylline, adenosine receptor antagonist prevents behavioral, biochemical and neurochemical changes associated with an animal model of tardive dyskinesia. Pharmacol Rep 59:181–191
Block ML, Zecca L, Hong J-S (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Bové J, Serrats J, Mengod G, Cortés R, Tolosa E, Marin C (2005) Neuroprotection induced by the adenosine A2A antagonist CSC in the 6-OHDA rat model of parkinsonism: effect on the activity of striatal output pathways. Exp Brain Res 165:362–374
Buu NT, Angers M (1987) Effects of different monoamine oxidase inhibitors on the metabolism of L-DOPA in the rat brain. Biochem Pharmacol 36:1731–1735
Cenci MA (2007) Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci 30:236–243
Chen J-F, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA (1999) A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19:9192–9200
Chen J-F, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N Jr, Schwarzschild MA (2001) Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci 21:RC143
Chen J-F, Steyn S, Staal R, Petzer JP, Xu K, Van der Schyf CJ, Castagnoli K, Sonsalla PK, Castagnoli N Jr, Schwarzschild MA (2002) 8-(3-Chlorostyryl)caffeine may attenuate MPTP neurotoxicity through dual actions of monoamine oxidase inhibition and A2A receptor antagonism. J Biol Chem 277:36040–36044
Chen J-F, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de Mendonça A (2007) Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol 83:310–331
Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ, Zhuang X (2008) Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci 28:425–433
Cleren C, Vilpoux C, Dourmap N, Bonnet J-J, Costentin J (1999) Acute interactions between L-DOPA and the neurotoxic effects of 1-methyl-4-phenylpyridinium or 6-hydroxydopamine in mice. Brain Res 830:314–319
Daré E, Schulte G, Karovic O, Hammarberg C, Fredholm BB (2007) Modulation of glial cell functions by adenosine receptors. Physiol Behav 92:15–20
Fahn S, Sulzer D (2004) Neurodegeneration and Neuroprotection in Parkinson’s Disease. NeuroRx 1:139–154
Gluck MR, Zeevalk GD (2004) Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson’s disease and catecholamine-associated diseases. J Neurochem 91:788–795
Gołembiowska K, Dziubina A (2004a) Effect of adenosine A2A receptor antagonist 8-(3-chlorostyryl)caffeine on L-DOPA biotransformation in rat striatum. Brain Res 998:208–217
Gołembiowska K, Dziubina A (2004b) Striatal adenosine A2A receptor blockade increases extracellular dopamine release following L-DOPA administration in intact and dopamine-denervated rats. Neuropharmacology 47:414–426
Halliwell B (2006) Oxidative stress and neurodegeneration: where we now? J Neurochem 97:1634–1658
Heikkila RE, Cohen G (1971) Inhibition of biogenis amine uptake by hydrogen peroxide: a mechanism for the toxic effects of 6-OHDA. Science 172:1257–1258
Ikeda K, Kurokawa M, Aoyama S, Kuwana Y (2002) Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J Neurochem 80:262–270
Jana S, Maiti AK, Bagh MB, Banerjee K, Das A, Roy A, Chakrabarti S (2007) Dopamine but not 3,4-dihydroxyphenylacetic acid (DOPAC) inhibits brain respiratory chain activity by autoxidation and mitochondria catalyzed oxidation to quinine products: implications in Parkinson’s disease. Brain Res 1139:195–200
Jones PA, Smith RA, Stone TW (1998) Protection against kainate-induced excitotoxicity by adenosine A2A receptor agonists and antagonists. Neuroscience 85:229–237
Kanda T, Jackson MJ, Smith LA, Pearce RK, Nakamura J, Kase H, Kuwana Y, Jenner P (1998) Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol 43:507–513
Levitt P, Pintar JE, Breakfield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79:6385–6389
LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM (2008) Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “Off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 63:295–302
Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, Bertorelli R, Monopoli A, Pedata F (2003) The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res 959:243–250
Migheli R, Godani C, Sciola L, Delogu MR, Serra PA, Zangani D, De Natale G, Miele E, Desole MS (1999) Enhancing effect of manganese on L-DOPA-induced apoptosis in PC12 cells: role of oxidative stress. J Neurochem 73:1155–1163
Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzchild MA (2007) Role of adenosine A2A receptors in parkinsonian motor impairment and L-DOPA-induced motor complications. Prog Neurobiol 83:293–309
Nakao N, Nakai K, Itakura T (1997) Metabolic inhibition enhances selective toxicity of L-DOPA toward mesencephalic dopamine neurons in vitro. Brain Res 777:202–209
Ochi M, Koga K, Kurokawa M, Kase H, Nakamura J, Kuwana Y (2000) Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience 100:53–62
Oreland L (1991) Monoamine oxidase, dopamine and Parkinson’s disease. Acta Neurol Scand 84(Suppl 136):60–65
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Phillis JW (1995) The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res 705:79–84
Pierri M, Vaudano E, Sager T, Englund U (2005) KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology 48:517–524
Pintor A, Galluzzo M, Grieco R, Pezzola A, Reggio R, Popoli P (2004) Adenosine A2A receptor antagonists prevent the increase in striatal glutamate levels induced by glutamate uptake inhibitors. J Neurochem 89:152–156
Pocock JM, Kettenmann H (2007) Neurotransmitter receptors on microglia. Trends Neurosci 30:527–535
Pollack AE, Fink JS (1995) Adenosine antagonists potentiate D2 dopamine-dependent activation of Fos in the striatopallidal pathway. Neuroscience 68:721–728
Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pèzzola L, Scarchilli L, Quarta D, Reggio R, Malchiodi-Albedi F, Falchi M, Massotti M (2002) Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci 22:1967–1975
Popoli P, Blum D, Pintor A, Tebano MT, Frank C, Gianfriddo M, Domenici MR, Schiffmann SN, Pedata F (2004) The controversial role of adenosine receptor antagonists as neuroprotective agents. Curr Med Chem – CNS Agents 4:35–45
Przedborski S, Jackson-Lewis V, Muthane U, Jiang H, Ferreira M, Naini A-B, Fahn S (1993) Chronic levodopa administration alters cerebral mitochondrial respiratory chain activity. Ann Neurol 34:715–723
Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung K-H, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR (2000) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283:2674–2679
Rowe DB, Le W, Smith RG, Appel SH (1998) Antibodies from patients with Parkinson’s disease react with protein modified by dopamine oxidation. J Neurosci Res 53:551–558
Saha AR, Ninkina NN, Hanger DP, Anderton BH, Davies AM, Bchman VL (2003) Induction of neuronal death by alpha-synuclein. Eur J Neurosci 12:3073–3077
Saura J, Angulo E, Ejarque A, Casadó V, Tussell JM, Moratalla R, Chen J-F, Schwarzschild MA, Lluis C, Franco R, Serratosa J (2005) Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem 95:919–929
Schwarzschild MA, Agnati L, Fuxe K, Chen J-F, Morelli M (2006) Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci 29:647–654
Serra PA, Esposito G, Enrico P, Mura MA, Migheli R, Delogu MR, Miele M, Desole MS, Grella G, Miele E (2000) Manganese increases L-DOPA auto-oxidation in the striatum of the freely moving rat: potential implications to L-DOPA long-term therapy of Parkinson’s disease. Br J Pharmacol 130:937–945
Simon P, Langwiński R, Boissier JR (1969) Comparaison de diffĕrents tests d’ĕvaluation de la catalepsie chez le rat. Thĕrapie 24:985–995
Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, Centonze D, Calabresi P, Borsini F (2007) Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology 53:783–789
Von Lubitz DK, Lin RC, Jacobson KA (1995) Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol 287:295–302
Wardas J, Konieczny J, Lorenc-Koci E (2001) SCH 58261, an A2A adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse 41:160–171
Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA 100:6145–6150
Xu K, Bastia E, Schwarzschild M (2005) Therapeutic potential of adenosine A2A receptor antagonists in Parkinson’s disease. Pharmacol Ther 105:267–310
Acknowledgments
The study was supported by the grant no 2PO5F 04427 awarded by the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gołembiowska, K., Dziubina, A., Kowalska, M. et al. Effect of Adenosine A2A Receptor Antagonists on l-DOPA-Induced Hydroxyl Radical Formation in Rat Striatum. Neurotox Res 15, 155–166 (2009). https://doi.org/10.1007/s12640-009-9016-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9016-2