Abstract
Background
The aim of this study was to verify the effects of protracted intake of chlorophyll on blood counts’ parameters and iron levels in endurance athletes, investigating supposed anti-anemic properties.
Methods
Twenty-two endurance athletes were randomly assigned into two groups in a double-blind study: the experimental group (EG) consumed chlorophyll, while the control group (CG) consumed a placebo, at a dose of 1.6 drops × kg per day for 120 days. Blood cell count and the serum iron analyses were carried out before starting the experiment, after 30 days and after 120 days.
Results
EG showed statistically significant increase in platelet distribution width (PDW, MD = 0.83, 95% CI 0.41, 1.38), mean platelet volume (MPV, MD = 0.41, 95% CI 0.19, 0.67) and platelet/large cell ratio (P-LCR, MD = 3.28, 95% CI 1.51, 5.25) after 120 days. No variations in CG were found during the follow-up.
Conclusions
The increase of platelet-related measures could positively influence the endurance performance by reducing pain and fatigue. The supposed ergogenic effects and anti-anemic properties however require further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focus on molecular structure
Chlorophyll is part of the human diet mainly in the form of fruits, vegetables and food coloring. The molecular structure of chlorophyll consists of a head (formed by a porphyrin ring) from which extends a long hydrophobic tail (phytol). At the center of the porphyrin ring there is a magnesium atom that gives stiffness to the structure avoiding dispersion of solar energy through heat. The porphyrin structure is very similar to the heme group of hemoglobin from which it differs for the presence of a magnesium atom instead of an iron atom [1].
Properties of chlorophyll
Several studies have demonstrated a potent anticancer action of chlorophyll and its derivatives due to its ability to form complex structures with some toxic substances, interfering with the gastrointestinal absorption of potential carcinogens and decreasing the amount of these substances in the susceptible tissues [2]. This anticancer activity has been observed especially in chlorophylline (CHL), a semi-synthetic derivative that differs from chlorophyll mainly for the substitution of the magnesium atom with a copper atom and for absence of the hydrophobic tail: precisely the absence of phytol could increase its effectiveness against carcinogenic and mutagenic agents [3].
The efficacy of chlorophyll and its derivatives has been demonstrated against dangerous substances for humans health such as: aflatoxins and mycotoxins [4]; polycyclic aromatic hydrocarbons formed as a result of incomplete combustion [5]; heterocyclic amines that are formed in high temperature cooked meat [6].
Chlorophyll is also a good source of antioxidants such as vitamin A, C and E, which help neutralize free radicals, that cause cellular damage [7, 8]. Many studies have shown antioxidant properties of chlorophyll and its derivatives [9] with a higher effect by pheophytin (chlorophyll in which the magnesium atom has been replaced by two hydrogen ions) [10]. The protective effect against oxidative damage by chlorophyll would be due to the stimulation of heme oxygenase-1 (HO-1), an enzyme that contributes to maintaining cell homeostasis and whose deficiency could make the organism more vulnerable to inflammatory stimuli and oxidative stress [11].
In addition, chlorophyll has been shown to be effective for the prevention of colorectal cancer [12], lung cancer [13], excretion of dioxins [14] and as antimicrobial [15].
Hypotheses of implication of physical activity
Although chlorophyll has deep been studied for its medicinal and therapeutic properties [16], there are currently no research that investigate its effects as sport supplement in physical activity or for its supposed ergogenic aid and anti-anemic properties.
The endurance training produces iron deficiency in post-exercise due to many factors such as hemolysis, hematuria and sweating. An additional factor is given by the role of hepcidin, a recently discovered hormone produced by liver that regulates iron homeostasis by inhibiting its cellular release and thus lowering blood levels [17]. The endurance activity produces an increase of hepcidin [18] in response to the inflammatory state caused by physical exercise, thereby it determines the condition (multifactorial and multi-causal) defined as “pseudo-anemia” by sports.
Objective of the study
The purpose of this study is to verify the effects of the protracted chlorophyll intake by athletes practicing endurance sports on blood count and serum iron values, to assess supposed ergogenic effects and contrasting “pseudo-anemia”.
Materials and methods
Subjects
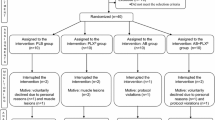
Twenty-two endurance athletes (15 males, 7 females) signed the informed consent and authorization to process personal data before the start of treatment. Participants were randomly divided into an experimental group (EG) and a control group (CG) considering: age, gender, weight, height, weekly training amount and discipline practiced. National and regional level athletes with at least five years of experience in endurance-related activities were included in the study and they were randomly divided into experimental group (EG) and control group (CG) considering: age (EG: 32 ± 11 years; CG: 31 ± 13 years), body weight (EG: 64 ± 11 kg; CG: 61 ± 7 kg), height (EG: 174 ± 8 cm; CG: 174 ± 7 cm), BMI (EG: 20.9 ± 2.7 kg/m2; CG: 20.2 ± 2.1 kg/m2) and weekly training amount (EG: 9.5 ± 3.0 h/w; 10.0 ± 3.0 h/w) as shown in Table 1. All participants did not change the diet and the amount of habitual calorie consumption. Inclusion criteria also considered sporting experience, competitive level and the absence of food allergies and sports injuries.
Experimental design
The experimental protocol was conducted using double-blind study design.
During the competitive period EG consumed daily an alcoholic extract of magnesiac chlorophyll (Herboristic laboratory Di Leo srl, Anzola dell’Emilia, BO, Italy) containing 86% water, alcohol (Vol. 34.4%) and 14% chlorophyll (extracted from festuca leaves) at a dosage of 1.6 drops × kg per day [19]; during the same period CG consumed a placebo containing 98% water, alcohol (Vol. 34.4%), 1.5% caramel , 0.4% yellow dye and 0.1% blue dye at the same dosage. The chlorophyll was guaranteed by the herboristic laboratory regarding the absence of contaminants that could have influenced the result.
Both the EG and the CG took the extract or placebo uninterruptedly for 120 days in the morning (from 7 am to 10 am) on an empty stomach and maintained their respective training program and usual diet. In addition, a daily diary was provided to monitor the time of consumption, training distance, duration of training, training type and fasting weight.
Three blood counts and serum iron tests were performed: before starting the intake (T0), after 30 days (T1) and after 120 days (T2).
For each examination 7 ml of blood was taken and analyzed (LARC, hematic laboratory in Turin) through the XE-2100D System (Sysmex, Kobe, Japan) to evaluate the following parameters: leukocytes (WBC), erythrocytes (RBC), hemoglobin (Hb), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width (RDW-SD, RDW-CV), platelets (PLTS), platelet distribution width (PDW), mean platelet volume (MPV), platelet/large cell ratio (P-LCR), and serum iron (Fe).
The amount of unbound iron (Fe) was analyzed by the ARCHITECT system (c8000, Abbott Laboratories, Abbott Park, Illinois, USA).
Statistical analysis
Statistical analysis was done using the R software (version 3.0.1, R Core Team, Foundation for Statistical Computing, Vienna, Austria). Blinded randomization procedures were performed to minimize selection bias. The normality assumption of the data was analyzed by the Kolmogorov–Smirnov and Shapiro–Wilk tests; the homogeneity of the variables was analyzed by the Levene and Brown–Forsythe tests. Intra-group differences were investigated by the analysis of variance (ANOVA) for repeated measurements. The level of significance for all tests was set at p < 0.05. The Tukey test was used for multiple comparisons.
Power calculation
Threshold probability for rejecting the null hypothesis (Type I error rate) was set at α (two-tailed) = 0.05. Probability of failing to reject the null hypothesis under the alternative hypothesis (Type II error rate) was set at β = 0.20. Standard deviation of the change in the outcome is S(Δ) = 0.40. This study has 80.0% power to detect an effect size of effect size E = E/S(Δ) × S(Δ) = 0.375. R package ‘pwr’ was used to estimates the power of the study [20].
Results
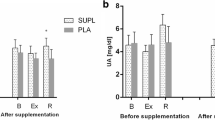
The EG and the CG did not show significant differences at T0. In intra-group analysis (T2 vs T0), significant increases were seen only in the experimental group regarding PDW (0.83, 95% CI [0.41, 1.38], p < 0.01), MPV (0.41, 95% CI [0.19, 0.67], p < 0.05) and P-LCR (3.28, 95% CI [1.51, 5.25], p < 0.001). The control group did not show statistically significant differences during follow-up. The results are reported from Figs. 1, 2 and 3.
Discussion
Blood count parameters, normally related to performance or as detecting elements of a pseudo-anemia state (in particular Ht, Hb, MCH, MCHC and Fe), showed no significant changes in intra-group analysis. In particular, hemoglobin (Hb) values remained stable in both groups; this parameter has a strong correlation with VO2Max [21] and performance in lactic acid tolerance [22]. About serum iron, it is important to point up how the iron turn-over is accelerated during exercise [23] and the deficiencies of this mineral can adversely affect performance [24], whereas iron homeostasis contributes to the production of free radicals, causing oxidative damage [25]; however no statistically significant difference was observed between the two groups or over time. The total number of platelets (PLTS) did not show significant intra-group variations, whereas there was a significant increase over T0 only in the experimental group about platelet distribution width (PDW), mean platelet volume (MPV), and platelet/large cell ratio (P-LCR); these parameters can be considered as a platelet activation index. Platelet-rich plasma has anti-inflammatory and anabolic effects [26] and several studies show its effectiveness in the healing process by muscle injury [27], tendon injury [28] and in the treatment of osteoarthritis [29]. A recent study shows a significant correlation between MPV and the running time in a half marathon [30], while in short-term performance at maximum intensity it appears no significant relationships between PLTS, MPV and PDW with VO2Max, resistance and running speed [31]. These results suggest that platelets may play a role in the performance of medium-long term by promoting the gradual release of growth factors and thereby relieving muscular pain and/or fatigue, or that MPV increase could be attributed to a greater turn-over of platelets that could reflect other chronic physical adaptation without necessarily having a direct ergogenic effect. In the present study, however, only the experimental group obtained a significant increase, indicating a role of chlorophyll in the modification of the above factor. It has been shown that exercise increases PLTS [32] due to adrenergic stimulation (due to epinephrine and norepinephrine activity) that induces splenic contractions resulting in PLTS release into blood circulation [33]; however, the PLTS and MPV values after exercises would return to basal levels after 3 h from the end of activity [34].
Conclusions
Protracted chlorophyll intake in endurance athletes has not shown significant changes about the hemochromocytometric and sideremic parameters. These results could indicate the absence of a direct ergogenic potential of chlorophyll in endurance sports. In the experimental group there was a significant increase in some platelet-related variables, which could affect sport performance and mitigate the pseudo-anemic effects of endurance sport practice.
Chlorophyll supplement can be considered in endurance sports for the proprieties previously described. However, the role of platelets in sports performance is not yet clear and the few findings currently available in the literature are conflicting. It should also be considered that MPV is used as a bio-marker of cardiovascular disease [35] and its eventually sudden increase, due to chlorophyll intake, could induce a false positive in the clinical monitoring.
References
Hendry GA, Jones OT (1980) Haems and chlorophylls: comparison of function and formation. J Med Genet 17(1):1–14
Ferruzzi MG, Blakeslee J (2007) Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr Res 27(1):1–12
Nagini S, Palitti F, Natarajan AT (2015) Chemopreventive potential of chlorophyllin: a review of the mechanisms of action and molecular targets. Nutr Cancer 67(2):203–211
Jubert C, Mata J, Bench G, Dashwood R, Pereira C, Tracewell W, Turteltaub K, Williams D, Bailey G (2009) Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B1 pharmacokinetics in human volunteers. Cancer Prev Res 2(12):1015–1022
Tachino N, Guo D, Dashwood WM, Yamane S, Larsen R, Dashwood R (1994) Mechanisms of the in vitro antimutagenic action of chlorophyllin against benzo [a] pyrene: studies of enzyme inhibition, molecular complex formation and degradation of the ultimate carcinogen. Mutat Res Fundam Mol M 308(2):191–203
Shaughnessy DT, Gangarosa LM, Schliebe B, Umbach DM, Xu Z, MacIntosh B, Knize MG, Matthews PP, Swank AE, Sandler RS, DeMarini DM, Taylor JA (2011) Inhibition of fried meat-induced colorectal DNA damage and altered systemic genotoxicity in humans by crucifera, chlorophyllin, and yogurt. PLoS One 6(4):e18707
Inanç AL (2011) Chlorophyll: structural properties, health benefits and its occurrence in virgin olive oils. Akademik Gıda 9(2):26–32
McCook JP, Stephens TJ, Jiang LI, Law RM, Gotz V (2016) Ability of sodium copper chlorophyllin complex to repair photoaged skin by stimulation of biomarkers in human extracellular matrix. Clin Cosmet Investig Dermatol 9:167
Zhang Y, Guan L, Wang X, Wen T, Xing J, Zhao J (2008) Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radic Res 42(4):362–371
Hsu CY, Yang CM, Chen CM, Chao PY, Hu SP (2005) Effects of chlorophyll-related compounds on hydrogen peroxide induced DNA damage within human lymphocytes. J Agric Food Chem 53(7):2746–2750
Zhang YL, Guan L, Zhou PH, Mao LJ, Zhao ZM, Li SQ, Xu XX, Cong CC, Zhu MX, Zhao JY (2012) The protective effect of chlorophyllin against oxidative damage and its mechanism. Zhonghua Nei Ke Za Zhi 51(6):466–470
Balder HF, Vogel J, Jansen MC, Weijenberg MP, van den Brandt PA, Westenbrink S, van der Meer R, Goldbohm RA (2006) Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol Biomark Prev 15(4):717–725
Das J, Samadder A, Mondal J, Abraham SK, Khuda-Bukhsh AR (2016) Nano-encapsulated chlorophyllin significantly delays progression of lung cancer both in in vitro and in vivo models through activation of mitochondrial signaling cascades and drug-DNA interaction. Environ Toxicol Pharmacol 46:147–157
Kitamura K, Nagao M, Hayatsu H, Morita M (2005) Effect of chlorophyllin-chitosan on excretion of dioxins in a healthy man. Environ Sci Technol 39(4):1084–1091
Lopez-Carballo G, Hernandez-Munoz P, Gavara R, Ocio MJ (2008) Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int J Food Microbiol 126(1):65–70
Mysliwa-Kurdziel B, Solymosi K (2016) Chlorophylls and Their Derivatives Used in Food Industry and Medicine. Mini Rev Med Chem 17(13):1194–1222
Buratti P, Gammella E, Rybinska I, Cairo G, Recalcati S (2016) Recent advances in iron metabolism: relevance for health, exercise, and performance. Med Sci Sports Exerc 47(8):1596–1604
Kong W, Gao G, Chang Y (2014) Hepcidin and sports anemia. Cell Biosci 4:19
Wood R, Foster L, Damant A, Key P (2004) Analytical methods for food additives. Elsevier, Amsterdam
Chow S-C, Shao J, Wang H (2008) Sample size calculations in clinical research, 2nd edn. Chapman & Hall/CRC, Boca Raton (section 3.1.1, p 50)
Schmidt W, Prommer N (2010) Impact of alterations in total hemoglobin mass on VO2max. Exerc Sport Sci Rev 38(2):68–75
Novack V, Finestone AS, Constantini N, Shpilberg O, Weitzman S, Merkel D (2007) The prevalence of low hemoglobin values among new infantry recruits and nonlinear relationship between hemoglobin concentration and physical fitness. Am J Hematol 82(2):128–133
Duca L, Da Ponte A, Cozzi M, Carbone A, Pomati M, Nava I, Cappellini MD, Fiorelli G (2006) Changes in erythropoiesis, iron metabolism and oxidative stress after half-marathon. Intern Emerg Med 1(1):30–34
LaManca JJ, Haymes EM (1993) Effects of iron repletion on VO2max, endurance, and blood lactate in women. Med Sci Sports Exerc 25(12):1386–1392
Sen CK (1995) Oxidants and antioxidants in exercise. J Appl Phys 79(3):675–686
Cugat R, Cuscó X, Seijas R, Álvarez P, Steinbacher G, Ares O, Wang-Saegusa A, García-Balletbó M (2015) Biologic enhancement of cartilage repair: the role of platelet-rich plasma and other commercially available growth factors. Arthroscopy 31(4):777–783
Cunha RC, Francisco JC, Cardoso MA, Matos LF, Lino D, Simeoni RB, Pereira G, Irioda AC, Simeoni PR, Guarita-Souza LC, Carvalho KA (2014) Effect of platelet-rich plasma therapy associated with exercise training in musculoskeletal healing in rats. In Transplant Proc 46(6):1879–1881
Hamid A, Mohamed Ali MS, Yusof MR, George A, Lee J (2014) LP. Platelet-rich plasma injections for the treatment of hamstring injuries a randomized controlled trial. Am J Sports Med 42(10):2410–2418
Raeissadat SA, Rayegani SM, Hassanabadi H, Fathi M, Ghorbani E, Babaee M, Azma K (2015) Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord 8:1–8
Lippi G, Salvagno GL, Danese E, Skafidas S, Tarperi C, Guidi GC, Schena F (2014) Mean platelet volume (MPV) predicts middle distance running performance. PLoS One 9(11):1–6
Alis R, Sanchis-Gomar F, Risso-Ballester J, Blesa JR, Romagnoli M (2015) Effect of training status on the changes in platelet parameters induced by short-duration exhaustive exercise. Platelets 29:1–6
Kratz A, Wood MJ, Siegel AJ, Hiers JR, Van Cott EM (2006) Effects of marathon running on platelet activation markers direct evidence for in vivo platelet activation. Am J Clin Pathol 125(2):296–300
Bakovic D, Pivac N, Zubin Maslov P, Breskovic T, Damonja G, Dujic Z (2013) Spleen volume changes during adrenergic stimulation with low doses of epinephrine. J Physiol Pharmacol 64(5):649–655
Lippi G, Salvagno GL, Danese E, Tarperi C, Guidi GC, Schena F (2014) Variation of red blood cell distribution width and mean platelet volume after moderate endurance exercise. Adv Hematol 192173:1–4
Sharma G, Berger JS (2011) Platelet activity and cardiovascular risk in apparently healthy individuals: a review of the data. J Thromb Thrombolysis 32(2):201–208
Acknowledgements
The authors thank the Herboristic laboratory Di Leo srl for the supply of chlorophyll and for sustaining the direct costs needed to carry out this study.
Funding
The authors have no funding to declare.
Author information
Authors and Affiliations
Contributions
GC performed the data analysis, conceived the statistical analysis, interpreted the results and drafted the manuscript. MI contributed to the experimental design, undertook the data collection, interpreted the results and drafted the manuscript. FM undertook the data collection, interpreted the results and analyzed the literature. FB and VC provided critical comments and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors declare no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cugliari, G., Messina, F., Canavero, V. et al. Relationship of chlorophyll supplement and platelet-related measures in endurance athletes: a randomized, double-blind, placebo-controlled study. Sport Sci Health 14, 449–454 (2018). https://doi.org/10.1007/s11332-018-0477-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-018-0477-7