Abstract
Non-dairy sources of prebiotics and probiotics impart various physiological functions in the prevention and management of chronic metabolic disorders, therefore nutraceuticals emerged as a potential industry. Extraction of prebiotics from non-dairy sources is economical and easily implemented. Waste products during food processing, including fruit peels and fruit skins, can be utilized as a promising source of prebiotics and considered “Generally Recognized As Safe” for human consumption. Prebiotics from non-dairy sources have a significant impact on gut microbiota and reduce the population of pathogenic bacteria. Similarly, next-generation probiotics could also be isolated from non-dairy sources. These sources have considerable potential and can give novel strains of probiotics, which can be the replacement for dairy sources. Such strains isolated from non-dairy sources have good probiotic properties and can be used as therapeutic. This review will elaborate on the potential non-dairy sources of prebiotics and probiotics, their characterization, and significant physiological potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prebiotics are non-digestible food constituents (such as inulin, and fructooligosaccharides), which have potential effects on the host’s health, particularly enhancing the growth of specific bacteria (Bifidobacteria) in the gastrointestinal tract (GI) [1]. Gibson and Roberfroid [1] used the term prebiotic for the first time. Carbohydrates, which are used as dietary fibers, are considered potential prebiotics. Prebiotics and dietary fibers are exchangeable terms used for those food components, which are not digested in the GI tract [2]. The prebiotics is grouped as disaccharides, oligosaccharides, and polysaccharides. Fructooligosaccharide products, including inulin, and oligofructose, are potent prebiotics. Moreover, glucooligosaccharides, xylooligosaccharides, glycol oligosaccharides, maltooligosaccharides, trans-galactooligosaccharides, stachyose, and raffinose also have the prebiotic potential [3]. Various in-vitro and in-vivo studies evidence that oligosaccharides are promising prebiotic options, which include galactooligosaccharides, trans-galactooligosaccharides, xylooligosaccharides, fructooligosaccharides, soybean oligosaccharides, and maltooligosaccharide [4]. Several fruits and vegetables contain an appropriate amount of oligofructose and inulin, usually, leeks, onion, wheat, garlic, and banana are the common sources of prebiotics [5]. Lee and Salminen [6] categorized prebiotics as only partially digested or undigested in the GI tract; small intestine not absorbed them; bacteria in the oral cavity poorly ferment them; they are well fermented by useful intestinal microflora, or potential bowel pathogens poorly ferment them [7,8,9].
Conventionally, yogurt and dairy-based products are considered valuable sources of probiotics. Though, nondairy foods are alternative sources of probiotics and have potential probiotic characteristics. Fruits and vegetables lack lactose, allergens, and cholesterol, which have an adverse effect on a dietary-restricted group of individuals [10]. Fruits and vegetables have phytochemicals and sugar contents, which support the growth of probiotics [11]. After fruit processing, about 70% of the weight consists of peels, and seeds remain as waste, but fruit peels have a high amount of fructooligosaccharides, pectin, and insoluble dietary fiber, other than proteins, vitamins, phenolic compounds, and minerals [12]. Plant waste materials have valuable compounds like disaccharides, oligosaccharides, and non-digestible oligosaccharides, which are currently categorized as prebiotics due to their indigestibility inside the colon and fermented mostly by lactic acid bacteria (LAB) and Bifidobacteria, therefore promoting health [1]. This review paper aims to highlight the potential non-dairy sources of prebiotics and probiotics that are natural, cheap, and generally recognized as safe. This review paper also highlights non-dairy-based foods, alternates of dairy food, including fruits, vegetables, cereals, etc. for both probiotics and prebiotics potential.
Need for Prebiotics and Probiotics
Dairy-probiotic products carry some potential health risks, including lactose intolerance, milk protein allergies, and high fat/cholesterol levels. However, lactose intolerance is the main health problem of dairy probiotics [13]. Lactose intolerance has associated with the inability to break down lactose into glucose and galactose due to low levels of the lactase enzyme. It is feasible to create probiotic food products without dairy, enabling those with intolerances or allergies to milk components to consume the healthy microbes [14]. Numerous alternative non-dairy-based probiotic foods have been developed to alleviate the drawbacks of dairy-based foods. Alternatives to dairy-based probiotic products are still being researched, and consumers may prefer non-dairy-based probiotic products, particularly those that use fruit and/or vegetable as a primary ingredient. Fruit and vegetable-based, cereal-based, and soy-based prebiotics and probiotics are becoming more popular among non-dairy source foods [15]. Fruits, vegetables, and grains are naturally occurring sources of prebiotics. They are not the only source of energy but also provide health and well-being to the consumers. They are helpful to reduce the occurrence and frequency of diarrhea and curing inflammation and other bowel disorders [16]. Prebiotics also promote the uptake and bioavailability of minerals, also lowering the risk of cardiovascular disease and controlling appetite and weight loss [17]. Prebiotics has a direct, indirect, or collective effect on the immune system, as well as increase the gut microbiota and lower the population of pathogens (Fig. 1). It is documented in different studies that mannose monomers reduce the adhesion of Salmonella in GIT [18]. Fructo, oligo, di, monosaccharides, and polyols (FODMAPs) can target the gut flora to treat intestinal bowel syndrome [19]. Animal model studies have proven that inulin-type fructans help in modulating carbohydrates and lipids. Oligofructose (OFS) has an anti-diabetic effect in both high fat and streptozotocin-treated mice [20].
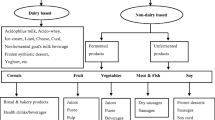
Prebiotics Potential of Non-Dairy Products
Fruits and vegetables being a great source of vitamins, antioxidants, minerals, and bioactive with hydrating qualities and tasty flavors, simultaneously offer an excellent dairy replacement and a good option for the human population, especially those who are lactose intolerant, allergic to milk proteins, and strict vegetarians.
Fruits
Mango
Mango (Mangifera indica) juices and concentrates are industrial products of mango, enriched with phenols and dietary fibers. Approximately 50—55% of the fruit is wasted as by-products, including seeds, peels, and paste. Mangoes peels contain about 40% of dietary fibers and have prebiotic potential. Mango peels have the potential to enhance the growth of Bifidobacterium and Lactobacillus. Indigestible ingredients of mango peels have the potential to be used as prebiotics. Mango peels are used in Kefir and promote the threefold higher growth of LAB [21]. Mango’s pulp has potential prebiotic effects by increasing the total bacterial counts, Lactobacilli, and Bifidobacteria counts in flavored probiotic yogurt [22]. Mango’s peels are enriched with nutrients and compounds having nutraceutical value that exerts antimicrobial, antioxidant, anti-inflammatory, and prebiotic properties [23]. Probiotic growth can be enhanced by using mango selective cultivars having superior prebiotics and antioxidant properties [24]. Polyphenols are potentially beneficial and have effects on the gut microbes significantly enhancing the proliferation of the Bifidobacterium spp. In vitro studies also indicate that mangoes have prebiotic benefit. Evidence shows that polyphenols are utilized by gut microbes and produce metabolites [25, 26]. Further research would be promising to evaluate the health effects of mangoes or their products, which might have phenolic properties or effects on gut microbiota.
Banana
Banana peels are also a good source of fructooligosaccharides (FOS) and generally recognized as safe (GRAS) status can be obtained from banana peels [26]. Banana is a source of prebiotic and oligosaccharides that promote the growth of Lactobacillus paracasei [27]. Green banana pulp, which contains resistant starch, has prebiotic potential. The synbiotic supplement prepared by green banana resistant starch and Bacillus coagulans spores exerts anti-inflammatory in mice models suffering from gut inflammation [28]. Banana has compounds categorized as prebiotic, for example, FOS and inulin [29]. Thus, the green banana pulp has shown prebiotic potential without affecting its physicochemical and sensorial properties [30].
Orange
Orange peel wastes produce pectin oligosaccharides (POS) through hydrothermal treatment, which promotes the growth of Lactobacilli and Bifidobacteria [31]. Prebiotic POS has emerged as a functional food ingredient, produced by hydrolyzing pectin-rich agricultural wastes to partially depolymerize them. Fruit wastes carry important components, mainly pectin that boosts the growth of Lactic acid bacteria (Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum), proving its prebiotic potential. Fruits peels of Citrus lanatus, Citrus limetta, and Musa spp. and rotten fruits of Psidium guajava and Solanum lycopersicum are used for extracting pectin [32]. POS significantly increases Lactobacillus fermentum compared to sugar, exerting great probiotic potential [33]. POS also exerts anti-microbial potential. Orange peels hydrolyzed using multi-enzyme complexes from Aspergillus japonicas also provide consistent POS release [34, 35]. Guava, passion fruit, and orange fruit’s by-products promote bacterial growth during fermentation and enhance probiotic tolerance in simulated conditions of the GI tract. Such findings make the by-products of the fruits useful [36].
Dry Fruits and Nuts
Almonds and their skins also have prebiotic potential. Their intake increases the total fecal count of Lactobacillus spp. and Bifidobacterium spp. Almonds and their skins can also improve the gut microbiota population; therefore, exert considerable prebiotic potential [37]. The dietary fibers in Natural and blanched almond skins significantly promote Bifidobacteria and Clostridium coccoides growth [38], whereas raw and roasted almonds also increase Lactobacillus acidophilus and Bifidobacterium breve, decreasing Enterococcus spp. and Escherichia coli [39]. Similarly, walnuts also have prebiotic potential as it increases Lactobacillus, Ruminococcaceae, and Roseburia, while decreasing Bacteroides, Anaerotruncus, and Alphaproteobacteria [40]. A randomized controlled trial also shows that Butyrate-producing bacteria, Ruminococcaceae and Bifidobacteria, were increased, but Clostridium spp. decreased [41]. The cashew apple as an agro-industrial by-product has prebiotic properties and has shown a significant prebiotic impact on probiotic strains. It can be a value-added ingredient for the food industry [42]. Also, the chestnut extract has a substantial impact on lactobacilli’s improvement in stomach tolerance [43]. The soluble crude polysaccharides from coconut kernel cake increase Lactobacillus plantarum and Lactobacillus rhamnosus CFU [44]. In addition, crude polysaccharides from defatted coconut residue exert positive effects on the growth of Lactobacillus casei Shirota and Lactobacillus bulgaricus [45].
Others
Different cultivars of the kiwifruit are used for their polyphenol and fiber contents to maintain microbial metabolism in the gut, confirming the prebiotic potential [46]. Strawberries have polyphenols, which have prebiotic activities that enhance the gut microbiota [47]. Strawberry supplements given into the diet of diabetic mice boost the amount of Bifidobacterium that plays a vital role in anthocyanin metabolism [48]. Ten percent Papaya pulp is used as prebiotics, while stevia leaves extract 1.0% used as a natural sweetener. The beverage is evaluated for sensory properties and viable count of probiotics during 10 days of cold storage. Such probiotic beverage, which is fortified with papaya pulp and stevia leaves extract, is healthy for consumption [49]. The peels of yellow watermelon, papaya, and honeydews significantly stimulate the growth of Lactobacillus rhamnosus and Bifidobacterium bifidum [50]. Thus, fruits and particularly their by-products exert considerable prebiotics and probiotics potential (Table 1).
Vegetables
Dairy products are inedible for several groups of people, including those who are lactose intolerant, allergic to milk proteins, and strict vegetarians. As a result, there is a need to investigate other non-dairy sources of prebiotics, such as vegetables, to provide consumers with an alternative to dairy products.
Gourd
Vegetables are a rich source of soluble and insoluble dietary fibers. Gourd family vegetables have prebiotic potential. Five gourd family vegetables Trichosanthes anguina, Benincasa hispida, Momordica charantia, and Lagenaria siceraria were blended with Escherichia coli, Bifidobacterium breve, Lactobacillus fermentum, and Clostridium acetobutilicum spp. During in vitro fermentation, all five gourd family vegetables produce short-chain fatty acid (SCFA). Among the five gourd vegetables, B. hispida produces the highest SCFA. Thus, gourd family vegetables have the prebiotic ability [51].
Leafy Green Vegetables
Leafy vegetables can also stimulate the growth of Bifidobacteria and lactobacilli in their pure culture. Such vegetables contain inulin, which is evident in their prebiotic potential [52]. Leafy vegetables are also known to promote the growth of Bifidobacteria and lactobacilli. They are grown in pure culture and supplemented with inulin. Such infusion might be tested as a potential prebiotic source using culture microorganisms or humans as host machinery [52].
Legumes
Many nations around the world rely heavily on legumes as a food source. Beans, chickpeas, peas, lentils, cowpeas, and soybeans are the most often consumed types. The overall composition of legumes is high in carbohydrates (30–60%), dietary fiber (9–25%), and protein (19–36%), which contains essential amino acids including lysine, leucine, and arginine. Bioactive chemicals found in legumes contribute significantly to human metabolism. Healthy components include dietary fiber, resistant starch, polyphenols, and phytosterols [53, 54]. One hundred grams of lentils (Lens culinaris L.) typically contains 4071 mg of raffinose family oligosaccharides (RFOs), 1423 mg of sugar alcohol (SA), 62 mg of fructooligosaccharides (FOSs), and 7500 mg of resistant starch (RS), among other prebiotic carbohydrates [54, 55]. By encouraging the production of Lactobacilli and Bifidobacteria healthy for the intestines and preventing the growth of putrefactive Enterobacteria in the colon, these oligosaccharides and dietary fiber may have prebiotic effects. In vitro studies revealed that red kidney beans have prebiotic potential. Water extractable polysaccharides, obtained from red kidney beans, stimulated the growth of probiotic strains Lactobacillus fermninetum and Lactobacillus plantarum, inhibiting the formation of biofilm by Escherichia coli. Polysaccharides obtained from red kidney beans might be a novel addition to synbiotic product development [56]. By promoting the growth of the advantageous bacteria Enterococcus, Ruminococcus, Blautia, and Bacteroides and inhibiting the growth of the potentially pathogenic bacteria Escherichia Shigella, mung bean seed coat extract influenced the composition of the gut microbiota [57].
Sweet Potato
Orange fleshed sweet potato OFSP has prebiotic potential that promotes the growth of gut microbiota Bifidobacterium genus. It also triggers the production of short-chain fatty acids (SCFA), which is promising for human gut health [58]. White and purple peel sweet potato root flours promoted probiotics growth (Lactobacillus acidophilus, L. casei, and Bifidobacterium animalis) with enhanced metabolic activities (increased acetic, formic, lactic, malic, succinic, and tartaric acids and decreased fructose, glucose, and maltose) [59]. Lactobacillus paracasei, Staphylococcus simulans, and Streptococcus thermophiles survive and proliferate in potato peel supplemented media [60].
Carrot
Because carrots are simple to digest and have a low energy content per 100 g of edible components, they are very beneficial for newborns, elders, and those trying to lose weight. Carotenoids, a type of lipophilic antioxidant, and hydrophilic antioxidants are both abundant in carrots (phenolic compounds) [61]. A carrot-derived pectic polysaccharide, known as cRG-I, supplemented with rhamnogalacturonan-I (RG-I) significantly enhanced Bifidobacterium longum, Bifidobacterium adolescentis, Bacteroides dorei, Bacteroides ovatus, Roseburia hominis, Faecalibacterium prausnitzii, and Eubacterium hallii [62]. The cRG-I administered via SHIME® (Simulator of Human Intestinal Microbial Ecosystem) technology has shown a significant increase in Bacteroides dorei and Prevotella sp. [63]. Carrot juice, both straight and concentrated, fermented by Lactobacillus gasseri DSM 20,604 create a nutrient-rich beverage with fewer calories, increased carotenoid concentration, and prebiotic potential [64].
Cereals
Cereals are recognized as a novel group of prebiotics. Mostly dairy products, vegetables, and fruits have prebiotic potentials and are used as prebiotic carriers [65] (Table 1). Several different kinds of studies are in the process of finding and identifying new sources, which have prebiotic potential. Different cereals grains like wheat, corn, barley, oat, and rice can be used to develop prebiotics [66]. Grains are a promising source of potential prebiotics because cereals are enriched with resistant starch, soluble and insoluble fibers, Fructooligosaccharide (FOS), and galactooligosaccharides [67]. De-lignified wheat bran (DWB) has prebiotic properties and is used in fermented pomegranate juice. Lactobacillus plantarum ATCC 14917 was used as a probiotic strain. DWB gives the protection of probiotic cells. Synbiotic pomegranate juice provided a good sensory evaluation of functional pomegranate juice. DWB is also used as a synbiotic with Lactobacillus paracasei. DWB also exerts prebiotic potential by enhancing the viability of Lactobacillus paracasei [68, 69]. Hemicelluloses have important constituents such as arabinoxylans. They are found in endosperm and outer covering of cereals grains, including wheat, corn, rye, oat, rice, and barley. The enzymatic hydrolysis of arabinoxylans yields arabinoxylan oligosaccharides that have prebiotic potentials, antioxidant activities, and immunomodulatory properties [70]. Arabinogalactan peptide (AGP) found in wheat has shown prebiotic potential. In vitro study has shown that AGP has a potential prebiotic effect on Eubacterium and Bifidobacterium genera. It also increased the concentration of short-chain fatty acids (SCFAs) [71]. Immature wheat grains (IWG) can have an impact on probiotic strains that might be used to enhance the growth and survival of probiotic strains Lactobacillus acidophilus 20079 (L20079) and Lactobacillus acidophilus NCFM (LNCFM) in yogurt [72]. Non-polysaccharides such as β glucan and arabinoxylan (AX) are present in cereal grains. Arabinoxylan (AX) is found in rye and wheat, whereas β glucan is present in oat and barley. In vitro studies on β-glucan and arabinoxylan have shown prebiotic potential. Arabinoxylan (AX) exerts bifidogenic activity and boosts the concentration of SCFAs [71]. Fiber-based oat flour used in low oil salad emulsion supports the growth of Lactobacillus paracasei subsp. Paracasei DC 412. The oat flour enhances the viability of probiotic strain. Viable cell counts are 108 CFU/g under simulated gastrointestinal tract at 7.5 weeks of refrigeration storage [73]. Hard and soft wheat contains water-soluble extractable arabinoxylans (WE-AX). WE-AX has prebiotic potential. AX extracted from hard wheat have more prebiotic efficiency than those from soft wheat. It suppresses the growth of intestinal pathogens and promotes the production of SCFAs in the intestines [74].
Miscellaneous Sources
Flaxseeds (Linum usitatissimum L.) and their oil are considered a source of prebiotics, as it has antimicrobial activities but also stimulate LAB growth. Crude oil of flaxseed is used in in vitro study with Lactobacillus plantarum KCTC3105, Lactobacillus brevis KCT3102, and Lactobacillus kefiranofaciens DN1; Lactobacillus bulgaricus KCTC3635 shows a significant boost in the growth of these lactic acid bacteria [79]. Flaxseed comprises phenolic antioxidants and fiber gives it prebiotic potential. Synbiotic product with dark chocolate and flaxseed is fabricated with Leuconostoc mesenteroides and used as probiotics. Synbiotic dark chocolate gives numerous gut health effects as well as exceptional nutritional value [80]. The synbiotic yogurts are produced by starter cultures of probiotic strains. Synbiotic yogurt made with flaxseed and Lactobacillus acidophilus enhances the growth of Lactobacillus acidophilus [81]. Olive oil is enriched with unsaturated fatty acids and has a bifidogenic effect on gut microbiota to elevate the levels of Bifidobacterium [82].
Spices also have well-documented prebiotic potential. Seven spices investigated for their prebiotic potential includes cinnamon (Venturella), cayenne pepper (CAP), black pepper (BLP), ginger (GIN), turmeric (TUR), rosemary (ROS), and Mediterranean oregano (ORE). Cayenne pepper, black pepper, ginger, and oregano have prebiotic potential as well as antimicrobial effects and promote the intestinal microbiota and eliminate the pathogenic bacteria [83]. Consumption of mixed spices also has a strong influence on gut microbiota and is considered as prebiotic doses [84]. Turmeric (Curcuma longa) is an herbaceous medicinal plant, which has an anti-inflammatory effect. Turmeric extract has shown exceptional resistance again simulated gastrointestinal environment compared to control prebiotic inulin. It also enhances the growth of Bifidobacterium animalis BB12 and Lactobacillus rhamnosus GG (LGG). Ghiamati Yazdi and Soleimanian-Zad [85] first time reported that turmeric extract has prebiotic potential. Black pepper (Piper nigrum), Tulsi (Ocimum sanctum), and Ginger (Zingiber officinale) have prebiotic potential. Tulsi and ginger have high prebiotic activity as FOS with Bifidobacterium and Lactobacillus. Black pepper has similar prebiotic activity as FOS. Therefore, these herbs can be used to promote gut bacteria growth [86]. Turmeric and garlic have prebiotic properties by reducing the population of the intestinal pathogen and stabilizing the gut microbiota [87]. Dandelion roots (Taraxacum officinale) and Elecampane (Inula helenium) contain fructans. Dandelion roots 34% and Elecampane 44% fructans herbs have the prebiotic potential [88]. Cress seed gum (CSG) and coriander extract (CE) affect Lactobacillus plantarum LS5 growth and viability in yogurt drinks (Doogh). CE at 0.05% and CSG at 0.5% level give the highest probiotic count [89]. Fenugreek seeds have prebiotic potential as contain galactomannan. It is the first scientifically documented proof that galactomannan extracted from fenugreek seeds has prebiotic potential. It can play a vital role in the growth of gut microbiota and serve as a substrate for probiotic bacteria such as Bacillus coagulans MTCC [90]. Haghshenas [91] incorporated alginate-psyllium biopolymers with prebiotic fenugreek and inulin and the probiotic Enterococcus durans was microencapsulated. The study has shown that herbs fenugreek and psyllium might be used with alginate, as they exhibit prebiotic properties, and growth enhancers of probiotic bacteria in the harsh gastrointestinal environment [91].
Olive tree pruning biomass (OTPB) yields oligosaccharides by autohydrolysis that have prebiotic potential. Two fractions were obtained and used as in vitro fermentation of Bifidobacterium longum and Bifidobacterium adolescentis, increasing Bifidobacteria [92]. During the olive oil extraction process, olive mill wastewater is obtained, which is a good source of polysaccharides. It has prebiotic potential, and antioxidant properties, shows resistance under a simulated human GI environment, and also has the capacity to ferment by Lactobacilli strains [93]. Aloe vera is also used as a prebiotic source. Aloe vera is enriching with antibiotics activities. Aloe vera fructans can significantly enhance microbial growth compared to inulin [94]. Persian Gum has prebiotic effects and is used as encapsulation material with alginate. Probiotic strain Lactococcus lactis is encapsulated, and it increases the survival rate in the gastrointestinal environment, as observed by Nami et al. [95]. Recent research is focusing on introducing prebiotic chocolates, which served as functional foods. Various in vivo and in vitro studies were conducted to evaluate its pre and probiotic characteristics as in synbiotic chocolates [96]. Recent research documented that dark coffee beans roasted, brewed, and spent coffee grounds have a wide variety of oligosaccharides, which can be utilized as prebiotics [97]. Seaweed (Gelidium sesquipedale) and brown seaweed (Ecklonia radiate, Osmundea pinnatifida) are excellent prebiotic sources because they enhance gut microflora [98]. Polysaccharides and proteoglycans are found in mushrooms, which indicate prebiotic properties in mushrooms [99]. They can also induce antitumor and immunomodulation factors [100].
Physiological Potential of Probiotics
The outcomes of clinical trials are evident that probiotics have potential health benefits in humans (Table 2). In the modern era, probiotic captures public attention as playing a key role in promoting gut health and the supplement of prebiotics and probiotics known as synbiotics [101]. Ingestion of probiotics has been recommended to enhance general health and the body’s immune system [102]. All probiotic strains have specific genomic and microbiological properties, which influence human gut health [103]. The significant interest of customers in non-dairy probiotics is induced due to the vegetarian trend and lactose intolerance. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the USA National Institutes of Health claimed that about 75% of the world population suffers from lactose intolerance. The milk allergens make dairy base probiotics unfit for consumers who have specific health issues. Probiotics obtained from vegetables and fruits may be considered more suitable as free from allergens, cholesterol, and disaccharide [104]. Though, all food products that contain potential probiotic strains should meet the established guidelines by FAO/WHO. Those guidelines are for commercial products as supplemented with probiotics strains: it should be safe from screening to isolation, correct identification, and have antimicrobial susceptibility; it should be stable in GI environment and keep intestinal epithelial adhesion characteristics; should be producing lactic acid and inhibitory potential against pathogens; should have a property to lower down cholesterol levels and boost up the immunity; and it should have anticarcinogenic and antimutagenic properties [105]. LAB probiotics exhibit anti-inflammatory, antimicrobial, anti-osteoporotic activities, and lactose intolerance. LAB can also weight, total cholesterol levels, and serum glucose [13, 106, 107]. The efficacy of probiotics depends on in vitro methods and under control conditions [108].
Probiotics microorganisms produce a wide range of inhibitory substances such as bacteriocins, peroxide, and organic acids. These compounds are helpful to inhibit the undesirable microbial population in the GI tract. Probiotics can provide immunity to encounter enteric diseases [113, 114]. LABs trigger the anticancer immune pathway (Fig. 2). LAB induce the production of different kind of immunity cells. Probiotics and pathogenic bacteria were grown in growing broth with supplementing carbon to promote the probiotics bacteria, indicating strong prebiotic potential and can be used as alternative commercial prebiotics [115].
Selection and Characterization of Probiotics
Isolation of potential probiotic strains has always been a keen interest for food technologists as well as pharmacists. Diverse sources for the isolation of LAB are taking the attention of researchers. LAB are widely distributed non-spore-forming rods or cocci, catalase-negative, anaerobic, gram-positive microbes that produce lactic acid by carbohydrate fermentation with other bioactive compounds, including organic acid and bacteriocins. Probiotics contribute to raised antioxidant, bioactive proteins, and exopolysaccharides levels, modulating oxidative stress and protecting cells from oxidative stress [116]. LAB cannot be only isolated from animal fecal and gut, mother’s milkfishes, shrimps, milk, and milk products, but also from non-dairy sources, including, fruits, vegetables, oil-industrial residues, pickles, fermented meat products, and cereal (Fig. 3). An effective probiotic bacterium must possess features, such as survival in GIT low pH, adhesion ability with intestinal epithelial cells, antibiotic resistance, and antimicrobial activity. It is advisable to consume 106 to 107 CFU/mL of probiotics to get the targeted health benefits [117]. There are various probiotic properties that a LAB strain should pass through during the screening process, including simulated gastric environment (acid and bile tolerance), antibiotics, antimicrobial, antibacterial, auto-aggregation, and co-aggregation, surface hydrophobicity, and safety evaluation [118].
Molecular Characterization of Probiotics
Probiotics identification has a significant role in food-related industries, which cannot be ignored. Therefore, accurate taxonomic identification is essentially required for the precise identification of new probiotic candidate strains, also metabolic features, and genomic information. The typical phenotypic assays do not provide sufficient species-level identification of probiotic LAB or Bifidobacterium strains. Phenotypic characterizations are considered old-fashioned and may have many challenges, such as they can produce ambiguity in the results toward misidentifying a target and then limiting their applications. Therefore, species molecular techniques, such as pulsed-field gel electrophoresis and randomly amplified polymorphic DNA analysis, are most effective for strain differentiation. A variety of other molecular methods, either based on fingerprinting or on sequencing, has also been used for the identification of probiotic Bifidobacteria and LAB. These methods were prompted by the shortcomings of phenotypic approaches and the growing significance of molecular tools in the field of bacterial systematics. Molecular biology has contributed to our understanding of probiotics when comparing the microbial communities identified by conventional approaches with those characterized by molecular technologies [119]. Genotyping is a worldwide technique used for identification, and it differentiates among microorganisms. Using methods, such as DNA-DNA hybridization or DNA sequencing that encodes 16S rRNA, and strain identification methods like pulsed-field gel electrophoresis or randomly amplified polymorphic DNA, probiotic strains can be identified, characterized, particularly physiological characteristics, microbial phenotype, enzymatic activity, etc. [120]. The primers used in Ribotyping are universal to amplify the 16S rRNA gene (27 FYM 5′-AGAGTTTGATYMTGGCTCAG-3′ and 1429R 5′-GGTTACCTTGTTGTTACGACTT-3′) as reported by Behabahani et al. [118]. Currently, one of the most effective and reliable approaches for species identification is the comparison of rDNA gene sequences. The enormous quantity of sequences in the GeneBank database enables accurate strain comparison and identification when DNA genomic similarity is at least 70% [121]. As opposed to PCR and cloning/sequencing analyses, non-selective medium and anaerobic incubation have led to improved detection. Since whole genome sequencing is becoming more widely available, probiotic bacterial species will undergo extensive sequence-based identification research in the coming years. There are certain genotyping tools used for the LAB characterization and identification discussed in Table 3.
Safety Concerns
Probiotic strains must satisfy both safety and functioning criteria, as well as those related to their technological usefulness, as per recommendations of the World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO), and European Food Safety Authority (EFSA) in their selection process [128]. The selective stimulation of intestinal bacteria’s growth may be linked to wellness and health protection. The safety of probiotics is dependent on their intended usage, which takes into account the consumer’s potential vulnerability, the dose, and length of consumption, as well as the method and frequency of administration. Probiotics are distinct from other food products or medicine that potentially spread infection or produce toxins in the body while being consumed. Since many kinds of bacteria can be employed as probiotics, safety is also closely related to the type of microbe that is being utilized. The safety evaluation of bacteria employed as probiotics includes factors such as antibiotic resistance expression and the transferability of antibiotic resistance determinants from fed probiotic strains to commensal microbiota in vivo. For instance, in vivo transfer of the vancomycin resistance gene, vanA, from an Enterococcus strain to L. acidophilus in mice model harboring enterococci-free human microbiota [129]. Moreover, Enterococci may also possess a wide range of virulence factors, such as hemolysin, gelatinase, or DNAse activity as well as the structural genes cylL, ace, asal, and esp [130]. Depending on the traits of the genus and species of the microbe being utilized, it is necessary to evaluate the probiotic’s genetic stability over time, detrimental metabolic activities, and the potential for pathogenicity. Probiotic traits are not linked to a microorganism’s genus or species, but rather to a few, carefully chosen strains of that species. The majority of human probiotic bacteria are from the genera Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus, and Enterococcus. The LAB and Bifidobacteria are probiotic microorganisms used essentially for human health. The origin of strain, lack of interaction with pathogenic strains, and antibiotic resistance profile all contribute to its safety [131]. For possible probiotic strains, the easily accessible and cost-effective genome sequencing methods would confirm the lack of genes of concern. Since entire bacterial genome sequences are becoming more widely available, sequence-based identification methods have been frequently utilized in probiotics identification and selection. It is anticipated that more probiotic candidate strains will be discovered by whole-genome sequencing as a result of improvements in DNA sequencing technologies that considerably increase capacity and speed at lower costs.
Bacteriocin Production and Probiotics
The production of specialized chemicals, antimicrobial peptides, which are active against foodborne pathogens may be a useful and optional factor for selecting probiotic strains. Despite the capacity to synthesize these antimicrobials and the health-promoting qualities, Bifidobacterium spp. is known to generate functional bacteriocins. Numerous bacteriocinogenic probiotic LAB, such as Pediococcus pentosaceus, Pediococcus acidilactici, Lactobacillus plantarum, Lactobacillus lactis, and Enterococcus faecium, are effective against foodborne pathogens [132, 133]. Probiotic bacteria can secrete a several bacteriocins, including short-chain fatty acids, hydrogen peroxide, and nitric oxide, which may improve their capacity to compete with other GI microbes and may suppress pathogenic bacteria. It has been suggested that E. faecium KH24 could be used as a probiotic and that bacteriocinogenic Enterococci might assist in beneficially controlling the local microbiota [134]. In the human GI tract, bacteriocin synthesis may aid in bacteria survival. Bifidobacterium longum subsp. longum DJO10A, an intestinal isolate, suggested that bacteriocin synthesis might be a crucial adaption for GI survival [135]. Fusobacterium, Bacteroides, and Bifidobacterium are a few of the significant gut genera that can be changed by bactofencin A-producing strains, Staphylococcus aureus, and L. monocytogenes, having a favorable impact on gut populations [136]. The Lactobacillus salivarius strains can produce certain bacteriocins under several stressful circumstances found in the gut, including Abp118 [137]. In several disease states, including the targeting of recently emerging pathobionts involved in a range of gastrointestinal illnesses, bacteriocins, and bacteriocin-producing probiotics could be a novel therapeutic approach [137].
Probiotic Potential of Non-Dairy Products
Fruits and vegetables are excellent sources of nutrients and sugar contents. These constituents have been considered considerable for the growth of probiotics, and the blended form provides a fast passage in harsh acidic stomach conditions [11]. As fruits and vegetables do not have inherent cholesterol, lactose, and other allergens, which have health hazards to a certain group of individuals [10]. Vegetables are considered a good source of minerals, sugars, vitamins, and other health-supporting compounds. Tomato, ginger, carrot root, cabbage, onion, and peanuts are used to isolate the probiotics. The lactic acid bacteria such as Lactobacillus plantarum, Lactobacillus casei, Lactobacillus acidophilus, and Bifidobacterium longum are mostly used for probiotic food based on vegetables [10].
Fruits
Several LABs have been isolated from mango brine and identified as PUFSTP38 Bacillus amyloliquefaciens (KT921420), PUFSTP35 Bacillus licheniformis (KT921419), PUFSTP Bacillus subtilis (KT921421), PUPUFSTP 70 Enterobacter cloacae (KU748633), PUFSTP Enterobacter cloacae (KU748634), and PUFSTP44 Bacillus methylotrophicus (KT921422). PUFSTP71 Bacillus safensis (KT92143), Bacillus licheniformis (KT921424) through 16 s rDNA gene sequencing. Most of these strains have antioxidant activity, hydroxyl radical scavenging ability, superoxide anion scavenging ability, plasma lipid peroxidation ability, and linoleic acid. The strain PUFSTP35 (Bacillus licheniformis KT921419) exhibits anticancer properties on the HT-29 colon cancer cell line [138].
Fruits and vegetables are used for the isolation of lactic acid bacteria Lactobacillus pentosus are isolated by chilly and Nagi Naspati (Pyrus communis), and Lactobacillus fermentum is isolated by PRSI Peru (Pyrus pyraste) using 16S rDNA [139]. Banana, cherry, orange, plum, and sapota fresh fruits are the vector for the isolation of Weissella paramesenteroides, which are valued by having bacteriocin and exopolysaccharide production ability. Five Weissella paramesenteroides strains are isolated from fruits. These strains are screened and selected based on tolerance at high acidic conditions, high cell surface hydrophobicity, bile-induced biofilm formation, and antimicrobial activity (AMA). Furthermore, these strains do not possess virulent traits, such as hemolysis, DNase production, and biogenic amine production [140]. Cardinal and red globe varieties, grown in Tunisia, were used to isolate the lactic acid bacteria. Screened lab strains have antifungal activities. Aspergillus carbonarius and Aspergillus niger are used fungal strains. The Pediococcus pentosaceus RG7B has the potential to detoxify ochratoxin A among all 18 strains. It can be considered a significant probiotic strain due to its stability in gastric juice and antimicrobial properties [141].
Vegetables
Lactic acid bacteria are isolated from fresh vegetables such as cauliflower, cluster beans, gherkins, fenugreek, bitter gourd, cowpea, French beans, tomatoes, cucumber, rigid gourd, and bottle gourd. Phenotypic and molecular characterization identified genera Lactobacillus, Enterococcus, and Weissella, which possess antibacterial properties against human and plant pathogens [142]. Vegetable juices are a good source of probiotics. Kale (Brassica oleracea L. var. acephala L.) vegetable juice is used to isolate LAB. Mainly Lactobacillus coryniformis, Lactobacillus plantarum, and Lactobacillus sakei are dominant strains. Among them, Lactobacillus plantarum has shown broad-spectrum antimicrobial activities. It also exhibits acid tolerance and bile tolerance characteristics. This study reveals that probiotics obtained from such vegetable sources can be considered a good probiotic potential source that can use as a starter culture [143]. Carrot juice inhibits the growth of Bifidobacterium catenulatum. Without affecting the probiotic species, carrots selectively prevented the growth of undesired intestinal bacteria [61]. Carrot juice increases both Lactobacillus rhamnosus and Lactobacillus bulgaricus probiotic bacterial strains up to levels of almost 5 × 109 CFU [144].
Cereals and Legumes
Lactic acid bacteria from legumes were isolated from Sweden, and found that Enterococcus and Pediococcus were predominant and closely associated with Bacillus. Tolerance toward low pH, bile salt hydrolase activity, and phenol are analyzed. Enterococcus strains were tested for resistance to antibiotics, whereas Pediococcus were evaluated for fermentation of bean beverages. Among 25 strains, five have the potential to resist at low pH, phenol, and bile also can survive even in GIT [145]. Leguminous fermented foods may be one of the probiotic bacteria carriers that are good for persons who cannot consume dairy products. Red kidney and green mung bean-based beverage KB (7:3) significantly improved the growth of probiotic Lactobacillus casei ATCC 335 and prevented the growth of intestinal Escherichia coli. Additionally, it had a significant amount of total reducing sugars and total carbs, which gave probiotics an energy source [146]. Prebiotics include the well-known soybean oligosaccharides, raffinose, and stachyose can be found in soygerm powder, which is also high in isoflavones [147]. Moreover, an enzyme, endogalactanases, produces arabinogalactan-oligosaccharides. The inclusion of Lactobacillus reuteri in a synbiotic with soygerm powder became more bile salt-resistant after being exposed to soygerm powder (4 g/l) [148]. L. rhamnosus GG, a probiotic strain, could proliferate and perform metabolic processes during the fermentation of leguminous porridges. Except for red bean porridge, where a drop in cell counts after 19 days was noticed, L. rhamnosus GG’s cell counts remained consistent during storage [149]. The porridges have the potential to produce lactic acid during the storage period, i.e., LAB could be proliferated in porridges. However, soybean and speckled beans cannot facilitate the production of lactic acid. Chickpea porridge produced the most lactic acid during storage at a rate of 6.54 mg kg−1 h−1. Amaranth flour and whole buckwheat flour showed lactic acid production. Also, no significant changes in lactic acid levels during storage were found in cereal and pseudocereal porridges fermented with L. rhamnosus GG [150]. Furthermore, the stability of the lactic acid bacteria was greatly improved by encapsulating in microparticles made of soybean protein concentrate [151]. The solid-state fermentation of the whole soybean with LAB mixed with B. subtilis natto facilitates the growth of LAB, i.e., the viable counts of B. animalis, L. casei, and L. plantarum were 1.41 × 108 CFU/g, 1.74 × 1010 CFU/g, and 2.19 × 1010 CFU/g, respectively [152]. The combination of soy with probiotics considerably reduces cholesterol levels, whereas soy enriched with prebiotics reduces total and low-density lipoprotein cholesterol in hypercholesterolemic patients [153]. The combination of extra-virgin olive oil with probiotics also improves dyspeptic symptoms [154]. In addition to having a higher nutritional value than other conventional fermented foods, olives are a promising functional food that transports probiotic microorganisms. The probiotic LAB strains included L. plantarum, L. pentosus, L. paracasei, L. rhamnosus, and L. coryniformis, which were largely isolated from diverse fermented olive varietals [155]. The vitality of probiotics that have been encapsulated and their resilience to environmental stresses, such as simulated gastrointestinal conditions, are improved by multi-layered fish oil microcapsules [156].
Meat Products
Fresh and raw meat, cooked, matured, dried, or fermented meat products are used to isolate LAB-producing bacteriocins. The five strains are identified as Lactococcus lactis subsp. hordinae CTC 484, Lactococcus lactis subsp. cremoris CTC 204 and CTC 483, and Lactobacillus plantarum CTC 368 and CTC 469 based on morphological, biochemical, and physiological characteristics. Whole-cell proteins (SDS-PAGE) and random amplified polymorphic (RAPD-PCR) analysis have shown the similarity among Lactobacillus strains and variation between Lactococcus strains. Among these, all CTC 469 have shown more acid bile tolerance even at 0.1%, whereas all other strains have shown a good survival rate at 4 ℃, 25 ℃, and 37 ℃. Due to the lower level of biogenic amine production by CTC368 and CTC 204 can have the greatest potential to use as probiotics [96]. In a study from Serbia, 21 Enterococcus faecium strains were analyzed for the simulated gastrointestinal environment, antibiotics, and antimicrobial resistance. The study observed that all 21 strains showed tolerance in the simulated gastrointestinal environment and susceptibility against ofloxacin, amoxicillin, and penicillin antibiotics. The study revealed that Enterococcus faecium strains obtained from fermented sausage have shown potential to study further probiotic properties and might be a possible probiotic [157]. In a study from Germany, the lactic acid bacteria were isolated from fermented sausages from traditional and industrial processing lines. Lactic acid bacteria have antibacterial properties against Listeria innocua DSM 20649, Pseudomonas aeruginosa DSM 939, Escherichia coli DSM 1103, Listeria monocytogenes DSM 19094, Salmonella typhimurium DSM 19,587, and Staphylococcus aureus DAM 799. Real-time PCR has also performed the confirmation of the structural genes for bacteriocin. Thus, German fermented meat products have potential strains of lactic acid bacteria with bacteriocin production ability [158]. The lactic acid bacteria can reduce nitrite production in fermented meat products. Thai fermented sausage, locally known as E-sarn sausages, was used for the isolation and molecularly identification of lactic acid bacteria. These strains were identified as Lactobacillus plantarum, Pediococcus pentosaceus, Enterococcus durans, and Lactobacillus brevis. The identified strains have great potential to reduce and degrade nitrite, therefore used as starter culture in Thai meat fermented products [159]. Raw shrimps were used to isolate Enterococcus lactis Q1 & 4CP3. E. lactis can form biofilms against the strains of methicillin-resistant Staphylococcus aureus (MRSA). Enterococcus lactis Q1 & 4CP3 have significant potential to form biofilm alone as well as in combination [160].
Vegan Food
Vegetarian and/or vegan lifestyles are on the rise as more people choose to reject items made from animals. An increasing number of consumers rely on plant-based milk substitutes due to concerns about sustainability, health, lifestyle, and diet, which has led to an abundance of products made from nuts, seeds, or beans [161, 162]. The food items with added value and/or nutritional balance are of significant interest. Probiotics could a potential option due to their effectiveness, significant physiological properties, and the flexibility of the probiotic cultures to varied dietary matrices. The most common vegan probiotic carriers are fermented and non-fermented beverages, in which the survival of probiotics in vegan goods is influenced by the food matrix and processing. Vegan probiotic products may strengthen the immune system and lipid metabolism [162, 163]. The viability of Lactobacillus sp., L. acidophilus, L. casei, Bifidobacterium, and B. longum exceeded 7 log10 CFU/mL in the presence of FOS, inulin, mannitol, maltodextrin, and pectin. Maltodextrin, pectin, mannitol, and FOS supplementation considerably boosted the growth of LAB, also increasing lactic acid concentrations. Maltodextrin and FOS supplementation also raised the α-galactosidase activity of probiotics, resulting in improved soy oligosaccharide hydrolysis and utilization [164]. In another study, various strains of L. acidophilus (FTDC 1331, FTDC 2631, FTDC 2333, FTDC 1331) and L. bulgaricus FTCC 0411 were immobilized on dried and ground wastes of mangosteen, durian, jackfruit, and inoculated into soy milk. LAB that had been immobilized demonstrated improved growth, and enhanced reduction of sucrose and glucose, with higher lactic and acetic acid concentrations, resulting in lower pH in soy milk. Therefore, agro wastes might be used to immobilize probiotics with improved growth, substrate utilization, and organic acid generation [165]. Non-dairy plant-based milk, which is mostly made from fruits and seeds, such as soy, coconut, almond, rice, peanut, and cashew, can be used to substitute conventional milk products. The formation of lactic acid and other physiologically active substances contribute toward nutritional and physiological value results from the starter cultures’ bacterial activity, giving fermented plant-based milk its distinctive flavor [166]. Soy milk also significantly increases the probiotics’ ability to tolerate bile and acid [167]. With cell viability of 7.55 ± 0.07 log CFU/mL, coconut milk served as an acceptable substrate for L. reuteri growth without the need for additional nutrients. After storage, L. reuteri showed a significant pH decline and post-acidification. However, due to poor cell viability preservation, coconut milk may not be accepted as a temporary transport medium [168].
Innovative Probiotic Functional Foods
Day by day, there is an increase in vegetarianism (or vegetarian or veganism) and consumers are in search of alternative plant-based food that is loaded with nutrition and functional values, such as probiotics. Currently, probiotic products make up between 60 and 70% of the whole functional food industry [169]. As fortification of food products can enhance the nutraceutical value, such fortified products provide health benefits as well as possess excellent organoleptic values (Table 4). Functional foods have considerable nutritious values that positively affect human health and at the same time the state of mind satisfied and the well-being of consumers [170]. Fruits, vegetables, seeds, nuts, spices, and herbs are considered improved functional foods [171]. On the other hand, modified functional foods are those food products having additional bioactive components, including minerals, vitamins, fibers, probiotics, etc. to improve health conditions. Modified functional food, also known as fortified food, has a higher amount of nutrients compared to natural food. Various fortified food products have been developed, such as fortified juices, fortified grains, fortified cereal, fortified milk alternatives, etc., to combat malnutrition, hunger, and poverty worldwide [172, 173]. Plant-based fortified foods have become progressively prevalent over the last decade [174].
The probiotic juice’s sensory qualities can vary depending on the type of fruit, probiotic organism, temperature at which they are stored, and whether prebiotics and protectants are included. It has been demonstrated that the probiotics’ survival and stability during the preparation and storage of probiotic fruit, vegetable, and cereal foods depend on the food matrix, water activity, pH of the finished goods, and choice of the probiotic strains. Fruit and vegetable juices may provide some necessary nutrients, but other circumstances, such as low pH, which is linked to high quantities of organic acids and dissolved oxygen, may limit the survival of probiotics [185]. Non-dairy probiotics food is the healthy food component with high fiber content and causes no allergy. However, the low pH of non-dairy foods and the presence of antimicrobial compounds might affect the viability of LAB. The probiotic juice’s sensory qualities can vary depending on the type of fruit, probiotic organism, temperature at which they are stored, and whether prebiotics and protectants are included. By adding a pleasant aroma and volatile chemicals that can hide (or mask) the presence of probiotics, it may be feasible to lessen the perception of unpleasant flavors and odors in non-dairy probiotic diets. Particularly, the aroma and flavor of the finished product may benefit from the addition of tropical fruit juices like pineapple, mango, or passion fruit [186].
Synbiotics
Synbiotics are the blended products of prebiotics and probiotics that aid in survival and anchor microbes into the GI tract (Fig. 4). The term synbiotic, which suggests synergy, describes a combination of prebiotics and probiotics in which the prebiotic component favors the probiotic bacteria’s ability to survive in the gastrointestinal tract [187]. Synbiotics have typically 10 g/kg of prebiotics. Different prebiotics will promote the development of various native gut microbiota, but these changes take place at the level of specific strains and species. A synbiotic containing Bifidobacterium longum and oligofructose increased feces bifidobacteria by 1.4 CFU/g, whereas oligofructose alone caused a 1.6 CFU/g rise in fecal bifidobacteria [188]. The combination of prebiotic and probiotic exerts more promising synergistic benefits in the living system of the host. Synbiotic association between probiotics and prebiotics leads to numerous health benefits. The functional foods comprising synbiotics have gained commercial interest due to their beneficial outcomes such as a healthy gut, prevention, and therapy of diseases [189]. Prebiotics also affects the immunological functions of several organisms. Various studies conducted on fishes demonstrated that prebiotics stimulates epithelial cells to secrete cytokines that modulate immune cells. Synbiotics also stimulate the degranulation of hemocytes in shrimps [190]. Synbiotics were developed to address potential issues with the survival of probiotics in the digestive tract since they have both probiotic and prebiotic qualities. Lactic acid production is boosted by the synergistic impact of synbiotics to reduce internal inflammation. LAB must pass through the digestive tract after being consumed, enter the colon in sufficient numbers (109 CFU/day), and then cling to and colonize the gut epithelium [191]. The probiotic bacteria’s capacity to agglutinate might enhance their chances of interacting with the host. Autoagglutination and co-agglutination causes brief colonization and extends the time to reach the gut, exerting desired beneficial effects. This temporary colonization favors the local action of probiotic metabolites, such as SCFA. However, prebiotic oligosaccharides may also improve probiotics’ ability to adhere to surfaces, indicating that development of new synbiotic products may extend the time of probiotic bacteria in the gut [192]. In synbiotics, combination of prebiotics and probiotics, prebiotics amounts diminish during storage because probiotics consume prebiotics as a source of carbon. The viability of probiotics in a product during consumption is critical; therefore, probiotics must withstand the mechanical stress during food processing and storage. By choosing effective probiotic strains, physically coating them, and adding the right prebiotics, the viability can be preserved [193]. Retaining cells in a protective membrane through the process of microencapsulation helps to prevent damage and death. The probiotics are preserved by encapsulation, which also increases their viability throughout processing and long-term storage and maintains their metabolic activity intact both before and after intake. Additionally, encapsulation enhances and maintains the sensory qualities of food and aids in the uniform distribution of probiotics throughout the final product [193]. For long-term storage, drying the synbiotics product creates an ideal condition for the preservation of probiotics. After intake, the cells begin to divide once more as regular cells, which is advantageous to the host.
Prebiotics Encapsulation
Several prebiotics, such as FOS, inulin, and GOS, are used as coating materials for the microencapsulation of probiotics. As inulin combined with biopolymer alginate capsules elevates viability levels of Lactobacillus acidophilus at storage [194]. Similarly, FOS is used in the encapsulation of Bifidobacterium bifidum to give protection during encapsulation [195]. Bifidobacterium adolescentis successfully encapsulated with FOS and pea protein isolate-alginate matrix [196]. Bifidobacterium longum, Lactobacillus reuteri, and Lactobacillus acidophilus have protective capacity due to supplementation of alginate matrix with FOS, pectic-oligosaccharides, and inulin [197]. The encapsulation matrices pectin + inulin and pectin + rice bran give the maximum encapsulation efficiency, i.e., 90.59% and 91.24%, respectively. Thus, exerting the highest protection to the encapsulated microorganisms when placed in the simulated gastrointestinal tract [198, 199] developed a synbiotic formula, which has beads of inulin and alginate mixture. The most researched technique to improve probiotics’ capacity to survive under challenging circumstances, such as low pH, oxygen, and extreme temperatures during manufacture, storage, and gastrointestinal transit, is microencapsulation. Additionally, microencapsulation might be a fascinating substitute to lessen the undesirable effects that probiotic microorganisms might have on food goods [185].
Bacterial Aggregation
Many bacteria have the capacity to bind to themselves in addition to binding to host cells, the extracellular matrix of host tissues, or inorganic surfaces, known as autoaggregation or autoagglutination [200]. Autoaggregation, a self-recognition process, is one of the initial steps in the development of biofilms, along with surface colonization. Similar types of bacteria, such as those in pure culture, create similar clumps during autoaggregation [201]. However, in co-aggregation, bacteria of different strains or even distinct species clump together [202]. Depending on the bacteria, the autoaggregative phenotype may be constitutive or may be brought about by specific circumstances, such as stress, oxygen supply, or a change in temperature [201]. Both environmental and pathogenic bacteria may benefit from autoaggregation since it typically defends against external pressures, especially in situations like food deprivation or oxidative stress. Surface proteins typically can act as autoagglutinins and mediate autoaggregation through homotypic interactions. In some circumstances, autoaggregation can also be induced by carbohydrates, especially exopolysaccharides [201].
Postbiotics
Any probiotic-produced, soluble metabolite due to the metabolic activity or fragment of LAB that has a positive impact on the host, either directly or indirectly, is referred to as a postbiotics [203]. Postbiotics can be cell-free supernatants, exopolysaccharides, enzymes, cell-wall fragments, SCFAs, bacterial lysates, vitamins, amino acids, etc. Postbiotics have pleotropic effects ranging from immunomodulatory effects, antitumor effects, antiatherosclerotic effects, wound healing to broad-spectrum antimicrobial effects [204]. Postbiotics may have a great potential to signal various organs and tissues in the host, inducing a variety of biological reactions, given their advantageous absorption, metabolism, distribution, and excretion properties. Additionally, postbiotics can replicate probiotics’ health benefits without requiring the introduction of live microbes [205]. Although Streptococcus and Faecalibacterium sp. have also been described as sources of postbiotics, Lactobacillus and Bifidobacterium strains are the most common sources of postbiotics [203]. The cell-free supernatants of different L. plantarum strains have potential to inhibit pathogenic bacterial growth, including Listeria monocytogenes, Salmonella enterica, Escherichia coli, and vancomycin-resistant Enterococci [206]. The interaction of postbiotics and probiotics may exerts protective effects and improves host health by delivering improved particular physiological effects [205]. Physiological protective effects of postbiotics may include antioxidant, anti-inflammatory, anti-proliferative, anti-hypertensive properties.
Consumer Awareness and Adaptation of Probiotics
As there is tremendous research done on probiotics and their therapeutic effect on human and animal health, but still, they are not globally standardized for their safety evaluation. Safety regularizations have diversity from country to country or region [207]. The FAO/WHO has been endorsed that probiotic products should contain this information on their labels: genus, species, and isolation source of strain; the dosage of viable cell count of probiotic strain; shelf life at storage conditions; and cooperate contact information [208]. Probiotic products often mention the viable cells counts as dose but found are significantly having lesser than on labels. The major threat for probiotic strains is that during food processing and handling, they might lose their functional characteristics and get contaminated [209]. Certain probiotics species may create a problem as they can transmit their antibiotics gene to pathogenic microbes and produce biogenic amine [210]. Antimicrobial resistance has been considered as a safety concern about probiotic consumption. As antimicrobial resistance horizontal gene can be transferred from probiotics to pathogenic microbes in the gastrointestinal tract [211].
Nowadays, consumers are becoming well aware of the food and its nutritional value. Thus, this enforces the manufacturers to focus on the advancement of functional foods. Hence, the successful marketing depends upon the acceptance of novelty in food products that have food quality and also value food functionalities [212]. Probiotic products have diverse food matrices. The international probiotic market is divided into different segments like probiotic dietary supplements, probiotic animal feeds, probiotic beverages, and probiotic foods. It can be further categorized into probiotics cereals, bakery products, probiotic baby food, and infant formula. Probiotics drinks are divided into fruit-based probiotic drinks, vegetable-based probiotic drinks, and dairy-based probiotic drinks. A diverse group of consumers are attracted to probiotic products, including children, by producing probiotic chocolates, jellies, and jams. Probiotic product markets capture the consumers of all age groups, particularly health issues and restricted diet individual to a child. Versatility in probiotic products also serves the vegan market. Various studies revealed that supplementing with probiotic cultures did not affect the organoleptic acceptance of probiotic products [213, 214]. Commercial non-dairy products contain more probiotic health-promoting microorganisms as a result of consumer demand for nutritious food options that enhance digestion, intestinal function, and general health. A significant portion of the global consumer population today consumes fruit juice, which is promoted as a nutritious food product. Additionally, juice does not include any dairy allergies (such as lactose) that can deter some population groups from consuming it. The consumer’s sensory acceptance of non-dairy probiotic products during their development may depends upon appearance, aroma, texture, and taste.
Challenges
Non-dairy probiotic-potent products are becoming a necessity due to the considerable attention from consumers. However, technological feasibility and the development of technologies that may change with progress are not compatible. Although current research is progressing at a phenomenal rate, non-dairy probiotics/prebiotics still lack credibility when measured against the output of dairy sources [215]. Fruit and vegetable juices are good substrates and provide certain necessary nutrients, but other factors, such as low pH, which is linked to high quantities of organic acids and dissolved oxygen, may restrict the survival of probiotics [216]. Therefore, the survival of probiotics during processing and storage depends on the choice of the food matrix, pH, temperature, antimicrobial components, preservatives, natural/artificial flavors, and dyes, etc. [14, 217]. Fermentation, encapsulation, drying, rehydration, and storage are recent technologies that significantly protect some non-dairy probiotics from environmental factors. Several disadvantages of using such methods to develop probiotic non-dairy meals still need to be addressed. For instance, during fermentation, increasing temperature, lowering pH, or changing O2/CO2 concentrations could have adverse effects on the integrity of prebiotics/probiotics [218]. Moreover, oxidative stress, osmotic pressure, or mechanical stress can pose serious problems for probiotic survival. In addition, the survivability of microorganisms in various food products can be impacted by water activity. Many food additives, such as salt and sugar, may generate dry conditions by binding with water, improving the survival of microbes [219]. Moreover, the probiotic cell viability is probably assured for the duration of the product’s shelf life. Non-dairy probiotics are generally stored at lowered temperatures, such as 5 °C. However, storage or preservation at room temperature may also affects the growth and survival of probiotic bacterial species [220]. There are still numerous challenges that need to be resolved for effectively developing and preserving non-dairy probiotic foods. When developing new probiotic products, technological concerns that affect the survival of probiotic strains during the manufacturing process and storage period should also be taken into consideration. Moreover, probiotic bacteria strains used for the preparation as well as the cultivar of the fruit or vegetable should be chosen selectively for significant production of probiotics and prebiotics. However, in comparison to dairy products, non-dairy sources make it harder to sustain the vitality of probiotic microorganisms. To ensure probiotic viability and produce acceptable organoleptic qualities (mostly scent and flavor) that can be altered by fermentation, the physicochemical parameters need to be closely monitored [221].
Conclusion and Future Conclusion
Non-dairy sources of prebiotic have not been given much attention. Fruits, vegetables, herbs, culinary spices have great prebiotic potential. Certain agro-industrial wastes products, which are produced during food processing, such as fruit peels, cereal bran/husk, have also prebiotic potential and contain polysaccharides, and oligosaccharides, which increase the level of SCFAs by probiotics. They are considered a growth promoter of probiotic bacteria and flourish in the population of gut microbiota in the gastrointestinal tract. Certain herbs are used as encapsulation matrices of lactic acid bacteria with splendid prebiotic properties. Recent trends in the food industry also give the diversity in probiotics world. The sources which acquire the novel strains gained attention from the researchers. In previous ages, dairy products were used as the source of probiotics, but nowadays, there are diverse sources that produce novel strains of probiotics, having countless health benefits like it can be effective even for the control of colon cancer. The strains isolated from fruits, vegetables, pickles, cereals, meat, etc. have great antimicrobial potential by producing bacteriocins, acid bile tolerance, possessing antifungal properties, and strong adhesion with intestinal epithelium. The prebiotics and probiotics obtained from non-dairy sources have significant gut health scope. Furthermore, the research gap exists; future research is of utmost demand in this field.
To find new possible probiotic microbes and new components as potential substrates, research on non-dairy probiotic beverages is still in its early stages and focuses on conventional non-dairy probiotics from around the world. The development of creative, economic, and technological matrices is absolutely necessary to develop non-dairy probiotic foods as healthy alternatives to dairy probiotic meals. The development of creative, economic, and technological matrices is an absolute necessity. Research is also required to find solutions to several issues with probiotic bacterial survival in harsh non-dairy substrates, such as the low pH of fruit juices, etc. Researchers must therefore search for probiotic microbes in numerous substrates to see which might be most suitable for global health. The development of creative, economic, and technological matrices is necessary. Functionality, stability, and sensory acceptance, particularly flavor, appeal, and pricing have to be considered while developing as these aspects play a significant influence in their successful commercialization. The development of new non-dairy-based probiotic products with enhanced flavor and taste will have substantial nutritional value.
References
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Ouwehand A, Derrien M, De Vos W, Tiihonen K, Rautonen N (2005) Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol 16:212–217
Collins MD, Gibson GR (1999) Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69(5):1052s-s1057
Patterson JA, Burkholder K (2003) Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631
van Loo J, Coussement P, de Leenheer L, Hoebregs H, Smits G (1995) On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit Rev Food Sci Nutr 35(6):525–552
Lee YK, Salminen S (2009) Handbook of probiotics and prebiotics: John Wiley & Sons
Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ et al (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods (Basel, Switzerland) 8(3):92
Markowiak P, Śliżewska K (2018) The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathogens 10(1):21
Crittenden R, Playne M, Lee Y, Salminen S (2009) Handbook of probiotics and prebiotics. 535–84
Panghal A, Janghu S, Virkar K, Gat Y, Kumar V, Chhikara N (2017) Potential non-dairy probiotic products—a healthy approach. Food Biosci 21
Kandylis P, Pissaridi K, Bekatorou A, Kanellaki M, Koutinas A (2016) Dairy and non-dairy probiotic beverages. Curr Opin Food Sci 7:58–63
do Espírito Santo AP, Perego P, Converti A, Oliveira MN (2012) Influence of milk type and addition of passion fruit peel powder on fermentation kinetics, texture profile and bacterial viability in probiotic yoghurts. LWT 47(2):393–9
Oak S, Jha R (2019) The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 59:1675–1683
Aspri M, Papademas P, Tsaltas D (2020) Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 6(1)
Vijaya Kumar B, Vijayendra SV, Reddy OV (2015) Trends in dairy and non-dairy probiotic products—a review. J Food Sci Technol 52(10):6112–6124
Peña AS (2007) Flora intestinal, probióticos, prebióticos, simbióticos y alimentos novedosos. Revista Espanola De Enfermedades Digestivas - Rev Espan Enferm Dig 99
Pokusaeva K, Fitzgerald GF, van Sinderen D (2011) Carbohydrate metabolism in Bifidobacteria. Genes Nutr 6(3):285–306
Kanjan P, Sahasrabudhe NM, de Haan BJ, de Vos P (2017) Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J Functional Foods 37:433–440
Zahedi MJ, Behrouz V, Azimi M (2018) Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol 33(6):1192–1199
Delzenne NM, Cani PD, Neyrinck AM (2007) Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. J Nutr 137(11 Suppl):2547s-s2551
Vicenssuto G, Castro R (2020) Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal Agric Biotechnol 24:101564
Saleh I, Abdelwahed EM, Rabie AMH, Abou El-Ella WM (2018) Fortification of probiotic stirred yoghurt by addition of apple and mango pulps. Zagazig J Agric Res 45(2):625–635
Lebaka VR, Wee YJ, Ye W, Korivi M (2021) Nutritional composition and bioactive compounds in three different parts of mango fruit. Int J Environ Res Public Health 18(2)
Hu K, Dars AG, Liu Q, Xie B, Sun Z (2018) Phytochemical profiling of the ripening of Chinese mango (Mangifera indica L.) cultivars by real-time monitoring using UPLC-ESI-QTOF-MS and its potential benefits as prebiotic ingredients. Food Chem 256:171–80
Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C et al (2015) A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int 2015:850902
Kurtoǧlu G, Yıldız Ş (2011) Extraction of fructo-oligosaccaride components from banana peels. Gazi Univ J Sci 24:877–882
Budhisatria R, Rosaria R, Jap L, Jan TT (2017) In vitro and in vivo prebiotic activities of purified oligosaccharides derived from various local bananas (Musa sp.): Tanduk, Uli, Raja Sereh, and Cavendish. Microbiol Indonesia 11(2):3
Shinde T, Perera P, Vemuri R, Gondalia S, Beale D, Karpe A et al (2020) Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur J Nutr 59
Dalu K, Nurhayati N, Jayus J (2019) In vitro modulation of fecal microflora growth using fermented “Pisang Mas” banana and red guava juices. Curr Res Nutr Food Sci J 7:449–456
Costa ELD, Alencar NMM, Rullo BGDS, Taralo RL (2017) Effect of green banana pulp on physicochemical and sensory properties of probiotic yoghurt. Food Sci Technol 37:363–8
Gómez B, Gullón B, Remoroza C, Schols HA, Parajó JC, Alonso JL (2014) Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J Agric Food Chem 62(40):9769–9782
Chatterjee E, Manuel S (2016) Effect of fruit pectin on growth of lactic acid bacteria. J Probio Health 04
Sarkar R, Nain L, Kundu A, Dutta A, Das D, Sethi S et al (2022) De-oiled citrus peels as feedstock for the production of pectin oligosaccharides and its effect on Lactobacillus fermentum, probiotic source. Frontier Nutr 9
Li P-J, Xia J-L, Nie Z-Y, Shan Y (2016) Pectic oligosaccharides hydrolyzed from orange peel by fungal multi-enzyme complexes and their prebiotic and antibacterial potentials. LWT - Food Sci Technol 69
Sabajanes M, Yáñez R, Alonso J, Parajó J (2012) Pectic oligosaccharides production from orange peel waste by enzymatic hydrolysis. Int J Food Sci Technol 47
Casarotti S, Borgonovi T, Batista C, Penna A (2018) Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidification and properties of probiotic fermented products. LWT 98
Liu Z, Lin X, Huang G, Zhang W, Rao P, Ni L (2014) Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 26:1–6
Mandalari G, Faulks RM, Bisignano C, Waldron KW, Narbad A, Wickham MSJ (2010) In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol Lett 304 2:116–22
Liu Z, Wang W, Huang G, Zhang W, Ni L (2016) In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J Sci Food Agric 96(5):1836–1843
Byerley LO, Samuelson D, Blanchard E, Luo M, Lorenzen BN, Banks S et al (2017) Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem 48:94–102
Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Fischer S et al (2018) A Walnut-enriched diet affects gut microbiome in healthy caucasian subjects: a randomized, controlled trial. Nutrients 10(2)
Duarte FND, Rodrigues JB, da Costa Lima M, Lima MDS, Pacheco MTB, Pintado MME et al (2017) Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus species. J Sci Food Agric 97(11):3712–9
Blaiotta G, La Gatta B, Di Capua M, Di Luccia A, Coppola R, Aponte M (2013) Effect of chestnut extract and chestnut fiber on viability of potential probiotic Lactobacillus strains under gastrointestinal tract conditions. Food Microbiol 36(2):161–169
Abbasiliasi S, Tan JS, Bello B, Ibrahim TAT, Tam YJ, Ariff A et al (2019) Prebiotic efficacy of coconut kernel cake’s soluble crude polysaccharides on growth rates and acidifying property of probiotic lactic acid bacteria in vitro. Biotechnol Biotechnol Equip 33(1):1216–1227
Mohd Nor NN, Abbasiliasi S, Marikkar MN, Ariff A, Amid M, Lamasudin DU et al (2017) Defatted coconut residue crude polysaccharides as potential prebiotics: study of their effects on proliferation and acidifying activity of probiotics in vitro. J Food Sci Technol 54(1):164–173
Parkar S, Simmons L, Herath T, Phipps J, Trower T, Hedderley D et al (2017) Evaluation of the prebiotic potential of five kiwifruit cultivars after simulated gastrointestinal digestion and fermentation with human faecal bacteria. Int J Food Sci Technol 53
MacNeill MT (2019) Strawberries and gut health in postmenopausal women
Petersen C, Wankhade U, Bharat D, Wong K, Mueller J, Chintapalli S et al (2019) Dietary supplementation with strawberry induces marked changes in the composition and functional potential of the gut microbiome in diabetic mice. J Nutr Biochem 66
Bahnas WM, Abbas KA, Metry WA, H. Elewa NA (2019) A novel bio-fermented beverages from dairy by-products based with papaya pulp and stevia leaves. J Food Dairy Sci 10(12):467–72
Hao CL, Esah EM, Tajarudin HA, Akter B, Salleh RM (2021) Effect of potential prebiotics from selected fruits peel on the growth of probiotics. J Food Process Preserv 45(6):e15581
Km S, Lele S (2013) Prebiotic activity of gourd family vegetable fibres using in vitro fermentation. Food Biosci 1:26–30
Kassim MA, Baijnath H, Odhav B (2014) Effect of traditional leafy vegetables on the growth of lactobacilli and bifidobacteria. Int J Food Sci Nutr 65(8):977–980
Cichońska P, Ziarno M (2022) Legumes and legume-based beverages fermented with lactic acid bacteria as a potential carrier of probiotics and prebiotics. Microorganisms 10(1)
Johnson N, Johnson CR, Thavarajah P, Kumar S, Thavarajah D (2020) The roles and potential of lentil prebiotic carbohydrates in human and plant health. Plants, People, Planet 2(4):310–319
Johnson CR, Thavarajah D, Combs GF, Thavarajah P (2013) Lentil (Lens culinaris L.): a prebiotic-rich whole food legume. Food Res Int 51(1):107–13
Jayamanohar J, Devi PB, Kavitake D, Priyadarisini VB, Shetty PH (2019) Prebiotic potential of water extractable polysaccharide from red kidney bean (Phaseolus vulgaris L.). LWT 101:703–10
Charoensiddhi S, Chanput WP, Sae-tan S (2022) Gut microbiota modulation, anti-diabetic and anti-inflammatory properties of polyphenol extract from mung bean seed coat (Vigna radiata L.). Nutrients 14(11)
Muchiri M, McCartney A (2017) In vitro investigation of orange fleshed sweet potato prebiotic potential and its implication on human gut health. Functional Foods Health Dis 7:833
de Albuquerque TMR, Borges CWP, Cavalcanti MT, Lima MdS, Magnani M, de Souza EL (2020) Potential prebiotic properties of flours from different varieties of sweet potato (Ipomoea batatas L.) roots cultivated in Northeastern Brazil. Food Biosci 36:100614
Thakur K, Xu GY, Zhang JG, Zhang F, Hu F, Wei ZJ (2018) In vitro Prebiotic effects of bamboo shoots and potato peel extracts on the proliferation of lactic acid bacteria under simulated GIT conditions. Front Microbiol 9:2114
Duda-Chodak ADA, Tarko T, Wajda L, Kręcioch B (2015) The impact of carrot juices on intestinal and probiotic bacteria. Potravìnárstvo 9:337–341
Van den Abbeele P, Verstrepen L, Ghyselinck J, Albers R, Marzorati M, Mercenier A (2020) A Novel non-digestible, carrot-derived polysaccharide (cRG-I) selectively modulates the human gut microbiota while promoting gut barrier integrity: an integrated in vitro approach. Nutrients 12(7)
Van den Abbeele P, Duysburgh C, Cleenwerck I, Albers R, Marzorati M, Mercenier A (2021) Consistent prebiotic effects of carrot RG-I on the gut microbiota of four human adult donors in the SHIME(®) model despite baseline individual variability. Microorganisms 9(10)
Xu Y, Hlaing MM, Glagovskaia O, Augustin MA, Terefe NS (2020) Fermentation by probiotic Lactobacillus gasseri strains enhances the carotenoid and fibre contents of carrot juice. Foods 9(12)
Das A, Raychaudhuri U, Chakraborty R (2012) Cereal based functional food of Indian subcontinent: a review. J Food Sci Technol 49(6):665–672
Neeraj G, Ravi S, Somdutt R, Ravi S, Vinoth Kumar V (2017) Immobilized inulinase: a new horizon of paramount importance driving the production of sweetener and prebiotics. Crit Rev Biotechnol 38:1–14
Reque P, Barreto Pinilla C, Gauterio G, Kalil S, Brandelli A (2019) Xylooligosaccharides production from wheat middlings bioprocessed with Bacillus subtilis. Food Res Int 126:108673
Mantzourani I, Terpou A, Alexopoulos A, Kimbaris A, Bezirtzoglou E, Koutinas AA et al (2019) Production of a potentially synbiotic pomegranate beverage by fermentation with Lactobacillus plantarum ATCC 14917 adsorbed on a prebiotic carrier. Appl Biochem Biotechnol 188(4):1096–1107
Mantzourani I, Terpou A, Bekatorou A, Mallouchos A, Alexopoulos A, Kimbaris A et al (2020) Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem 308:125658
Wang J, Bai J, Fan M, Li T, Li Y, Qian H et al (2020) Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci Technol 96:157–165
Harris S, Monteagudo-Mera A, Kosik O, Charalampopoulos D, Shewry P, Lovegrove A (2019) Comparative prebiotic activity of mixtures of cereal grain polysaccharides. AMB Express 9(1):203
Demirci T, Öztürk Negiş H, Oraç A, Konak Göktepe Ç, Sözeri Atik D, Aktaş K et al (2019) Immature wheat grain as a potential prebiotic ingredient in set-type yoghurts: impact on antioxidative, textural properties and survival of different probiotics. J Food Sci Technol 56(12):5474–5483
Mantzouridou F, Karousioti A, Kiosseoglou V (2013) Formulation optimization of a potentially prebiotic low-in-oil oat-based salad dressing to improve Lactobacillus paracasei subsp. paracasei survival and physicochemical characteristics. LWT - Food Sci Technol 53:560–8
Paesani C, Degano A, Salvucci E, Zalosnik I, Fabi J, Sciarini LS et al (2020) Soluble arabinoxylans extracted from soft and hard wheat show a differential prebiotic effect in vitro and in vivo. J Cereal Sci 93:102956
Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Advances in nutrition (Bethesda, Md) 3(4):506–516
Mohanty D, Misra S, Mohapatra S, Sahu PS (2018) Prebiotics and synbiotics: recent concepts in nutrition. Food Biosci 26:152–160
Aachary AA, Prapulla SG (2011) Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr Rev Food Sci Food Saf 10(1):2–16
George Kerry R, Patra JK, Gouda S, Park Y, Shin HS, Das G (2018) Benefaction of probiotics for human health: A review. J Food Drug Anal 26(3):927–939
Kim D-H, Jeong D, Oh Y-T, Song K-Y, Kim H-S, Chon J-W et al (2017) Stimulating the growth of Kefir-isolated lactic acid bacteria using addition of crude flaxseed (Linum usitatissimum L.) Extract. J Milk Sci Biotechnol 35:93–7
Waghmode M, Gunjal A, Patil N (2020) Probiotic sugar confectionery fortified with flax seeds (Linum usitatissimum L.). J food Sci Technol 57(5):1964–70
Mousavi M, Heshmati A, Daraei Garmakhany A, Vahidinia A, Taheri M (2018) Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT 102
Patterson E, Doherty R, Murphy E, Wall R, O’Sullivan O, Nilaweera K et al (2014) Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr 111:1–13
Lu QY, Summanen PH, Lee RP, Huang J, Henning SM, Heber D et al (2017) Prebiotic potential and chemical composition of seven culinary spice extracts. J Food Sci 82(8):1807–1813
Lu QY, Rasmussen AM, Yang J, Lee RP, Huang J, Shao P et al (2019) Mixed spices at culinary doses have prebiotic effects in healthy adults: a pilot study. Nutrients 11(6)
Ghiamati Yazdi F, Soleimanian-Zad S, van den Worm E, Folkerts G (2019) Turmeric extract: potential use as a prebiotic and anti-inflammatory compound? Plant Foods Hum Nutr (Dordrecht, Netherlands) 74(3):293–299
Narendra Babu K, Hemalatha R, Satyanarayana U, Shujauddin M, Himaja N, Bhaskarachary K et al (2018) Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J Herbal Med 13:42–51
Kurhekar J (2013) Curcuma longa and Allium sativum as prebiotics. Bionano Front 6:327–329
Petkova N, Ivanov I, Vrancheva R, Denev P, Pavlov A (2017) Ultrasound and microwave-assisted extraction of elecampane (Inula helenium) roots. Nat Prod Commun 12:171–174
Shariati Z, Jouki M, Rafiei F (2019) Flavored functional drinking yogurt (Doogh) formulated with Lactobacillus plantarum LS5, cress seed gum, and coriander leaves extract. Food Sci Nutr 8
Majeed M, Majeed S, Nagabhushanam K, Arumugam S, Natarajan S, Beede K et al (2018) Galactomannan from Trigonella foenum-graecum L. seed: prebiotic application and its fermentation by the probiotic Bacillus coagulans strain MTCC 5856. Food Sci Nutr 6(3):666–73
Haghshenas B, Abdullah N, Nami Y, Radiah D, Rosli R, Yari KA (2015) Microencapsulation of probiotic bacteria Lactobacillus plantarum 15HN using alginate-psyllium-fenugreek polymeric blends. J Appl Microbiol 118(4):1048–1057
Ruiz E, Gullón B, Moura P, Carvalheiro F, Eibes G, Cara C et al (2017) Bifidobacterial growth stimulation by oligosaccharides generated from olive tree pruning biomass. Carbohydr Polym 169
Nadour M, Laroche C, Pierre G, Delattre C, Farida M-M, Michaud P (2015) Structural characterization and biological activities of polysaccharides from olive mill wastewater. Appl Biochem Biotechnol 177:431–445
Quezada M, Salinas C, Gotteland M, Cardemil L (2017) Acemannan and fructans from aloe vera (Aloe barbadensis Miller) plants as novel prebiotics. J Agric Food Chem 65
Nami Y, Lornezhad G, Kiani A, Abdullah N, Haghshenas B (2020) Alginate-Persian gum-prebiotics microencapsulation impacts on the survival rate of Lactococcus lactis ABRIINW-N19 in orange juice. LWT 124:109190
Pérez Montoro B, Benomar N, Caballero Gómez N, Ennahar S, Horvatovich P, Knapp CW et al (2018) Proteomic analysis of Lactobacillus pentosus for the identification of potential markers involved in acid resistance and their influence on other probiotic features. Food Microbiol 72:31–38
Tian T, Freeman S, Corey M, German B, Barile D (2017) Chemical characterization of potentially prebiotic oligosaccharides in brewed coffee and spent coffee grounds. J Agric Food Chem 65
Okolie C, C. K. Rajendran SR, Udenigwe C, Aryee A, Mason B (2017) Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J Food Biochem 41:e12392
Khursheed R, Singh SK, Wadhwa S, Gulati M, Awasthi A (2020) Therapeutic potential of mushrooms in diabetes mellitus: role of polysaccharides. Int J Biol Macromol 164:1194–1205
Ayeka PA (2018) Potential of mushroom compounds as immunomodulators in cancer immunotherapy: a review. Evidence-based complementary and alternative medicine : eCAM 2018:7271509
Swanson K, Gibson G, Hutkins R, Reimer R, Reid G, Verbeke K et al (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17:1–15
Ashaolu TJ (2020) Immune boosting functional foods and their mechanisms: a critical evaluation of probiotics and prebiotics. Biomed Pharmacother 130:110625
Castro-López C, García HS, Guadalupe Martínez-Ávila GC, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A (2021) Genomics-based approaches to identify and predict the health-promoting and safety activities of promising probiotic strains—a probiogenomics review. Trends Food Sci Technol 108:148–163
Esaiassen E, Hjerde E, Cavanagh JP, Simonsen GS, Klingenberg C (2017) Bifidobacterium bacteremia: clinical characteristics and a genomic approach to assess pathogenicity. J Clin Microbiol 55(7):2234–2248
Bai JC, Ciacci C (2017) World gastroenterology organisation global guidelines: celiac disease February 2017. J Clin Gastroenterol 51(9):755–768
Aminlari L, Shekarforoush SS, Hosseinzadeh S, Nazifi S, Sajedianfard J, Eskandari MH (2019) Effect of probiotics Bacillus coagulans and Lactobacillus plantarum on lipid profile and feces bacteria of rats fed cholesterol-enriched diet. Probiot Antimicrob Proteins 11(4):1163–1171
Lee CS, Kim SH (2020) Anti-inflammatory and anti-osteoporotic potential of Lactobacillus plantarum A41 and L. fermentum SRK414 as probiotics. Probiot Antimicrob Proteins 12(2):623–34
Kubota M, Ito K, Tomimoto K, Kanazaki M, Tsukiyama K, Kubota A et al (2020) Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a DOUBLE-blind and randomized clinical trial. Nutrients 12(1)
Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ (2020) Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother 129:110409
Li T, Teng D, Mao R, Hao Y, Wang X, Wang J (2020) A critical review of antibiotic resistance in probiotic bacteria. Food research international (Ottawa, Ont) 136:109571
Jakubczyk D, Leszczyńska K, Górska S (2020) The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)—a critical review. Nutrients 12(7)
Tijjani K, James M, Altın C (2020) Probiotics and their attributes in human health therapy: review. Int J Res - Granthaalayah 8:158–164
Fijan S, Frauwallner A, Langerholc T, Krebs B, Ter Haar Née Younes JA, Heschl A et al (2019) Efficacy of using probiotics with antagonistic activity against pathogens of wound infections: an integrative review of literature. BioMed Res Int 2019:7585486
Hamouda I, Amel D (2018) Characterization and screening of the potential probiotic Lactic acid bacteria and Bifidobacterium strains isolated of different biotopes. Mediterr J Nutr Metab 11:1–29
Powthong P, Jantrapanukorn B, Suntornthiticharoen P, Laohaphatanalert K (2020) Study of prebiotic properties of selected banana species in Thailand. J Food Sci Technol 57
Meng X, Li S, Li Y, Gan RY, Li HB (2018) Gut microbiota's relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients 10(10)
Gharbi Y, Fhoula I, Ruas-Madiedo P, Afef N, Boudabous A, Gueimonde M et al (2019) In-vitro characterization of potentially probiotic Lactobacillus strains isolated from human microbiota: interaction with pathogenic bacteria and the enteric cell line HT29. Ann Microbiol 69(1):61–72
Hojjati M, Behabahani BA, Falah F (2020) Aggregation, adherence, anti-adhesion and antagonistic activity properties relating to surface charge of probiotic Lactobacillus brevis gp104 against Staphylococcus aureus. Microb Pathog 147:104420
Donelli G, Vuotto C, Mastromarino P (2013) Phenotyping and genotyping are both essential to identify and classify a probiotic microorganism. Microb Ecol Health Dis 24(1):20105
Stefanis C, Mantzourani I, Plessas S, Alexopoulos A, Galanis A, Bezirtzoglou E (2016) Reviewing classical and molecular techniques regarding profiling of probiotic character of microorganisms. Curr Res Nutr Food Sci J 4(1):27–47
Del Piano M, Morelli L, Strozzi GP, Allesina S, Barba M, Deidda F et al (2006) Probiotics: from research to consumer. Dig Liver Dis 38:S248–S255
Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P et al (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70(4):2782–2858
Stefanis C, Mantzourani I, Plessas S, Alexopoulos A, Galanis A, Bezirtzoglou E et al (2016) Reviewing classical and molecular techniques regarding profiling of probiotic character of microorganisms. Curr Res Nutr Food Sci J 4:27–47
Sharma A, Lee S, Park Y-S (2020) Molecular typing tools for identifying and characterizing lactic acid bacteria: a review. Food Sci Biotechnol 29(10):1301–1318
Sharma, Kaur J, Lee, Park (2019) Tracking of intentionally inoculated lactic acid bacteria strains in yogurt and probiotic powder. Microorganisms 8:5
Maleki Kakelar H, Barzegari A, Hanifian S, Barar J, Omidi Y (2019) Isolation and molecular identification of Lactobacillus with probiotic potential from abomasums driven rennet. Food Chem 272:709–714
Buron-Moles G, Chailyan A, Dolejs I, Forster J, Mikš MH (2019) Uncovering carbohydrate metabolism through a genotype-phenotype association study of 56 lactic acid bacteria genomes. Appl Microbiol Biotechnol 103(7):3135–3152
Pineiro M, Asp N-G, Reid G, Macfarlane S, Morelli L, Brunser O et al (2008) FAO Technical meeting on prebiotics. J Clin Gastroenterol 42:S156–S159
Mater DD, Langella P, Corthier G, Flores MJ (2005) Evidence of vancomycin resistance gene transfer between enterococci of human origin in the gut of mice harbouring human microbiota. J Antimicrob Chemother 56(5):975–978
Franz CM, Muscholl-Silberhorn AB, Yousif NM, Vancanneyt M, Swings J, Holzapfel WH (2001) Incidence of virulence factors and antibiotic resistance among Enterococci isolated from food. Appl Environ Microbiol 67(9):4385–4389
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9(9)
Dobson A, Cotter PD, Ross RP, Hill C (2012) Bacteriocin production: a probiotic trait? Appl Environ Microbiol 78(1):1–6
Pinto A, Barbosa J, Albano H, Isidro J, Teixeira P (2020) Screening of bacteriocinogenic lactic acid bacteria and their characterization as potential probiotics. Microorganisms 8(3)
Bhardwaj A, Gupta H, Kapila S, Kaur G, Vij S, Malik RK (2010) Safety assessment and evaluation of probiotic potential of bacteriocinogenic Enterococcus faecium KH 24 strain under in vitro and in vivo conditions. Int J Food Microbiol 141(3):156–164
Lee JH, Karamychev VN, Kozyavkin SA, Mills D, Pavlov AR, Pavlova NV et al (2008) Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth. BMC Genomics 9:247
Guinane CM, Lawton EM, O’Connor PM, O’Sullivan Ó, Hill C, Ross RP et al (2016) The bacteriocin bactofencin A subtly modulates gut microbial populations. Anaerobe 40:41–49
Hegarty JW, Guinane CM, Ross RP, Hill C, Cotter PD (2016) Bacteriocin production: a relatively unharnessed probiotic trait? F1000 Res 5:2587
Kessavane R, Kandasamy S, Palanisamy B, Shetty Halady PK (2020) Evaluation of functional properties of potential probiotic isolates from fermented brine pickle. Food Chem 311:126057
Boricha AA, Shekh SL, Pithva SP, Ambalam PS, Vyas BRM (2019) In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. LWT 106:201–208
Pabari K, Pithva S, Kothari C, Purama RK, Kondepudi KK, Vyas B et al (2020) Evaluation of probiotic properties and prebiotic utilization potential of Weissella paramesenteroides isolated from fruits. Probiot Antimicrob Proteins 12
Bouzaiene T, Lasram S, Ziadi M, Ouzari HI, Hamdi Z, Moktar H (2018) Isolation of lactic acid bacteria from grape fruit: antifungal activities, probiotic properties, and in vitro detoxification of ochratoxin A. Ann Microbiol 69
Junnarkar M, Pawar S, Gaikwad S, Nawani N (2019) Probiotic potential of lactic acid bacteria from fresh vegetables: application in food preservation. Indian J Exp Biol 57:825–838
Szutowska J, Gwiazdowska D (2021) Probiotic potential of lactic acid bacteria obtained from fermented curly kale juice. Arch Microbiol 203
Nazzaro F, Fratianni F, Sada A, Orlando P (2008) Synbiotic potential of carrot juice supplemented with Lactobacillus spp. and inulin or fructooligosaccharides. J Sci Food Agric 88(13):2271–6
Labba IM, Andlid T, Lindgren Å, Sandberg AS, Sjöberg F (2020) Isolation, identification, and selection of strains as candidate probiotics and starters for fermentation of Swedish legumes. Food Nutr Res 64
Chaturvedi S, Chakraborty S (2002) Evaluation of prebiotic properties of legume-based synbiotic beverages. n/a(n/a):e16685
Rastall RA, Maitin V (2002) Prebiotics and synbiotics: towards the next generation. Curr Opin Biotechnol 13(5):490–496
De Boever P, Wouters R, Verstraete W (2001) Combined use of Lactobacillus reuteri and soygerm powder as food supplement. Lett Appl Microbiol 33(6):420–424
Petrulakova M, Valík Ľ (2015) Legumes as potential plants for probiotic strain Lactobacillus rhamnosus GG. Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis 63:1505–1511
Kocková M, Dilongová M, Hybenová E, Valík L (2013) Evaluation of cereals and pseudocereals suitability for the development of new probiotic foods. J Chem 2013:414303
González-Ferrero C, Irache JM, Marín-Calvo B, Ortiz-Romero L, Virto-Resano R, González-Navarro CJ (2020) Encapsulation of probiotics in soybean protein-based microparticles preserves viable cell concentration in foods all along the production and storage processes. J Microencapsul 37(3):242–253
Zhang S, Shi Y, Zhang S, Shang W, Gao X, Wang H (2014) Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control 41:1–6
Larkin TA, Astheimer LB, Price WE (2009) Dietary combination of soy with a probiotic or prebiotic food significantly reduces total and LDL cholesterol in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 63(2):238–245
Ianiro G, Pizzoferrato M, Franceschi F, Tarullo A, Luisi T, Gasbarrini G (2013) Effect of an extra-virgin olive oil enriched with probiotics or antioxidants on functional dyspepsia: a pilot study. Eur Rev Med Pharmacol Sci 17(15):2085–2090
Abouloifa H, Rokni Y, Ghabbour N, Karboune S, Brasca M, D'Hallewin G et al (2021) Probiotics from fermented olives. 215–29
Huang R-M, Feng K, Li S-F, Zong M-H, Wu H, Han S-Y (2021) Enhanced survival of probiotics in the electrosprayed microcapsule by addition of fish oil. J Food Eng 307:110650
Pavli F, Argyri A, Chorianopoulos N, Nychas G-J, Tassou C (2019) Evaluation of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 118:108810
Bungenstock L, Abdulmawjood A, Reich F (2020) Evaluation of antibacterial properties of lactic acid bacteria from traditionally and industrially produced fermented sausages from Germany. PLoS ONE 15(3):e0230345
Wanangkarn A, Tan FJ, Phumthong N, Boonsema P (2020) Identification of lactic acid bacteria isolated from Thai fermented sausage for nitrite degradation ability. Int J Agric Technol 16(3):761–770
Ben Braïek O, Cremonesi P, Morandi S, Smaoui S, Hani K, Ghrairi T (2018) Safety characterisation and inhibition of fungi and bacteria by a novel multiple enterocin-producing Enterococcus lactis 4CP3 strain. Microb Pathog 118:32–38
Ploll U, Petritz H, Stern T (2020) A social innovation perspective on dietary transitions: diffusion of vegetarianism and veganism in Austria. Environ Innov Soc Trans 36:164–176
Pimentel TC, Costa WKAd, Barão CE, Rosset M, Magnani M (2021) Vegan probiotic products: a modern tendency or the newest challenge in functional foods. Food Res Int 140:110033
Behera SS, Panda SK (2020) Ethnic and industrial probiotic foods and beverages: efficacy and acceptance. Curr Opin Food Sci 32:29–36
Yeo S-K, Liong M-T (2010) Effect of prebiotics on viability and growth characteristics of probiotics in soymilk. J Sci Food Agric 90(2):267–275
Teh S-S, Ahmad R, Wan-Abdullah W-N, Liong M-T (2009) Evaluation of agrowastes as immobilizers for probiotics in soy milk. J Agric Food Chem 57(21):10187–10198
Shori AB, Al Zahrani AJ (2021) Non-dairy plant-based milk products as alternatives to conventional dairy products for delivering probiotics. Food Sci Technol
Aboulfazli F, Baba AS (2015) Effect of vegetable milk on survival of probiotics in fermented ice cream under gastrointestinal conditions. Food Sci Technol Res 21(3):391–397
Saini D, Gadicherla P, Chandra P, Anandakrishna L (2017) Coconut milk and probiotic milk as storage media to maintain periodontal ligament cell viability: an in vitro study. Dent Traumatol 33(3):160–164
Damián MR, Cortes-Perez NG, Quintana ET, Ortiz-Moreno A, Garfias Noguez C, Cruceño-Casarrubias CE et al (2022) Functional foods, nutraceuticals and probiotics: a focus on human health. Microorganisms 10(5)
Arshad MS, Khalid W, Ahmad RS, Khan MK, Ahmad MH, Safdar S et al (2021) Functional foods and human health: an overview. Functional Foods Phytochem Health Promoting Potential 3
González N, Marquès M, Nadal M, Domingo JL (2019) Occurrence of environmental pollutants in foodstuffs: a review of organic vs. conventional food. Food Chem Toxicol 125:370–5
Palacios C, Cormick G, Hofmeyr GJ, Garcia-Casal MN, Peña-Rosas JP, Betrán AP (2021) Calcium-fortified foods in public health programs: considerations for implementation. Ann NY Acad Sci 1485(1):3–21
Vishwakarma S, Dalbhagat CG, Mandliya S, Mishra HN (2022) Investigation of natural food fortificants for improving various properties of fortified foods: a review. Food Res Int 111186
McClements DJ, Grossmann L (2021) A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci Food 5(1):1–10
Nguyen B, Bujna E, Fekete N, Thi Mai Anh T, Rezessy-Szabó J, Prasad R et al (2019) Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front Nutr 6:54
Thakur M (2017) Development of probiotic pomegranate beverage and its physico-chemical and microbial characterization. Int J Pure Appl Biosci 5:35–41
Xu X, Luo D, Bao Y, Liao X, Wu J (2018) Characterization of diversity and probiotic efficiency of the autochthonous lactic acid bacteria in the fermentation of selected raw fruit and vegetable juices. Front Microbiol 9:2539
Zieliński H, Surma M, Zielińska D (2017) The naturally fermented sour pickled cucumbers. 503–16
Kohajdová Z (2017) Fermented cereal products. Current developments in biotechnology and bioengineering: Elsevier 91–117
Khusro A, Aarti C, Dusthackeer A, Agastian P (2018) Anti-tubercular and probiotic properties of coagulase-negative staphylococci isolated from Koozh, a traditional fermented food of South India. Microb Pathog 114:239–250
Qandashtant R, Salehi E, Sani A, Mehraban M, Safari O (2020) Investigation on quality properties of traditional bulk bread covered with probiotics and soybean oil edible coating. Acta Aliment 49:144–153
Seyedain-Ardabili M, Sharifan A, Ghiassi TB (2016) The production of synbiotic bread by microencapsulation. Food Technol Biotechnol 54(1):52–59
Klindt-Toldam S, Larsen SK, Saaby L, Olsen LR, Svenstrup G, Müllertz A et al (2016) Survival of Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 encapsulated in chocolate during in vitro simulated passage of the upper gastrointestinal tract. LWT 74:404–410
Talebzadeh S, Sharifan A (2017) Developing probiotic jelly desserts with Lactobacillus acidophilus 41(1):e13026
Aspri M, Papademas P, Tsaltas D (2020) Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 6:30
Grujović MŽ, Mladenović KG, Semedo-Lemsaddek T, Laranjo M, Stefanović OD, Kocić-Tanackov SD (2022) Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: potential use as starters or probiotics. Compr Rev Food Sci Food Saf 21(2):1537–1567
Tufarelli V, Laudadio V (2016) An overview on the functional food concept: prospectives and applied researches in probiotics, prebiotics and synbiotics. J Exp Biol Agric Sci 4:273–278
Bielecka M, Biedrzycka E, Majkowska A (2002) Selection of probiotics and prebiotics for synbiotics and confirmation of their in vivo effectiveness. Food Res Int 35(2–3):125–131
Mishra A, Chakravarty I, Mandavgane S (2021) Current trends in non-dairy based synbiotics. Crit Rev Biotechnol 41(6):935–952
Huynh TG, Cheng AC, Chi CC, Chiu KH, Liu CH (2018) A synbiotic improves the immunity of white shrimp, Litopenaeus vannamei: metabolomic analysis reveal compelling evidence. Fish Shellfish Immunol 79:284–293
Rasika DMD, Vidanarachchi JK, Rocha RS, Balthazar CF, Cruz AG, Sant’Ana AS et al (2021) Plant-based milk substitutes as emerging probiotic carriers. Curr Opin Food Sci 38:8–20
Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103(16):6463–6472
Moumita S, Das B, Hasan U, Jayabalan R (2018) Effect of long-term storage on viability and acceptability of lyophilized and spray-dried synbiotic microcapsules in dry functional food formulations. LWT 96:127–132
Nazzaro F, Fratianni F, Coppola R, Sada A, Orlando P (2009) Fermentative ability of alginate-prebiotic encapsulated Lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J Functional Foods 1:319–323
Chen MJ, Chen KN, Kuo YT (2007) Optimal thermotolerance of Bifidobacterium bifidum in gellan-alginate microparticles. Biotechnol Bioeng 98(2):411–419
Kent RM, Doherty SB (2014) Probiotic bacteria in infant formula and follow-up formula: Microencapsulation using milk and pea proteins to improve microbiological quality. Food Res Int (Ottawa, Ont) 64:567–576
Chaluvadi S, Hotchkiss AT Jr, Call JE, Luchansky JB, Phillips JG, Liu L et al (2012) Protection of probiotic bacteria in a synbiotic matrix following aerobic storage at 4 °C. Beneficial Microbes 3(3):175–187
Atia A, Gomaa A, Fernandez B, Subirade M, Fliss I (2018) Study and understanding behavior of alginate-inulin synbiotics beads for protection and delivery of antimicrobial-producing probiotics in colonic simulated conditions. Probiot Antimicrob Proteins 10(2):157–167
Raddatz G, Fonseca B, Poletto G, Jacob-Lopes E, Cichoski A, Muller E et al (2019) Influence of the prebiotics hi-maize, inulin and rice bran on the viability of pectin microparticles containing Lactobacillus acidophilus LA-5 obtained by internal gelation/emulsification. Powder Technol 362
Kragh KN, Hutchison JB, Melaugh G, Rodesney C, Roberts AE, Irie Y et al (2016) Role of multicellular aggregates in biofilm formation. mBio 7(2):e00237
Trunk T, Khalil HS, Leo JC (2018) Bacterial autoaggregation. AIMS Microbiol 4(1):140–164
Vornhagen J, Stevens M, McCormick DW, Dowd SE, Eisenberg JN, Boles BR et al (2013) Coaggregation occurs amongst bacteria within and between biofilms in domestic showerheads. Biofouling 29(1):53–68
Tsilingiri K, Rescigno M (2013) Postbiotics: what else? Beneficial Microbes 4(1):101–107
Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W (2020) Postbiotics—a step beyond pre- and probiotics. Nutrients 12(8)
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B et al (2018) Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol 75:105–114
Kareem KY, Hooi Ling F, Teck Chwen L, May Foong O, Anjas AS (2014) Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut pathogens 6(1):1–7
Kolaček S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R et al (2017) Commercial Probiotic Products: A Call for Improved Quality Control. A position paper by the ESPGHAN working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr 65(1):117–24
Cremon C, Barbaro MR, Ventura M, Barbara G (2018) Pre- and probiotic overview. Curr Opin Pharmacol 43:87–92
Chen T, Wu Q, Zhou H, Deng K, Wang X, Meng F et al (2016) Assessment of commercial probiotic products in China for labelling accuracy and probiotic characterisation of selected isolates. Int J Dairy Technol 70
Zoumpopoulou G, Kazou M, Alexandraki V, Angelopoulou A, Papadimitriou K, Pot B et al (2018) Probiotics and Prebiotics: An Overview on Recent Trends. 1–34
de Melo Pereira GV, de Oliveira CB, Magalhães Júnior AI, Thomaz-Soccol V, Soccol CR (2018) How to select a probiotic? A review and update of methods and criteria. Biotechnol Adv 36(8):2060–2076
Bryant CJ (2019) We can’t keep meating like this: attitudes towards vegetarian and vegan diets in the United Kingdom. Sustainability 11(23):6844
Miranda JS, Costa BV, de Oliveira IV, de Lima DCN, Martins EMF, de Castro Leite Júnior BR et al (2020) Probiotic jelly candies enriched with native Atlantic Forest fruits and Bacillus coagulans GBI-30 6086. LWT 126:109275
Vivek K, Mishra S, Pradhan RC (2020) Characterization of spray dried probiotic Sohiong fruit powder with Lactobacillus plantarum. LWT 117:108699
Rouf S, Jan T, Sharma P (2018) Non-dairy probiotics—an emerging trend in health care products. Int J Curr Microbiol App Sci 7(10):131–145
Pereira ALF, Rodrigues S (2018) Turning fruit juice into probiotic beverages. Elsevier, Fruit juices, pp 279–287
Perricone M, Bevilacqua A, Altieri C, Sinigaglia M, Corbo MR (2015) Challenges for the production of probiotic fruit juices. Beverages 1(2):95–103
Min M, Bunt CR, Mason SL, Hussain MAJ (2019) Non-dairy probiotic food products: an emerging group of functional foods. Crit Rev Food Sci Nutr 59(16):2626–2641
Holck AL, Axelsson L, Rode TM, Høy M, Måge I, Alvseike O et al (2011) Reduction of verotoxigenic Escherichia coli in production of fermented sausages. Meat Sci 89(3):286–295
Vinderola G, Burns P, Reinheimer J (2017) Probiotics in nondairy products. Vegetarian and plant-based diets in health and disease prevention: Elsevier. 809–35
Valero-Cases E, Cerdá-Bernad D, Pastor J-J, Frutos M-J (2020) Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 12(6)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, F.F., Sohail, A., Ghazanfar, S. et al. Recent Innovations in Non-dairy Prebiotics and Probiotics: Physiological Potential, Applications, and Characterization. Probiotics & Antimicro. Prot. 15, 239–263 (2023). https://doi.org/10.1007/s12602-022-09983-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09983-9