Abstract
6-Hydroxydopamine (6-OHDA) is the most used toxin in experimental Parkinson’s disease (PD) models. 6-OHDA shows high affinity for the dopamine transporter and once inside the neuron, it accumulates and undergoes non-enzymatic auto-oxidation, promoting reactive oxygen species (ROS) formation and selective damage of catecholaminergic neurons. In this way, our group has established a 6-OHDA in vitro protocol with rat striatal slices as a rapid and effective model for screening of new drugs with protective effects against PD. We have shown that co-incubation with guanosine (GUO, 100 μM) prevented the 6-OHDA-induced damage in striatal slices. As the exact GUO mechanism of action remains unknown, the aim of this study was to investigate if adenosine A1 (A1R) and/or A2A receptors (A2AR) are involved on GUO protective effects on striatal slices. Pre-incubation with DPCPX, an A1R antagonist prevented guanosine effects on 6-OHDA-induced ROS formation and mitochondrial membrane potential depolarization, while CCPA, an A1R agonist, did not alter GUO effects. Regarding A2AR, the antagonist SCH58261 had similar protective effect as GUO in ROS formation and mitochondrial membrane potential. Additionally, SCH58261 did not affect GUO protective effects. The A2AR agonist CGS21680, although, completely blocked GUO effects. Finally, the A1R antagonist DPCPX, and the A2AR agonist CGS21680 also abolished the preventive guanosine effect on 6-OHDA-induced ATP levels decrease. These results reinforce previous evidence for a putative interaction of GUO with A1R-A2AR heteromer as its molecular target and clearly indicate a dependence on adenosine receptors modulation to GUO protective effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by bradykinesia, tremor, and rigidity, caused by the progressive loss of the dopaminergic neurons in the nigrostriatal pathway [1]. Although the etiology of PD is considered idiopathic, it can be related to other factors as genetics, environmental toxins, oxidative stress, or mitochondrial abnormalities [2]. The main therapeutic approach is based on dopamine replacement to promote motor symptoms relief; however, it does not stop the progression of the disease [3]. The molecular trigger to neuronal degeneration may involve the oxidative burst, mitochondrial dysfunction, and bioenergetics impairment [4,5,6]; thus, new strategies of treatment aiming protection of these biochemical unbalance in dopaminergic neurons are necessary.
The experimental study of PD relies, mainly, in the use of animal models administered with toxins to mimic the neurodegeneration in the nigrostriatal pathway. 6-hydroxydopamine (6-OHDA) was the first discovered drug that has specific toxicity for dopaminergic neurons [7], and it is the most used toxin in experimental PD models [8,9,10]. Because of the similar structure, 6-OHDA also shows affinity for the dopamine transporters [11] and it accumulates inside the neurons, where it undergoes an auto-oxidation, promoting selective damage of catecholaminergic neurons [8, 11].
In this way, our group has established a 6-OHDA in vitro model with rat brain slices, showing that in vitro incubation with 6-OHDA induced a decrease in cellular viability, increase in ROS production, and a disruption in mitochondrial membrane potential in striatal slices [12]. Moreover, we have shown that co-incubation with guanosine (GUO) prevented the 6-OHDA-induced damage in striatal slices [13]. GUO is a purine nucleoside, which has demonstrated neuroprotective effects in several animal and cellular models of neurotoxic conditions and neurodegenerative diseases [14].
Regarding PD, it is already known that GUO exerts protective effects against in vitro 6-OHDA toxicity in two cell lines (C6 glioma and dopaminergic human SH-SY5Y neuroblastoma cells) [15, 16]. Besides that, GUO also have effects on in vivo PD models. In unilaterally 6-OHDA-lesioned rats, GUO acutely administered increased L-DOPA sub-maximal response and decreased L-DOPA-induced dyskinesia, i.e., GUO potentialized the L-DOPA effects diminishing its side effects. In the same way, GUO also reversed reserpine-induced motor disturbance in mice [17]. In search for the molecular target of GUO, our group has already implied adenosine receptors modulation with GUO effects in an ischemic-like damage, in hippocampal slices and cortical astrocytes [18, 19]. In fact, adenosinergic transmission has been pointed out as a promising therapeutic strategy for motor symptoms of PD [20, 21]. This therapeutic potential is mainly due the fact that adenosine A1 and A2A receptors (A1R and A2AR) are largely expressed in the striatum and have a key role in modulation of dopaminergic transmission [22, 23].
Since the GUO mechanism of action it is still not clearly identified, it is of great interest to understand the signaling behind its effects acting as neuroprotective agent. Therefore, the aim of this study was to investigate if adenosine A1R and/or A2AR are involved on GUO protective effects on striatal slices against oxidative damage, mitochondrial dysfunction, and ATP depletion due to 6-OHDA-induced toxicity in vitro.
Materials and methods
Animals
Male Wistar rats (3 months old, 350–400 g) were obtained from our local colony, maintained in a 12-h dark/light cycle, at constant room temperature at 23 ± 1 °C and with food and water ad libitum. Experiments followed the ARRIVE Guidelines published in 2010 and were approved by the local Ethical Committee for Animal Research (CEUA/UFSC PP00955).
Brain slices
Animals were euthanized by decapitation and the whole brain were quickly removed and the striatum was rapidly dissected in ice-cold Krebs Ringer buffer (KRB) (122 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, 0.4 mM KH2PO4, 25 mM NaHCO3, and 10 mM D-glucose, bubbled with 95% O2/ 5% CO2 up to pH 7.4). Striatal slices (0.4 mm) were prepared using a McIlwain Tissue Chopper (The Mickle Laboratory Engineering Co. ltd., England) and separated in KRB at 4 °C. After sectioning, slices were randomly selected (3 slices per group) and incubated in 24-well cell culture plate with KRB (1 mL) for 30 min, at 37 °C, for metabolic recovery from slicing procedure.
Slices treatment
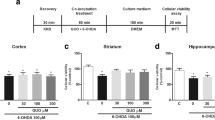
6-OHDA (Sigma, St. Louis, MO, USA) was solubilized in water at 0.1% sodium metabisulfite and stored at − 20 °C. For the experiment, 6-OHDA was diluted to 100 μM in KRB. To investigate 6-OHDA-induced damage, slices from the striatum were exposed to 6-OHDA (100 μM) during 1 h [12]. GUO (Sigma, St. Louis, MO, USA) was freshly prepared and diluted in KRB (100 μM) and co-incubated with 6-OHDA for 1 h to determine its neuroprotective effect. To investigate the role of A1R or A2AR in the GUO neuroprotective effect, slices were pre-incubated with agonists or antagonists of A1R (CCPA and DPCPX, respectively) and A2AR (CGS21680 and SCH58261) 15 min prior to the incubation with 6-OHDA and/or GUO (Fig. 1a). Concentrations of adenosine receptors ligands were selected based on previous studies [19, 24]. Slices of control group were incubated in a physiological KRB. All experimental groups were assayed in triplicates. After the 1 h of co-incubation with 6-OHDA and GUO, ROS production, mitochondrial membrane potential, or intracellular ATP levels were evaluated.
Effects of A1R modulation on 6-OHDA-induced toxicity in striatal slices. Experimental design is describe in a. Striatal slices were pre-incubated with A1R agonist CCPA (100 nM; b, c) or A1R antagonist DPCPX (250 nM; d, e). Slices were incubated with 6-OHDA (100 μM) and/or co-incubated with GUO (100 μM). 6-OHDA-induced mitochondrial membrane potential (ΔΨ) (b, d) and ROS levels (c, e). Data are expressed as percentage of controls normalized among individual experiments and represent means with SEM (n = 6). (*) when p < 0.05 compared with control or (#) compared to 6-OHDA group (one-way ANOVA followed by Tukey’s test)
ROS levels
ROS production was measured by using the molecular probe 2,7-dichlorofluorescein diacetate (H2DCFDA, Sigma Aldrich, St. Louis, MO, USA). H2DCFDA diffuses through the cell membrane and is hydrolyzed by intracellular esterases to the non-fluorescent form 2′,7′-dichlorofluorescein (DCFH). DCFH reacts with intracellular ROS (such as H2O2) to form dichlorofluorescein (DCF), a green fluorescent dye. DCF fluorescence intensity is proportional to the amount of ROS. Striatal slices were incubated with 80 μM of H2DCFDA diluted in KRB (1 mL) for 30 min at 37 °C. Slices were then washed with and maintained in KRB (1 mL) for fluorescence measurement. Fluorescence was read using excitation and emission wavelengths of 480 and 525 nm, respectively in a fluorescence microplate reader (TECAN®). Results were obtained as arbitrary unit of fluorescence and were expressed in percentage related to control levels.
Mitochondrial membrane potential
Mitochondrial membrane potential (ΔΨ) was measured by using the molecular probe tetramethylrhodamine ethyl ester (TMRE, Sigma Aldrich, St. Louis, MO, USA). TMRE is a cell-permeant, cationic, red-orange fluorescent dye that is readily sequestered by active mitochondria. Slices were incubated with 10 nM TMRE diluted in KRB (1 mL) for 30 min at 37 °C. Slices were then washed with and maintained in KRB (1 mL) for fluorescence measurement. Fluorescence was measured using wavelengths of excitation and emission of 550 and 590 nm, respectively. The results are expressed and normalized as percentages relative to the control conditions. Results were obtained as arbitrary unit of fluorescence and were expressed in percentage related to control levels.
ATP levels
After GUO and 6-OHDA treatment, brain slices from striatum were homogenized in trichloroacetic acid (TCA) 2% aqueous solution (350 μL). The homogenates were centrifuged at 14000 rpm at 4 °C for 3 min. The supernatants (100 μL) were used for determination of ATP levels, using bioluminescent assay kit according to the manufacturer’s recommendations (#FLAA, Sigma Aldrich, St. Louis, MO, USA). The amount of protein in each sample was measured using the method of [25] and the results are expressed in μmol ATP/μg of protein in each sample (3 slices for group).
Statistical analysis
Results are expressed as means ± standard error (SEM). Comparisons among experimental and control groups were performed by one-way ANOVA followed by the Tukey post hoc test. Statistical difference was accepted when p < 0.05.
Results
A1R modulation
As we previously shown, GUO (100 μM) protects striatal slices against in vitro 6-OHDA-induced mitochondrial membrane depolarization and increased ROS generation [13]. So, we aimed to investigate whether these effects were related to A1R modulation. Slices incubated with 6-OHDA (100 μM) showed a decrease in the florescence of the TMRE dye, that is related to a mitochondrial membrane depolarization, as the same effect was observed when slices were incubated with carbonyl-cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP, 10 μM), a mitochondrial oxidative phosphorylation uncoupler [12]. Interestingly, when slices were pre-incubated with the A1R agonist, CCPA (100 nM), it prevented the 6-OHDA-induced mitochondrial membrane depolarization (Fig. 1a). However, the pre-incubation with CCPA did not affect the GUO protective effect on mitochondrial membrane potential (ΔΨ). Regarding ROS levels, CCPA pre-incubation had no effect on 6-OHDA-induced ROS increase and did not alter the protective effect of GUO. These results suggest that activation of A1R does not counteract the GUO effects.
On the other hand, pre-incubation with DPCPX (250 nM), an A1R antagonist, showed to be ineffective in preventing the mitochondrial membrane depolarization and ROS increase caused by 6-OHDA (Fig. 2c, d). Besides that, DPCPX pre-incubation totally abolished GUO effects on 6-OHDA-induced mitochondrial membrane depolarization and ROS increase. These experiments indicate that blocking A1R also blocks the GUO effects, showing an A1R dependence on GUO protective effects.
Effects of A2AR modulation on 6-OHDA-induced toxicity in striatal slices. Experimental design is describe in a. Striatal slices were pre-incubated with of A2AR agonist CGS 21680 (CGS, 30 nM; b, c) or A2AR antagonist SCH 58261 (SCH, 50 nM; d, e). Slices were incubated with 6-OHDA (100 μM) and/or co-incubated with GUO (100 μM). 6-OHDA-induced mitochondrial membrane depolarization (b, d) and ROS levels increase (c, e). Data are expressed as percentage of controls normalized among individual experiments and represent means with SEM (n = 6). (*) when p < 0.05 compared with control or (#) compared to 6-OHDA group (one-way ANOVA followed by Tukey’s test)
A2AR modulation
A putative A2AR dependence on the GUO protective effects in the 6-OHDA in vitro protocol was also assessed in striatal slices (Fig. 2a). The pre-incubation with the A2AR agonist CGS 21680 (30 nM) exerted no effect on 6-OHDA-induced mitochondrial membrane potential (ΔΨ) depolarization and ROS levels increase; however, it completely blocked the GUO protective effect in both ΔΨ and ROS levels (Fig. 2b, c). These results indicate that A2AR activation can affect GUO effects. The blockade of A2AR is already seen as an anti-parkinsonian strategy, as it has protective effects on many PD models. In this way, pre-incubation of SCH58261 (50 nM), an A2AR antagonist, presented a protective effect upon OHDA-induced ΔΨ depolarization and ROS levels increase (Fig. 2c, d). Moreover, this protective effect of SCH58261 does not affect GUO effects.
ATP levels
As mitochondrial depolarization might cause ATP depletion and 6-OHDA also causes changes in the cellular energetic balance [13], we evaluate the effects of adenosine receptors ligands on 6-OHDA and/or GUO incubation, by measuring ATP levels in striatal slices. Since the blockade of A1R or activation of A2AR interfered on the GUO neuroprotective effect, we performed ATP measurements with DPCPX and CGS21680. As expected, 6-OHDA incubation decreased the ATP levels and GUO co-incubation prevented this decrease (Fig. 3). Although neither DPCPX nor CGS21680 presented effect on ATP levels decrease by 6-OHDA, both ligands impaired the capability of GUO to prevent it. These results are in line with the observed for ΔΨ and ROS levels, indicating that GUO effects are related to both A1R and A2AR.
A1R and A2AR modulation on ATP levels in striatal slices. Experimental design is describe in a. Effect of pre-incubation of A1R antagonist, DPCPX (250 nM) (b) or A2AR agonist, CGS21680 (CGS, 30 nM) (c) on 6-OHDA-induced ATP depletion. Slices were incubated with 6-OHDA (100 μM) and/or co-incubated with GUO (100 μM). Data are expressed as μmol ATP/μg of protein of each sample and represent means with SEM (n = 3). (*) when p < 0.05 compared with control or (#) compared to 6-OHDA group (one-way ANOVA followed by Tukey’s test)
Discussion
In this study, we investigated the modulation of A1R and A2AR upon GUO effects against the cellular damage caused by in vitro incubation with 6-OHDA in ex vivo slices obtained from rat striatum. This in vitro protocol of 6-OHDA-induced toxicity is a simple and sensitive protocol that completely suits our goal of evaluating the mechanism of neuroprotection afforded by GUO. We have already shown that co-incubation with GUO (100 μM) prevented the striatal slices against oxidative damage, mitochondrial dysfunction, and ATP depletion caused by 6-OHDA [13]. GUO is a naturally occurring guanine-based purine that has been pointed out to act as a neuromodulator and a neuroprotective agent [14, 26]. GUO have already shown to exert protective effect in different models of PD. In vitro 6-OHDA toxicity in two cell lines (C6 glioma and dopaminergic human SH-SY5Y neuroblastoma cells) was prevented by GUO incubation [15, 16]. In the same way, the toxicity induced by 1-methyl-4-phenylpyridinium (MPP+) in different cultured cells (SH-SY5Y and PC12 cells) was prevented by GUO [27, 28]. Protective effects of GUO were also showed in rodent models of PD [17, 29], and recently, we showed that GUO effects on reducing oral tremor in reserpinized mice were sensitive to adenosine receptors modulation [30].
Among the new classes of drugs developed to improve the clinical features of PD, A2AR antagonists appear to be the most promising. A2AR blockade has been demonstrated to be effective in both preclinical and clinical PD studies [31,32,33]. Indeed, istradefylline (KW6002), an A2AR antagonist, was already approved for clinical use in Japan, and recently, in the USA [34,35,36]. Interestingly, the mechanism behind A2AR antagonists in PD may rely in part to the existing functional and molecular interaction (i.e., heteromerization) of A2AR and D2R within postsynaptic striatal neurons [37, 38]. Moreover, a mutual trans-inhibition between these two receptors has been described [39]. Corroborating with this, in our results, the A2AR antagonist SCH58261 was effective in both parameters analyzed. More important, the protective effect of SCH58261 does not affect GUO effects, suggesting that they do not interfere in each other mechanism. Interestingly, we previously showed that submaximal doses of SCH58261 and GUO can potentiated each other effect in a PD-related behavioral test but not in ROS levels [30]. Taken together, these data suggest that GUO might be acting through a negative A2AR modulation.
In addition to this postsynaptic site of action, A2AR can also form heteromeric complexes with A1R in presynaptic neurons of the basal ganglia, where they can control glutamate release [40] and striatal circuits independently of dopaminergic signaling [41]. Regarding A1R, some rare mutations on this receptor gene could lead to PD [42]. Also, some studies show that A1R modulation could control and improve motor function associated with PD [43,44,45]. In fact, a lot of data show that A1R stimulation is neuroprotective [46,47,48,49]. Surprisingly, CCPA was unable to prevent the ROS increase induced by 6-OHDA, but it did protected mitochondrial function. However, CCPA did not alter the GUO protective effect on mitochondrial ΔΨ, and this result could be interpreted as a non-additive synergistic effect of CCPA and GUO. Nevertheless, the potential use of A1R-based therapies, by using A1R agonists, could lead to deleterious peripheral side-effects, once A1R is also expressed in the vascular system.
Evidences from other disease models also have pointed to GUO effects via adenosine receptors modulation. In an in vitro brain ischemia model, hippocampal slices subjected to oxygen/glucose deprivation presented increased ROS production prevented by GUO, but this effect is abolished by pre-incubation with DPCPX [19]. Notwithstanding, in the same protocol, not only DPCPX but also CGS21680 blunted the protective effect of GUO in hippocampal slices [19] and in cortical astrocytes [18]. The same pattern of results was seen in our study that used an in vitro PD model and evaluated other brain area. Despite the similarity of these results, one study, where staurosporine-induced apoptosis in astrocytes cultures were evaluated, showed that the neuroprotective effect of GUO was not affected by the selective A1R or A2AR antagonists [50]. On the other hand, still in the ischemia model, in hippocampal slices of A2AR-knockout animals subjected to oxygen/glucose privation, the GUO-protective effects is abolished, evidencing the importance of this receptor for GUO effects. Taken together, these observations strengths the possible mechanism of GUO-effects through the dependence of adenosine A1 and A2A receptors modulation.
Regarding GUO interaction with a putative specific receptor, it was showed that GUO presented selective binding site in cellular membrane that is not related to adenosine receptors [51, 52]; however, this possible GUO-specific receptor has not been fully described or characterized. Beside this and the controversy data regarding GUO effects via A1R or A2AR interaction, the possibility of GUO interaction with adenosine receptor heteromers appears to be the most likely scenario to explain this debate [53]. In fact, we recently showed that GUO-induced effects require both A1R and A2AR co-expression in transfected HEK293 cells [54]. Only in cells expressing both A1R and A2AR, GUO was able to decrease A2AR binding affinity and cAMP response evoked by a selective A2AR ligand. Also, GUO had no effect on A1R signaling through intracellular calcium increase, even in the presence or absence of A2AR co-expression [54]. Considering all these evidences, our working hypothesis is that we need to interpret GUO interaction with adenosine receptors results not as separated receptors but as a heteromeric entity. GUO could be acting as a negative modulator of A2AR, but only in the presence of A1R. It is feasible to speculate that the physical interaction between A1R and A2AR could lead to an increase of A2AR affinity for GUO. This could explain why GUO effects are blocked by CGS21680 and DPCPX (by causing allosteric modulation of GUO A2AR affinity) and not by SCH58261 and CCPA. Indeed, GUO modulation over A1R-A2AR heteromer or A1R or A2AR individual entities could vary among brain structures, once that it may depend on receptors expression. Therefore, we also cannot exclude the possibility of GUO acting through other heteromer, that could modulate or be associated with A1R or A2AR, and further investigations are necessary to detail GUO mechanism of action.
In conclusion, we demonstrated that GUO protective effects on oxidative damage, mitochondrial dysfunction and ATP depletion caused by 6-OHDA in rat striatal slices are sensitive of both A1R and A2AR modulation. These results could provide another possibility for GUO action through the A1R-A2AR heteromer and highlight its importance as a neuroprotective agent in PD.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- A1R:
-
Adenosine A1 receptors
- A2AR:
-
Adenosine A2A receptors
- CCPA:
-
4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl] benzene propanoic acid hydrochloride
- CGS21680:
-
4-(2-[7-Amino-2-(2-furyl)[1, 2, 4] triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol
- DPCPX:
-
1,3-Dipropyl-8-cyclopentylxanthine
- GUO:
-
Guanosine
- 6-OHDA:
-
6-Hydroxydopamine
- H2DCFDA:
-
2,7-Dichlorofluorescein diacetate
- KRB:
-
Krebs Ringer buffer
- ΔΨ:
-
Mitochondrial membrane potential
- MTT:
-
(4,5 Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- SCH58261:
-
5-Amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo(4,3-e)-1,2,4-triazolo(1,5-c)pyrimidine
- TMRE:
-
Tetramethylrhodamine ethyl ester
References
Hirsch EC, Mouatt A, Faucheux B, Bonnet AM, Javoy-Agid F, Graybiel AM, Agid Y (1992) Dopamine, tremor, and Parkinson’s disease,” (in eng). Lancet 340(8811):125–126
Pereira D, Garrett C (2010) Risk factors for Parkinson disease: an epidemiologic study,” (in por). Acta Medica Port 23(1):15–24
Poewe W (2009) Treatments for Parkinson disease--past achievements and current clinical needs,” (in eng). Neurology 72(7 Suppl):S65–S73. https://doi.org/10.1212/WNL.0b013e31819908ce
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration,”(in eng). Ann Neurol 58(4):495–505. https://doi.org/10.1002/ana.20624
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases,” (in eng). Nature 443(7113):787–795. https://doi.org/10.1038/nature05292
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases,” (in eng). J Pharmacol Exp Ther 342(3):619–630. https://doi.org/10.1124/jpet.112.192138
Ungerstedt U (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons,” (in eng). Eur J Pharmacol 5(1):107–110
Blandini F, Armentero MT, Martignoni E (2008) The 6-hydroxydopamine model: news from the past,” (in eng). Parkinsonism Relat Disord 14(Suppl 2):S124–S129. https://doi.org/10.1016/j.parkreldis.2008.04.015
Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordán J, Schrader M (2008) 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells,” (in eng). Free Radic Biol Med 44(11):1960–1969. https://doi.org/10.1016/j.freeradbiomed.2008.03.009
Mu X, He G, Cheng Y, Li X, Xu B, Du G (2009) Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro,” (in eng). Pharmacol Biochem Behav 92(4):642–648. https://doi.org/10.1016/j.pbb.2009.03.008
Lehmensiek V, Tan EM, Liebau S, Lenk T, Zettlmeisl H, Schwarz J, Storch A (2006) Dopamine transporter-mediated cytotoxicity of 6-hydroxydopamine in vitro depends on expression of mutant alpha-synucleins related to Parkinson’s disease,” (in eng). Neurochem Int 48(5):329–340. https://doi.org/10.1016/j.neuint.2005.11.008
Massari CM, Castro AA, Dal-Cim T, Lanznaster D, Tasca CI (2016) In vitro 6-hydroxydopamine-induced toxicity in striatal, cerebrocortical and hippocampal slices is attenuated by atorvastatin and MK-801,” (in eng). Toxicol in Vitro 37:162–168. https://doi.org/10.1016/j.tiv.2016.09.015
Marques NF, Massari CM, Tasca CI (2019) Guanosine protects striatal slices against 6-OHDA-induced oxidative damage, mitochondrial dysfunction, and ATP depletion,” (in eng). Neurotox Res 35(2):475–483. https://doi.org/10.1007/s12640-018-9976-1
Lanznaster D, Dal-Cim T, Piermartiri TC, Tasca CI (2016) Guanosine: a neuromodulator with therapeutic potential in brain disorders,” (in eng). Aging Dis 7(5):657–679. https://doi.org/10.14336/AD.2016.0208
Giuliani P et al (2015) Guanosine protects glial cells against 6-hydroxydopamine toxicity,” (in eng). Adv Exp Med Biol 837:23–33. https://doi.org/10.1007/5584_2014_73
Giuliani P et al (2012) Protective activity of guanosine in an in vitro model of Parkinson’s disease,” (in eng). Panminerva Med 54(1 Suppl 4):43–51
Massari CM, López-Cano M, Núñez F, Fernández-Dueñas V, Tasca CI, Ciruela F (2017) Antiparkinsonian efficacy of guanosine in rodent models of movement disorder,” (in eng). Front Pharmacol 8:700. https://doi.org/10.3389/fphar.2017.00700
Dal-Cim T, Poluceno GG, Lanznaster D, de Oliveira KA, Nedel CB, Tasca CI (2019) Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: involvement of A,” (in eng). Purinergic Signal. https://doi.org/10.1007/s11302-019-09679-w
Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, López MG, Tasca CI (2013) Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions,” (in eng). J Neurochem 126(4):437–450. https://doi.org/10.1111/jnc.12324
Yabe I, Kitagawa M, Takahashi I, Matsushima M, Sasaki H (2017) The efficacy of istradefylline for treating mild wearing-off in Parkinson disease,” (in eng). Clin Neuropharmacol 40(6):261–263. https://doi.org/10.1097/WNF.0000000000000249
Suzuki K, Miyamoto T, Miyamoto M, Uchiyama T, Hirata K (2018) Could istradefylline be a treatment option for postural abnormalities in mid-stage Parkinson’s disease?,” (in eng). J Neurol Sci 385:131–133. https://doi.org/10.1016/j.jns.2017.12.027
Palmer TM, Stiles GL (1995) Adenosine receptors,” (in eng). Neuropharmacology 34(7):683–694
Krügel U, Kittner H, Franke H, Illes P (2003) Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo,” (in eng). Synapse 47(2):134–142. https://doi.org/10.1002/syn.10162
Almeida RF et al (2016) Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems,” (in ENG). Mol Neurobiol. https://doi.org/10.1007/s12035-015-9660-x
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent,” (in eng). J Biol Chem 193(1):265–275
Tasca CI, Lanznaster D, Oliveira KA, Fernández-Dueñas V, Ciruela F (2018) Neuromodulatory effects of guanine-based purines in health and disease,” (in eng). Front Cell Neurosci 12:376. https://doi.org/10.3389/fncel.2018.00376
Pettifer KM, Jiang S, Bau C, Ballerini P, D’Alimonte I, Werstiuk ES, Rathbone MP (2007) MPP(+)-induced cytotoxicity in neuroblastoma cells: antagonism and reversal by guanosine,” (in eng). Purinergic Signal 3(4):399–409. https://doi.org/10.1007/s11302-007-9073-z
Li DW et al (2014) Guanosine exerts neuroprotective effects by reversing mitochondrial dysfunction in a cellular model of Parkinson’s disease,” (in eng). Int J Mol Med 34(5):1358–1364. https://doi.org/10.3892/ijmm.2014.1904
Su C, Elfeki N, Ballerini P, D'Alimonte I, Bau C, Ciccarelli R, Caciagli F, Gabriele J, Jiang S (2009) Guanosine improves motor behavior, reduces apoptosis, and stimulates neurogenesis in rats with parkinsonism,” (in eng). J Neurosci Res 87(3):617–625. https://doi.org/10.1002/jnr.21883
Massari CM et al (2020) Involvement of adenosine A,” (in eng). Purinergic Signal. https://doi.org/10.1007/s11302-020-09716-z
Vallano A, Fernandez-Duenas V, Pedros C, Arnau JM, Ciruela F (2011) An update on adenosine A2A receptors as drug target in Parkinson’s disease,” (in eng). CNS Neurol Disord Drug Targets 10(6):659–669
Pinna A (2014) Adenosine A2A receptor antagonists in Parkinson’s disease: progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued,” (in eng). CNS Drugs 28(5):455–474. https://doi.org/10.1007/s40263-014-0161-7
Jenner P (2014) An overview of adenosine A2A receptor antagonists in Parkinson’s disease,” (in eng). Int Rev Neurobiol 119:71–86. https://doi.org/10.1016/B978-0-12-801022-8.00003-9
Kondo T, Mizuno Y, J. I. S. Group (2015) A long-term study of istradefylline safety and efficacy in patients with Parkinson disease,” (in eng). Clin Neuropharmacol 38(2):41–46. https://doi.org/10.1097/WNF.0000000000000073
Hussar DA (2020) New Drugs 2020, part 1,” (in eng). Nursing 50(2):31–38. https://doi.org/10.1097/01.NURSE.0000651608.77613.29
Dungo R, Deeks ED (2013) Istradefylline: first global approval,” (in eng). Drugs 73(8):875–882. https://doi.org/10.1007/s40265-013-0066-7
Fuxe K, Ferré S, Canals M, Torvinen M, Terasmaa A, Marcellino D, Goldberg SR, Staines W, Jacobsen KX, Lluis C, Woods AS, Agnati LF, Franco R (2005) Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function,” (in eng). J Mol Neurosci 26(2-3):209–220. https://doi.org/10.1385/JMN:26:2-3:209
Fernández-Dueñas V et al (2015) Untangling dopamine-adenosine receptor-receptor assembly in experimental parkinsonism in rats,” (in eng). Dis Model Mech 8(1):57–63. https://doi.org/10.1242/dmm.018143
Ferré S et al (2016) Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer,” (in eng). Neuropharmacology 104:154–160. https://doi.org/10.1016/j.neuropharm.2015.05.028
Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers,” (in eng). J Neurosci 26(7):2080–2087. https://doi.org/10.1523/JNEUROSCI.3574-05.2006
Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S (2007) Adenosine A2A receptors and basal ganglia physiology,” (in eng). Prog Neurobiol 83(5):277–292. https://doi.org/10.1016/j.pneurobio.2007.05.001
Blauwendraat C et al (2017) ADORA1 mutations are not a common cause of Parkinson’s disease and dementia with Lewy bodies,” (in eng). Mov Disord 32(2):298–299. https://doi.org/10.1002/mds.26886
Mango D, Bonito-Oliva A, Ledonne A, Cappellacci L, Petrelli R, Nisticò R, Berretta N, Fisone G, Mercuri NB (2014) Adenosine A1 receptor stimulation reduces D1 receptor-mediated GABAergic transmission from striato-nigral terminals and attenuates l-DOPA-induced dyskinesia in dopamine-denervated mice,” (in eng). Exp Neurol 261:733–743. https://doi.org/10.1016/j.expneurol.2014.08.022
Rivera-Oliver M, Moreno E, Álvarez-Bagnarol Y, Ayala-Santiago C, Cruz-Reyes N, Molina-Castro GC, Clemens S, Canela EI, Ferré S, Casadó V, Díaz-Ríos M (2019) Adenosine A,” (in eng). Mol Neurobiol 56(2):797–811. https://doi.org/10.1007/s12035-018-1120-y
Cortés A, Casadó-Anguera V, Moreno E, Casadó V (2019) The heterotetrameric structure of the adenosine A,” (in eng). Adv Pharmacol 84:37–78. https://doi.org/10.1016/bs.apha.2019.01.001
Mitchell HL, Frisella WA, Brooker RW, Yoon KW (1995) Attenuation of traumatic cell death by an adenosine A1 agonist in rat hippocampal cells. Neurosurgery 36(5):1003–1007; (in eng), discussion 1007-8. https://doi.org/10.1227/00006123-199505000-00017
Kawamura M, Ruskin DN, Masino SA (2019) Adenosine A,” (in eng). J Neurophysiol 122(2):721–728. https://doi.org/10.1152/jn.00813.2018
Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration?,” (in eng). J Neurochem 139(6):1019–1055. https://doi.org/10.1111/jnc.13724
Duarte JM, Cunha RA, Carvalho RA (2016) Adenosine A1 receptors control the metabolic recovery after hypoxia in rat hippocampal slices,” (in eng). J Neurochem 136(5):947–957. https://doi.org/10.1111/jnc.13512
D’Alimonte I, Ballerini P, Nargi E, Buccella S, Giuliani P, di Iorio P, Caciagli F, Ciccarelli R (2007) Staurosporine-induced apoptosis in astrocytes is prevented by A1 adenosine receptor activation,” (in eng). Neurosci Lett 418(1):66–71. https://doi.org/10.1016/j.neulet.2007.02.061
Traversa U, Bombi G, Di Iorio P, Ciccarelli R, Werstiuk ES, Rathbone MP (2002) Specific [(3)H]-guanosine binding sites in rat brain membranes,” (in eng). Br J Pharmacol 135(4):969–976. https://doi.org/10.1038/sj.bjp.0704542
Volpini R, Marucci G, Buccioni M, Ben DD, Lambertucci C, Lammi C, Mishra RC, Thomas A, Cristalli G (2011) Evidence for the existence of a specific g protein-coupled receptor activated by guanosine,” (in eng). ChemMedChem 6(6):1074–1080. https://doi.org/10.1002/cmdc.201100100
Ciruela F (2013) Guanosine behind the scene,” (in eng). J Neurochem 126(4):425–427. https://doi.org/10.1111/jnc.12328
Lanznaster D et al (2019) Adenosine A,” (in eng). Cells 8(12). https://doi.org/10.3390/cells8121630
Acknowledgments
The research was supported by the Brazilian funding agencies, CAPES (CAPES/PAJT), CNPq (INCT-EN for Brain Diseases, Excitotoxicity and Neuroprotection), and FAPESC (NENASC/ PRONEX) to C.I.T. We thank LAMEB/UFSC team work for experimental support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The procedures used in this study complied with the guidelines on animal care of the UFSC Ethics Committee on the Use of Animals (CEUA), which follow the principles of laboratory animal care from NIH (2011).
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Massari, C.M., Constantino, L.C. & Tasca, C.I. Adenosine A1 and A2A receptors are involved on guanosine protective effects against oxidative burst and mitochondrial dysfunction induced by 6-OHDA in striatal slices. Purinergic Signalling 17, 247–254 (2021). https://doi.org/10.1007/s11302-021-09765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-021-09765-y