Abstract
Objectives
Platelets are playing a crucial role in acute cardiovascular events. We investigated if physical stress activates platelets and whether this activation can be inhibited by a polyphenol-enriched diet.
Methods
Blood samples were taken from a total of 103 athletes three weeks before, one day before, immediately as well as 24 hours and 72 hours after a marathon run. Participants were randomized, double-blinded and divided into two groups. One group received a polyphenol-rich beverage the other the same beverage without polyphenols. Besides analysis of platelet counts and impedance-aggregometric-measurement of platelet activity, soluble P-selectin and Endothelin-A measurements were performed.
Results
In the control group, runners showed a 2.2-fold increased platelet aggregation directly after completing a marathon and within the following three days when compared with baseline values (p<0.01). In accordance, significant increases in sP-selectin (57.52ng/ml vs. 94.86ng/ml;p<0.01) were detectable. In contrast, for the group consuming a beverage with increased polyphenol content (upper quartile of study beverage intake) we did not find any increase of platelet aggregation.
Discussion
Physical stress causes a significant increase in platelet activity. Our results demonstrate that a diet enriched in polyphenols is capable of preventing platelet activation. These findings might indicate a diminished cardiovascular stress-reaction following pre-exposition to polyphenol-enriched diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by early endothelial dysfunction (1, 2). In response to endothelial activation, monocytes and T-cells mediate the progression of atherosclerosis (1, 2). In addition to circulating immune cells, platelets also adhere to dysfunctional endothelium which activates their function. These activated platelets release cytokines, growth factors and free arachidonic acids that enhance the migration and proliferation of smooth-muscle cells, monocytes and T-cells (3). Free arachidonic acid can also be transformed into prostaglandins such as thromboxane A2, one of the most potent vasoconstricting and platelet-aggregating substances known (3).
Thus, platelets accumulate on the walls of arteries and recruit additional platelets into an expanding thrombus. By this, plaque rupture and thrombosis are notable complications of advanced lesions that lead to subtotal or complete vessel obstruction with consecutive clinical manifestations (4).
The cardio-protective potential of regular aerobic physical exercise is well known. However, extreme physical exercise can trigger the onset of acute cardiac events, especially in people who are habitually sedentary (5). Marathon running has become a very popular sport and represents prolonged exhaustive physical exercise being associated with a high cardiovascular burden: increased troponin and brain natriuretic peptide (BNP) levels can be detected after a marathon race as consequences of myocardial stress (6, 7).
Additionally, increased platelets clumps and increased plasmatic activity as a result of increased platelet activity after strenuous exercise have been observed (8, 9). This physical stress-induced platelet activity seems indeed to be of clinical relevance. Zwart et al. described several cases of acute stent thrombosis in the context of intense physical exercise despite dual platelet aggregation inhibition (10).
Flavonoids are an important sub-class of the polyphenols and can be divided into three main sub-classes: Flavanols (e.g., epigallocatechin-3-gallate), flavones (e.g., genistein), and flavonols (e.g., quercetin) (11).
Epidemiological studies have demonstrated that increased intake of flavonoids such as epigallocatechin-3-gallate (found, among others, in red wine and green tea) significantly correlated inversely with the incidence of cardiovascular diseases (11-13). Vasculoprotective effects of polyphenols have been attributed to their blood-pressure and cholesterol lowering potential (14, 15), as well as to their potential to decrease platelet aggregability and to improve endothelial dysfunction (13).
Beer is a widely consumed beverage and has been brewed in Bavaria since 1516 (Bavarian purity law), especially out of Hop. Hop is rich in polyphenolic compounds and acyl phloroglucides. The antioxidative action of hop cones, however, is poorly understood. There are some in-vitro studies indicating that the hop extract has protective effects in-vitro against damage to platelet proteins and lipids (16).
The aim of this study was to analyse the marathon-induced thrombocyte activation and to examine if pre-exposition to polyphenol-enriched non-alcoholic beer has the potential to diminish exercise-induced platelet activation.
Materials and Methods
Our study is part of the BeMaGIC Study [Beer Marathon Genetics Inflammation and Cardiovascular System] and was conducted in cooperation with the Department of Prevention and Sports Medicine, Klinikum rechts der Isar, Technische Universitaet Muenchen. The study protocol was approved by the ethics committee (reference number: 2384/09, University Hospital Klinikum rechts der Isar, Munich, Germany). For this prospective, randomized, volunteer- and observer-blinded, placebo-controlled trial, male volunteers aged between 20-60 years and a history of at least one successfully finished half marathon were included.
Exclusion criteria included known cardiac disease, pharmaceutical treatment for diabetes mellitus or arterial hypertension, musculoskeletal or psychiatric disease, neoplasia, acute or chronic infection or inflammatory disease, known malabsorption, use of medications or supplements influencing platelet or immune function, and history of alcohol and/or drug abuse or addiction.
A total of 277 athletes were randomly allocated to the following interventions: 1.0 to 1.5 liters of non-alcoholic beer per day (Erding, Germany) or the same amount of a control beverage identical in taste, color and foaming (placebo group), which differed only with regard to polyphenol content.
The overall polyphenol content of the placebo drink was zero, whereas that of the nonalcoholic beer, measured by the Folin–Ciocalteu test, was 326 ± 1 mg of gallic acid equivalents per liter (mg GAE/l) (17).
Furthermore, subjects were asked to refrain from all polyphenol-containing food, especially beverages such as wine, beer, and fruit juice, as well as fresh and dried fruits or vegetables. In addition, they were asked to minimize intake of fatty foods and large doses of vitamins and to refrain completely from mineral supplementation and probiotic yogurt during the entire study period. Subjects were instructed in multiple workshops by a nutrition scientist on how to record food intakes using standard nutrition diaries. All participants were asked not to change their dietary habits during the study period (17).
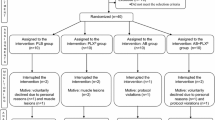
Three weeks before the race, participants were examined for inclusion and exclusion criteria, gave informed written consent, and were randomized in the two study groups and serum for the baseline values was drawn (visit 1). One the day before the marathon, after three weeks of drinking the respective beverage, serum for the 2nd baseline was taken (visit 2). Thereafter, serum was taken within one hour (visit 3), 24 (visit 4) and 72 hours after finishing the marathon (visit 5). Fasting blood samples (defined as abstinence from all food for at least 8 hours) were drawn from an antecubital vein with the subject in supine position at visits 1, 2, 4 and 5 and non-fasting blood samples were taken immediately after the race. For our analysis of platelet function and to exclude confounding by circadian rhythm and postprandial state, we only included subjects with blood drawn at visit 1, 2, 4 and 5 at approximately the same time of day (between 8 and 11 a.m.). All analyses were performed by investigators blinded to categorization (verum and placebo group; figure 1a).
Nutritional intake was recorded with a 3-day nutritional record before visit 2 and before the marathon. To ensure compliance of participants, telephone interviews asking for drinking protocols and a training diary were performed on a weekly basis. In addition, all participants kept a diary to document their exact fluid intake, including the study beverage (17).
Thrombocyte count
To address a possible bias of thrombocyte count by dehydration during the marathon run our department of clinical hematology used the method of Dill and Costill. These authors found (for exercise runners) that the changes in blood volume, cell volume and plasma volume can be calculated from measurements of hemoglobin and hematocrit (18).
Multiple electrode aggregometry
Measurements of the platelet function were performed within 3 hours after taking blood. Aggregation was assessed by a new generation impedance aggregometer (Multiplate, Verum Diagnostic, Munich, Germany). The detailed testing principle and procedure is described elsewhere (19-22).
Briefly, hirudine anticoagulated blood is filled in the test cells, diluted 1:1 with 0.9 % NaCl solution and incubated for 3 minutes under stirring. After 3 minutes, either ADP with a final concentration of 6.4 µM is added (ADPtest) or the measurement is started without further reagent (spontaneous aggregation). ADP is incubated with the thrombocytes and thus stimulating ADP-induced intracellular aggregation signal pathways. During the 6 minutes of measurement, increases of impedance are recorded. The results are given as the area under the curve (AUC) of the ensuing plot with the arbitrary unit “aggregation”.
P-Selectin, Endothelin-A and CGA measurement
Soluble P-Selectin, Endothelin-A and GGA were measured via an enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions. P-Selectin and Endothelin-A Elisa was purchased from R&D Systems (Wiesbaden, Germany). CGA ELISA was purchased from Cedex (France). Blood for measurement was centrifuged at 1000 g for 15 minutes at room temperature. The resulting serum was stored at -80°C. Analysis was performed by investigators blinded to participant allocation into verum and placebo group.
Statistics
The mean value and standard deviation for normally distributed data or the median and interquartile range (IQR) for non-normally distributed data were reported for descriptive purpose. Assumption of normal distribution of data was verified by using descriptive methods (skewness, outliers and distribution plots) and inferential statistics (Shapiro–Wilk test). Data not being normally distributed were analyzed using the Wilcoxon signed Rank Test for paired samples. Unpaired, not normally distributed samples were evaluated using the Mann-Whitney-U test. All statistical tests were conducted with two-sides at a 0.05 level of significance. Differences between means were considered significant with p<0.05 and highly significant with p<0.01. SPSS (Version 16, IBM-USA) was used for statistical analyses.
Results
We assessed 277 participants for eligibility, 27 were excluded from our study. From the remaining 250 participants, for logistic reasons, only 115 could be followed-up due to restrictions of sampling to the time frame of 8:00-11:00h a.m. This time frame was defined to rule out circadian rhythm-induced changes as well as postprandial changes (figure 1a, table 1).
Flow-diagram of probands recruitment. From 277 primary screened probands, 250 were finally included. Subsequently all probands who participated in visit 1,2,4,5 between 8:00 and 11:00 a.m. were included for aggregometry. For the final analyses, the data-records were only used when a drink diary was returned [total 103]
From these 115 participants, 103 handed back their drinkdiaries, by which the individual study-fluid intake could be calculated (figure 1a). In total, we included 103 athletes in our analyses. 50 of the 103 participants received the verum and 53 of 103 the placebo drink. The median of the study fluid intake was 1.2l/day for the verum and 1.28l/day for the placebo group (p=ns; figure 1b).
The participant characteristics are summarized in table 1. To rule out an influence of age differences on the main results of our survey we also analyzed results for four different age groups (20-29 years [n=15], 30-39 years [n=30], 40-49 [n=46], 50-59 [n=23]) and found no relevant differences.
Thrombocyte count
For the first visit we measured 210*103/µl, after the training period we found a significant increase to 217*103/µl (p<0.01). Directly after the marathon we found a further significant increase to 231*103/µl (compared to visit 2; p<0.01). 24h (228*103/µl, visit 4) and 72h (224*103/µl, visit 5) after the race, the platelet count decreased significantly and but was still increased 72h after the marathon compared to visit 1 and visit 2 (p<0.05; figure 1c).
Multiple electrode aggregometry
Spontaneous aggregation
Results are shown as area under the curves (AUC). The baseline at visit 1 was 8 and increased significantly to 11 on visit 2. After the Marathon (visit 3), we found a more than 100% increase reaching 24. 24h after the marathon the spontaneous aggregation was back to baseline-level (V4 versus V3 p<0.01; V4 versus V1 p=n.s.). 72h after marathon, levels were raised again to 13 (V 5 versus V4/V1 p<0.01; figure 2a).
For verum versus placebo analysis (without a differentiation for fluid intake) we found only a lower trend directly after the marathon run for the verum group. Both groups show the same significant changes like in the total population analysis (figure 2b).
For the high intake quartile (≥1.28l/day), we did not find an increase for the platelet aggregation in the verum group (p<0.05 versus placebo). We further did not find a training-induced increase for the verum group (V1 versus V2). The differences between V1 versus V3 or V5 were not significant either. The only significant change for the verum group was the decrease between V3 and V4 (figure 2c).
The placebo group showed the same significant changes as described above for the analyses without group differentiation.
ADPtest
The AUC of ADPtest was 63, 65, 90, 67 and 70 (V1-V5). The results without group differentiation are thus similar to the results of the spontaneous aggregation. The only difference is the missing increase from visit 1 versus visit 2 (figure 3).
For verum versus placebo analysis (without a differentiation for fluid intake), we found lower but not significantly different values for the verum group (figure 3).
Looking at the high intake quartile (≥1.28l), we did not find a difference between both groups (data not shown).
Soluble P-selectin-measurement
For the three week training phase (V1 versus V2), we found a decreased trend (62.17ng/ml versus 57.96ng/ml), which was not statistically significant. After the marathon (V3), the level increased significantly to 94.86ng/ml (p<0.01). 24h after the marathon (V4) the level decreased to 81.74ng/ml (verus V3 p=n.s.) and was still significantly (p<0.05) increased compared to V2. 72h after the marathon (V5) the CD62-levels (61.45ng/ml) returned to baseline levels again (figure 4).
For the subgroup analysis (figure 4) and the high intake analysis we did not find any inter-group differences for all 5 time points (data not shown).
Endothelin-A measurement
For the 3 week training phases (V1 versus V2), we did not find any significant changes (2.42 versus 2.30 pg/ml). After the marathon (V3), levels increased significantly to 2.60 pg/ml (p<0.01). 24h after the marathon (V4), levels decreased to 2.36 pg/ml (versus V3 p<0.05) and returned again to baseline after 72h (V5; 2.29 pg/ml) (figure 5).
For the subgroup analysis (figure 5) and the high intake analysis, we did not find inter-group differences for any of the 5 time points (data not shown).
CGA measurement
For CGA, we tested only the quartile of verum subjects with the highest beverage intake (> 1.28 l/day) plus a matched cohort from the placebo group. CGA was 55.7, 57.5, 75.4, 66, 54.6 pg/ml (V1-V5). The increase after the marathon is as highly significant as the decrease on Visit 4. For the comparison of high intake verum versus placebo group, we did not find any relevant differences (figure 6).
Analysis of CGA [ng/ml] for 12 subjects with verum beverage intake >1.28l/day and 12 subjects from placebo group matched to all criteria from table 1. **p<0.01
Discussion
In this study we examined the influence of physical stress on platelet counts and aggregation and the platelet relevant biomarkers p-selectin and Endothelin-A. We further analyzed the protective potential of polyphenolic compounds on stress-induced platelet aggregation after a marathon run.
We found a significantly increased aggregation of platelets post-marathon. The post-race platelet activation could be inhibited by administration of a polyphenol-enriched diet (≥417 mg GAE/day) for three weeks prior to the run.
With respect to the coagulation blood parameters following exercise, available studies show controversial results. Kratz et al. and Rocker et al. found exercise-induced increased platelets clumps and an increased plasmatic activity (8, 9). In contrast, Rock et al. found a decreased platelet aggregation in a small cohort of 14 participants who were examined merely 24h after the stress event (23). Our study of over 100 participants is the first to use the clinical gold standard of analyzing platelet function by multiple electrode aggregometry in a large cohort (24).
Our results show an increase of 120% for platelet aggregation measured immediately after the marathon and a recurrent increase of 62% after 72h. However, 24h after the marathon activation of thrombocytes was back to baseline, which helps explain the missing increase in platelet aggregation after 24h in the study of Rock et al. (23).
Anfossi et al. described that stress-hormones, like catecholamines, are able to activate platelets via a receptor on the surface of the platelets (25, 26). This seems to be capable to explain the increase of platelet aggregation after the marathon.
The recurrent platelet activation 72h after the marathon is most likely explained by the decelerated increase of the inflammatory cytokines such as IL-6 and TNF-alpha, which are responsible for a prolonged inflammatory state after prolonged exhaustive exercise (27, 28).
Finally, platelets can also be activated by endothelial dysfunction, which may be induced by marathon running. Increased Endothelin-A levels have previously been shown to represent endothelial dysfunction (29).
After the marathon run, we found a significant increase of Endothelin-A levels, which may result in hyper- as well as anticoagulative properties (30). The resulting preponderance is depending, amongst other influencing factors, on endothelial cells and their function. Endothelial dysfunction fosters a hyper-coagulable state with concomitant increased risk for acute cardiovascular events.
We did not find signs for a depletion of thrombocytes, which could be responsible for the intermittent normalized aggregation. Theoretically, activated thrombocytes could be depleted and newly recruited from the bone marrow. The number of thrombocytes would not be affected or even be increased like in our study.
P-selectin is stored in the a-granula of the platelets as well as in the Weibel Palade Bodies of endothelial cells (31). When P-Selectin is activated by inflammatory stimuli, the membrane is disconnected by proteolysis (32). Our results show elevated levels of P-selectin 24h after the marathon, possibly due to a degradation of platelets in a rising inflammatory milieu.
The moderate increase of spontaneous thrombocyte aggregation during the training period of three weeks most likely represents activation due to more than 60km/week of regular exercise training. In a previous study we were able to show that high training loads are associated with increased chromogranin A levels, which is a specific stress marker (33). The hypothesis of a training-induced increase of thrombocyte activity is supported by the fact, that even the Endothelin-A levels of our study population are around 100% higher [1,1pg/ml versus 2,3 pg/ml] than in a sedentary control collective (34).
In their studies, Li et al. were not able to inhibit the activation by the intake of acetylsalicylic acid (35, 36). This may help explain why the occurrence of a stent-thrombosis is possible during a dual platelet inhibiting regime after extreme exercise, as described by Zwart et al. (10). Our findings of an inhibition of activation by high polyphenol intake may therefore be of high clinical relevance.
The pathophysiological mechanisms that may help explain the effectiveness of the polyphenols inhibiting the exercise-induced platelet activation are complex. Possible mechanisms how polyphenols may reduce exercise-induced platelet aggregation are, for example, by increasing the intracellular concentration of cAMP (inhibition of decomposition and enhanced production) or by a consecutive increase of intracellular Ca2+-concentrations which in turn inhibits platelet aggregation (25, 37, 38). In addition, polyphenols are also able to inhibit the production of Thromboxane A2 (39) and Proteinkinase C (40) and potentially increase NO-production (41). Furthermore, polyphenols have a high antioxidative capacity whereby they neutralize reactive oxygen species (ROS), which are induced by nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidases), a proaggregation agent (37, 41).
In our setting, ADP-addicted pathway, Endothelin-A, P-selectin levels an CGA were not influenced by polyphenolintake.
Interestingly, we found an effect for high-intake of polyphenols on spontaneous platelet aggregation. High-intake was defined as more than 417 mg GAE per day. Data about a daily intake of polyphenols are conflicting but daily intake can be estimated as 800-1000 mg/d depending on examined population (42, 43). So the high intake quartile of athletes received an increase of about 50% of daily polyphenol intake.
Next to the quantity of polyphenol intake, the total antioxidant capacity plays a pivotal role in cardiovascular prevention, taking into account all antioxidants and the synergistic effects between them (44). The hop-based beer has relatively high gallic acid equivalents and, therefore, has high potential for vascular protection (45)
Although polyphenol supplementation was increased only for 3 weeks before the marathon run, the overall cumulative intake proved to be effective in a healthy pre-trained population. Further studies are warranted to investigate higher and longer-term intake of polyphenols in a population with an increased cardiovascular risk, such as in patients with coronary heart disease.
Acknowledgments: This work was supported in part by a grant from the Heinrich and Lotte Muehlfenzl Foundation to Thomas Nickel.
Ethics standards: The study protocol was approved by the ethics committee (reference number: 2384/09, University Hospital Klinikum rechts der Isar, Munich, Germany).
Conflicting of interests: A. Calatzis also held a position as chairman and founder of Verum Diagnostic (Munich, Germany), which let us use multiplate equipment for free, we paid only for the incidentals. The BeMaGIC Study (Beer Marathon Genetics Inflammation and Cardiovascular System) conducted by the Department of Prevention and Sports Medicine (M.Halle and J.Scherr) was funded by the Erdinger Weissbräu (beer company).
Abbreviations
- ADP:
-
adenosindiphosphat
- AUC:
-
area under the curve
- BeMaGIC:
-
Beer Marathon Genetics Inflammation and Cardiovascular System
- BNP:
-
brain natriuretic peptide
- cAMP:
-
cyclisches Adenosinmonophosphat
- CGA:
-
Chromogranin A
- ELISA:
-
enzyme linked immunosorbent assay
- GbIIb/IIIa:
-
glycol-protein IIb-IIIa complex
- GAE:
-
gallic acid equivalents
- IQR:
-
interquartile range
- NADPH:
-
nicotinamide adenine dinucleotide phosphate-oxidase
- NO:
-
nitric oxide
- ROS:
-
reactive oxygen species
References
Libby, P. and P. Theroux. Pathophysiology of coronary artery disease. Circulation. 2005;111(25): p. 3481–8.
Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16): p. 1685–95.
Bombeli, T., B.R. Schwartz, and J.M. Harlan. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med. 1998;187(3): p. 329–39.
Nickel, T., D. Schmauss, H. Hanssen, Z. Sicic, B. Krebs, S. Jankl, C. Summo, P. Fraunberger, A.K. Walli, S. Pfeiler, and M. Weis. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205(2): p. 442–50.
Mittleman, M.A., M. Maclure, G.H. Tofler, J.B. Sherwood, R.J. Goldberg, and J.E. Muller. Triggering of Acute Myocardial-Infarction by Heavy Physical Exertion-Protection against Triggering by Regular Exertion. New England Journal of Medicine. 1993;329(23): p. 1677–1683.
Trivax, J.E., B.A. Franklin, J.A. Goldstein, K.M. Chinnaiyan, M.J. Gallagher, A.T. deJong, J.M. Colar, D.E. Haines, and P.A. McCullough. Acute cardiac effects of marathon running. Journal of Applied Physiology. 2010;108(5): p. 1148–1153.
Hanssen, H., A. Keithahn, G. Hertel, V. Drexel, H. Stern, T. Schuster, D. Lorang, A.J. Beer, A. Schmidt-Trucksass, T. Nickel, M. Weis, R. Botnar, M. Schwaiger, and M. Halle. Magnetic resonance imaging of myocardial injury and ventricular torsion after marathon running. Clin Sci (Lond). 2011;120(4): p. 143–52.
Kratz, A., M.J. Wood, A.J. Siegel, J.R. Hiers, and E.M. Van Cott. Effects of marathon running on platelet activation markers: direct evidence for in vivo platelet activation. Am J Clin Pathol. 2006;125(2): p. 296–300.
Rocker, L., M. Taenzer, W.K. Drygas, H. Lill, B. Heyduck, and H.U. Altenkirch. Effect of prolonged physical exercise on the fibrinolytic system. Eur J Appl Physiol Occup Physiol. 1990;60(6): p. 478–81.
Zwart, B., T.C. Van Kerkvoorde, J.W. van Werkum, N.J. Breet, J.M. Ten Berg, and A.W. Van’t Hof. Vigorous exercise as a triggering mechanism for late stent thrombosis: A description of three cases. Platelets. 2010;21(1): p. 72–6.
Nickel, T., H. Hanssen, Z. Sisic, S. Pfeiler, C. Summo, D. Schmauss, E. Hoster, and M. Weis. Immunoregulatory effects of the flavonol quercetin in vitro and in vivo. Eur J Nutr. 2011;50(3): p. 163–72.
Oomen, C.M., M.C. Ocke, E.J. Feskens, F.J. Kok, and D. Kromhout. alpha-Linolenic acid intake is not beneficially associated with 10-y risk of coronary artery disease incidence: the Zutphen Elderly Study. Am J Clin Nutr. 72001;4(4): p. 457–63.
Nickel, T., C.L. Schlichting, and M. Weis. Drugs modulating endothelial function after transplantation. Transplantation. 2006;82(1 Suppl): p. S41–6.
Gaytan, R.J. and L.M. Prisant. Oral nutritional supplements and heart disease: a review. Am J Ther. 2001;8(4): p. 255–74.
Duffy, S.J. and J.A. Vita. Effects of phenolics on vascular endothelial function. Curr Opin Lipidol. 2003;14(1): p. 21–7.
Olas, B., J. Kolodziejczyk, B. Wachowicz, D. Jedrejek, A. Stochmal, and W. Oleszek. The extract from hop cones (Humulus lupulus) as a modulator of oxidative stress in blood platelets. Platelets. 2011;22(5): p. 345–52.
Scherr, J., D.C. Nieman, T. Schuster, J. Habermann, M. Rank, S. Braun, A. Pressler, B. Wolfarth, and M. Halle. Nonalcoholic beer reduces inflammation and incidence of respiratory tract illness. Med Sci Sports Exerc. 2012;44(1): p. 18–26.
Dill, D.B. and D.L. Costill. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2): p. 247–8.
Sibbing, D., S. Braun, S. Jawansky, W. Vogt, J. Mehilli, A. Schomig, A. Kastrati, and N. von Beckerath. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb Haemost. 2008;99(1): p. 121–6.
Sibbing, D., T. Morath, J. Stegherr, S. Braun, W. Vogt, M. Hadamitzky, A. Schomig, A. Kastrati, and N. von Beckerath. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost. 2009;101(4): p. 714–9.
Sibbing, D., S. Braun, T. Morath, J. Mehilli, W. Vogt, A. Schomig, A. Kastrati, and N. von Beckerath. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53(10): p. 849–56.
Siller-Matula, J.M., G. Christ, I.M. Lang, G. Delle-Karth, K. Huber, and B. Jilma. Multiple electrode aggregometry predicts stent thrombosis better than the vasodilator-stimulated phosphoprotein phosphorylation assay. J Thromb Haemost. 2010;8(2): p. 351–9.
Rock, G., P. Tittley, and A. Pipe. Coagulation factor changes following endurance exercise. Clin J Sport Med. 1997;7(2): p. 94–9.
Gremmel, T., S. Steiner, D. Seidinger, R. Koppensteiner, S. Panzer, and C.W. Kopp. Comparison of methods to evaluate aspirin-mediated platelet inhibition after percutaneous intervention with stent implantation. Platelets. 2011;22(3): p. 188–95.
Jurk, K. and B.E. Kehrel. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31(4): p. 381–92.
Anfossi, G. and M. Trovati. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur J Clin Invest. 1996;26(5): p. 353–70.
Nickel, T., H. Hanssen, I. Emslander, V. Drexel, G. Hertel, A. Schmidt-Trucksass, C. Summo, Z. Sisic, M. Lambert, E. Hoster, M. Halle, and M. Weis. Immunomodulatory effects of aerobic training in obesity. Mediators Inflamm. 2011: p. 308965.
Starkie, R.L., J. Rolland, D.J. Angus, M.J. Anderson, and M.A. Febbraio. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Physiol Cell Physiol. 2001;280(4): p. C769–74.
Petidis, K., S. Douma, M. Doumas, I. Basagiannis, K. Vogiatzis, and C. Zamboulis. The interaction of vasoactive substances during exercise modulates platelet aggregation in hypertension and coronary artery disease. BMC Cardiovasc Disord. 2008;8: p. 11.
el-Sayed, M.S. Effects of exercise on blood coagulation, fibrinolysis and platelet aggregation. Sports Med. 1996;22(5): p. 282–98.
Shimomura, H., H. Ogawa, H. Arai, Y. Moriyama, K. Takazoe, N. Hirai, K. Kaikita, O. Hirashima, K. Misumi, H. Soejima, K. Nishiyama, and H. Yasue. Serial changes in plasma levels of soluble P-selectin in patients with acute myocardial infarction. Am J Cardiol. 1998;81(4): p. 397–400.
Blann, A.D., W.M. Noteboom, and F.R. Rosendaal. Increased soluble P-selectin levels following deep venous thrombosis: cause or effect? Br J Haematol. 2000;108(1): p. 191–3.
Eifert, S., S. Kofler, T. Nickel, S. Horster, A.K. Bigdeli, A. Beiras-Fernandez, B. Meiser, and I. Kaczmarek. Gender-based analysis of outcome after heart transplantation. Exp Clin Transplant. 2012;10(4): p. 368–74.
Wilbert-Lampen, U., T. Nickel, D. Leistner, D. Guthlin, T. Matis, C. Volker, S. Sper, H. Kuchenhoff, S. Kaab, and G. Steinbeck. Modified serum profiles of inflammatory and vasoconstrictive factors in patients with emotional stress-induced acute coronary syndrome during World Cup Soccer 2006. J Am Coll Cardiol. 2010;55(7): p. 637–42.
Li, N., N.H. Wallen, and P. Hjemdahl. Evidence for prothrombotic effects of exercise and limited protection by aspirin. Circulation. 1999;100(13): p. 1374–9.
Perneby, C., N.H. Wallen, H. Hu, N. Li, and P. Hjemdahl. Prothrombotic responses to exercise are little influenced by clopidogrel treatment. Thromb Res. 2004;114(4): p. 235–43.
Nardini, M., F. Natella, and C. Scaccini. Role of dietary polyphenols in platelet aggregation. A review of the supplementation studies. Platelets. 2007;18(3): p. 224–43.
Landolfi, R., R.L. Mower, and M. Steiner. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure-activity relations. Biochem Pharmacol. 1984;33(9): p. 1525–30.
Chang, G.T., S.K. Kang, J.H. Kim, K.H. Chung, Y.C. Chang, and C.H. Kim. Inhibitory effect of the Korean herbal medicine, Dae-Jo-Whan, on platelet-activating factor-induced platelet aggregation. J Ethnopharmacol. 2005;102(3): p. 430–9.
Son, D.J., M.R. Cho, Y.R. Jin, S.Y. Kim, Y.H. Park, S.H. Lee, S. Akiba, T. Sato, and Y.P. Yun. Antiplatelet effect of green tea catechins: a possible mechanism through arachidonic acid pathway. Prostaglandins Leukot Essent Fatty Acids. 2004;71(1): p. 25–31.
Pignatelli, P., S. Di Santo, B. Buchetti, V. Sanguigni, A. Brunelli, and F. Violi. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. FASEB J. 2006;20(8): p. 1082–9.
Ovaskainen, M.L., R. Torronen, J.M. Koponen, H. Sinkko, J. Hellstrom, H. Reinivuo, and P. Mattila. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr. 2008;138(3): p. 562–6.
Scalbert, A. and G. Williamson. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130(8S Suppl): p. 2073S–85S.
Rautiainen, S., E.B. Levitan, N. Orsini, A. Akesson, R. Morgenstern, M.A. Mittleman, and A. Wolk. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med. 2012;125(10): p. 974–80.
Piazzon, A., M. Forte, and M. Nardini. Characterization of phenolics content and antioxidant activity of different beer types. J Agric Food Chem. 2010;58(19): p. 10677–83.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Both senior authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nickel, T., Lackermair, K., Scherr, J. et al. Influence of high polyphenol beverage on stress-induced platelet activation. J Nutr Health Aging 20, 586–593 (2016). https://doi.org/10.1007/s12603-016-0697-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-016-0697-y