Abstract
This paper reports on the extraction, partial characterization and the potential application of crude polysaccharides from defatted coconut residue as a prebiotic. The coconut residue was defatted and extracted to obtain the crude polysaccharides and its physicochemical properties were determined. The crude polysaccharides were assessed for monosaccharide composition, total carbohydrate content, reducing sugar concentration and protein content determination. The functional group and structural elucidation of crude polysaccharides was also done using Fourier transform infrared spectra analysis. The product was then subjected to artificial human gastric juice treatment to determine digestibility. Finally, an in vitro proliferation and acid production by two probiotic bacteria namely Lactobacillus casei Shirota and Lactobacillus bulgaricus were included in this study. It was found that the defatted coconut residue contained ash (0.54%), moisture (55.42%), protein (1.69%), crude fat (17.26%) and carbohydrate (25.73%). The percentage of crude polysaccharides extracted was 0.73 ± 0.04. The two fractions of monosaccharides obtained were glucose and fructose. Total carbohydrate content of DCR was 13.35% (w/v). The quantitative value of the reducing sugars obtained was 20.71%. Protein content in the crude polysaccharides was 0.009% and the peaks which indicated the presence of protein were observed at around 1640 cm−1 (amide I) and 1530 cm−1 (amide II). DCR crude polysaccharides were highly resistant (88%) to hydrolysis when subjected to artificial human gastric juice. The product was found to markedly stimulate two tested probiotics to proliferate and produce organic acids. All the above findings are supportive of the fact that polysaccharides extracted from DCR, an industrial waste, have a vast potential to be exploited as novel prebiotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Current trend amongst consumers is towards food products that promote health and wellness. These food products are those with benefits beyond basic nutritional needs and at the same time alleviate risks towards debilitating conditions and chronic diseases (Cencic and Chingwaru 2010). Foods of this nature is widely being researched by virtue of their containing a wide diversity of bioactive compounds. Of particular interest consumers are attracted to prebiotics and probiotics which are well known to confer health benefits over conventional food products (Ares et al. 2009).
Prebiotics are food ingredient which are not digestible but in the digestive tract they create an environment which promote the growth of specific probiotic microorganisms. Apart from activating bacterial metabolites in the intestinal tract (Charalampopoulos and Rastall 2012) prebiotics also facilitate bacterial fermentation in the colon (Forssten et al. 2011). Prebiotics are applied in the food industry for the growth of pure cultures, for the production of functional food used in veterinary practice to promote growth of young livestock, as therapeutics, restore unbalanced intestinal flora and regulate metabolic processes (Roberfroid et al. 2010).
To date most prebiotics are known to be carbohydrates. These are fructan-type oligosaccharides (Bielecka et al. 2002), soybean oligosaccharide (Su et al. 2007), galactooligosaccharides (Huebner et al. 2007), burdock inulin (Li et al. 2008), neoagaro-oligosaccharides (Hu et al. 2006), polysaccharides from Gigantochloa levis shoots (Azmi et al. 2012), kiwifruit pectins (Parkar et al. 2010) which demonstrated prebiotic activity and have been claimed to be potential novel prebiotics.
Coconut residue is a by-product waste following extraction of coconut milk from coconut kernal. Being nutritionally rich, cultivation of probiotics on this by-product could be a solution to transform the inedible waste into a commodity of high economic value. Utilization of such by-product which pose a major disposal problem for the coconut industry and its economic potential is an approach towards solving environmental pollution (Ng et al. 2010).
To our knowledge, information concerning the characterization of defatted coconut residue polysaccharides (DCRP) and their potential application as prebiotics have not been reported. Hence this study is directed towards (1) polysaccharides extraction from defatted coconut residue; (2) analysis of polysaccharides chemical composition; (3) evaluation of polysaccharides digestibility using artificial human gastric juice; and (4) investigation on proliferation and acid production by probiotic bacteria namely as Lactobacillus casei Shirota and Lactobacillus bulgaricus when cultured on the polysaccharides.
Materials and methods
Supplies
Coconut residue was obtained from a local market in Sri Serdang (Selangor, Malaysia). Fructooligosaccharides (FOS, purity ≥90%, polymerization degree <10) was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). de Man, Rogosa and Sharpe (MRS) and Luria–Bertani (LB) were sourced from Merck (Darmstadt, Germany). Glucose and inulin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Ethanol, petroleum ether, sulfuric acid, phenol, sodium hydroxide, 3,5-dinitrosalicylic acid (DNS) and all other chemicals used in this study were of analytical grade and purchased from Merck (Darmstadt, Germany).
Bacterial strains
Three bacterial strains, Lactobacillus casei Shirota isolated from Yakult cultured milk drink (Yakult, Malaysia), Lactobacillus bulgaricus isolated from Nestle fruit yogurt and Escherichia coli (ATCC1129) were used in this study. The presumptive colonies of the two isolates were identified using the API 50 CHL micro-identification system and combination of colony morphology. The two lactic acid bacteria (LAB) strains and E. coli were stored at −80 °C in MRS broth and LB broth containing 10% glycerol respectively, until use. Prior to their assays, the bacteria were activated according to the procedures described by Huebner et al. (2008).
Preparation of defatted coconut residue crude polysaccharides
The coconut residue samples were dried and ground into a fine powder using a Perten 120 lab mill (Perten, Sweden) to fine powder (passed through a No. 50 sieve). The extraction procedure of defatted coconut residue crude polysaccharides was conducted according to the method by Al-Sheraji et al. (2012a, b) and Azmi et al. (2012) with minor modifications. Briefly, the coconut residue was defatted using 10 times of 80% (v/v) ethanol for 48 h. The defatted residue was heated to 80 °C in distilled water. The supernatant was then separated from the residue and concentrated to 1/5 of its original volume in a rotary evaporator (IKA HB® 10 digital, Germany). It was then centrifuged (Eppendorf, Hamburg, Germany) at 5432 × g for 10 min at 25 °C and dialyzed using 12-14 kDa dialysis bags (Medicell international LTD,UK) against deionized water at 4 °C for 24 h. The dialyzed solution was then filtered using Whatmann No. 1, and concentrated to about 1/5 of its original volume by a rotary evaporator (at 175 MPa and 60 °C). This is followed by adding 5 times the original volume of 85% (v/v) ethanol at 4 °C for 18 h. The mixture was then centrifuged at 5432 × g for 10 min at 25 °C. The precipitate obtained was then dried and grounded manually and DCR crude polysaccharides were kept in room temperature until used.
Physicochemical properties of defatted coconut residue
Moisture and ash content were determined according to the method of Association of Official Analytical Chemists (AOAC 1997). The protein content was estimated using the Kjeldahl method (Havilah et al. 1977) with a conversion factor of 6.25. The fat content was determined using Soxtec method (AOAC 1997) and total carbohydrates (TCO) were calculated using Eq. (1).
Monosaccharide composition of crude polysaccharides
Coconut crude polysaccharides (CCP) were hydrolyzed before being assessed for monosaccharide composition. About 10 mg of each fraction were dissolved in 10 mL portions of 2 mol/L trifluoroacetic acid (TFA) and hydrolyzed at 100 °C for 6 h. The mixture was then evaporated in a rotary evaporator at 60 °C to completely remove excess TFA from the solution. The hydrolysates were then dissolved in 1 mL of distilled water. High performance liquid chromatography (HPLC) (Waters 2696, Milford, USA) was used to determine the quantity of monosaccharide compositions. The HPLC system was equipped with Purospher® star NH2 (250 mm × 4.6 mm, 5 µm) (Merck, Darmstadt, Germany) and a refractive index detector. The mobile phase composed of acetonitrile–water (75:25, v/v) at a flow rate of 1 mL/min was used and the detection was read after 15 min retention time (Al-Sheraji et al. 2012a, b). All measurements were performed in triplicates.
Determination of total carbohydrate contents
Phenol–sulphuric method (Dubois et al. 1956) was used to determine total carbohydrate contents using glucose as a standard. Briefly, 2 mL of CCP solution was added to 1 mL of 5% phenol and 5 mL of 95% of sulphuric acid (H2SO4), vortexed and the mixture placed in a water bath at 25 °C for 20 min. Absorbance was measured at 490 nm using UV–VIS spectrophotometer (Hitachi U 2810, Tokyo, Japan) (Albalasmeh et al. 2013). Total carbohydrate content was calculated using a standard curve of d-glucose.
Determination of reducing sugar of crude polysaccharides
The method of Robertson et al. (2001) was used to determine reducing sugar concentration. Briefly 1 mL of CCP was added to 2 mL of 3,5-dinitrosalicylic acid (DNS), boiled for 5 min and cooled at room temperature. The mixture was then topped up with 7 mL of distilled water. Absorbance was read at 540 nm using UV–VIS spectrophotometer (Hitachi U 2810, Tokyo, Japan). Total reducing sugar was calculated with d-glucose as a standard.
Determination of protein content of crude polysaccharides
Bradford reagent (Essex, UK) with albumin as a standard protein (Bradford 1976) was used to determine total protein concentration. A total of 20 μL of the sample was added to 200 μL of Bradford reagent in a microtiter plate and incubated at 37 °C for 15 min. Absorbance was measured at 590 nm.
Fourier transform infrared (FTIR) spectra analysis
The functional group and structural elucidation were identified using FTIR spectrophotometer (A Nicolet 6700 from Thermo Nicolet Corp., Madison, WI, USA) equipped with an OMNIC operating system (Version 7.0 Thermo Nicolet). The sample was prepared by mixing 95 mg of potassium bromide (KBr) and 5 mg of crude polysaccharides. The mixture was grounded, pelleted, and characterized with the spectrum ranging between 500 and 4000 cm−1.
Hydrolysis of coconut residue crude polysaccharides by artificial human gastric juice
Procedure described by Korakli et al. (2002) was employed to evaluate hydrolysis of CRCP by artificial human gastric juice. FOS was used as a positive control. Crude polysaccharides obtained from coconut residue was dissolved in distilled water to make a 1% (w/v) solution. Artificial human gastric juice was prepared as follows: 8 g of NaCl, 0.2 g of KCl, 8.25 g of Na2HPO4·2H2O, 14.35 g of NaHPO4, 0.1 g of CaCl2·2H2O and 0.18 g of MgCl2·6H2O were dissolved in distilled water to make a 1000 mL solution. The pH of the solution was adjusted to 1, 2, 3, 4 and 5 using 6 M HCl, respectively. Subsequently 5 mL of the solution at each pH was added onto 5 mL of CRCP (Korakli et al. 2002). The reaction mixture was incubated in a water bath at 37 °C for 6 h. 3 mL of the reaction mixture was collected at 0 and 30 min and 1, 2, 5 and 6 h to determine the reducing and total sugar. The degree of hydrolysis of samples were calculated according to the Eq. (2) as follows:
where reducing sugar released was the difference between its final and initial content.
Proliferation and acidifying activity of probiotics on DCR crude polysaccharides
Lactobacillus casei Shirota and Lactobacillus bulgaricus, as probiotics and E. coli as a potential pathogenic control of prebiotic activity were used to investigate the proliferative effect of DCR polysaccharides. Carbohydrate-free MRS and LB broths were used as basal culture media and blank controls for Lactobacillus sp. and E. coli, respectively while glucose (as a monosaccharide) and inulin (as a polysaccharide) were used as positive controls. The carbohydrates (glucose, inulin, DCR) were filter-sterilized and added to the sterilized basal media (carbohydrate-free MRS and LB) to make final concentrations of 0.25, 0.5 and 1% (w/v). The media were inoculated with 1 × 106 CFU mL−1 of the probiotics and incubated at 37 °C anaerobically in 85% N2, 10% CO2 and 5% H2. Bacterial numbers and pH of the medium were determined after incubation for 0 h, 24 h and 48 h. Bacterial enumeration was performed as follows: One milliliter of the culture was transferred to sterile test tubes and serially diluted with sterile 1% (w/v) Ringer solution (Merck, Darmstadt, Germany). 100 μL of each dilution was then spread on the plates of MRS agar and anaerobically incubated at 37 °C for 48 h. Bacteria counts were expressed in colony forming units per milliliter (CFU mL−1). The increase of bacterial numbers between 0 and 48 h was calculated according to the Eq. (3).
where A is the bacterial number at 0 h (CFU mL−1) and B is the bacterial number after incubation for 48 h (CFU mL−1).
Statistical analysis
Each experiment was repeated three times and the results were reported as ±standard deviation (SD) of triplicate independent extractions. Data obtained were analyzed by one-way ANOVA using Minitab Software Version 16.0 (Minitab Inc., USA). All statistics were based on a confidence level of 95%, and P < 0.05 were considered statistically significant.
Results and discussion
Chemical compositions of defatted coconut residue
The chemical composition of DCR is shown in Table 1. The percentage of ash in DCR was low (0.54%) which was an indication of low mineral content especially the macro-minerals. The moisture content was 55.42% which was higher (50.26%) compared to a previous study which could be due to the high water-holding capacity, water retention and swelling capacity (Ng et al. 2010). The protein content was 1.69% which was lower compared to that of fruits and vegetables (2.70–24.9%). The crude fat content of DCR was 17.26% which was higher than that of fruits and vegetables (0.5–10.9%) reported by Grigelmo-Miguel and Martin-Belloso (1999). The carbohydrate content of DCR was 25.73% which was lower than that of fruits and vegetables. Most fruits however showed higher carbohydrate contents (>72.3%) compared to vegetables with the exception of apples which contain only 25.8% carbohydrate (Ng et al. 2010). Overall, these observed variations in composition values might have resulted from geographic, climatic and seasonal variations.
Monosaccharide composition of DCR crude polysaccharides
SI. 1 (see supplementary information) shows the monosaccharide composition of DCR crude polysaccharides which comprised of two monosaccharides—glucose and fructose—with concentration fractions of 1.66 and 19.16%, respectively. Previous study showed that the yield and composition of soluble polysaccharides are strongly dependent on the extraction conditions such as temperature and time (Graham et al. 1988). For several polysaccharides there was generally no sharp distinction between soluble (or extractable) and insoluble fractions. The ratio between the two was dependent on conditions used (e.g., physical treatment, enzymatic treatment, temperature and time) in the solubilisation procedure. Thongsook and Chaijamrus (2014) reported that the major monosaccharides of coconut meal are mannose, followed by glucose, galactose and some other sugars. Khuwijitjaru et al. (2012) revealed that carbohydrate in the coconut meal contained mannose, glucose, galactose and small amount of arabinose.
Total carbohydrate content, reducing sugar and protein content of DCR crude polysaccharides
The percentage of crude polysaccharides extracted in this study was 0.73 ± 0.04. Extraction yields usually depended upon the ratio of water to raw material, extraction temperature and time (Cai et al. 2008). Total carbohydrate content of DCR after milk extraction in this study was about 13.35% (w/v) which is lower than that 18.8% of total sugar after extraction of oil reported by Yalegama and Chavan (2006). The extraction methods could be affected to the content of total carbohydrate as other compounds, apart from polysaccharide, reducing sugar and protein are easier to solubilise by hot water extraction and in some cases it was found that the highest yield of crude polysaccharides will achieve with the lowest content of total carbohydrate (Thetsrimuang et al. 2011). The quantitative value of the reducing sugars obtained was approximately 20.71%. Protein content in the DCR crude polysaccharides is in a minute, about 0.009%. This could be due to heating the extracted crude polysaccharides using rotary evaporator (Azmi et al. 2012).
Fourier transform infrared (FTIR) spectra analysis
FTIR analysis results are presented in SI. 2. The absorption near 894.8 cm−1 was caused by β-type glycosidic bond while the absorption exhibited in the region of 843.2 cm−1 was caused by α-glycosidic band. It has been reported that C–H bond in α-style usually exhibited an absorption band nearby 844 cm−1 in IR spectra, while C–H bond in β-style usually exhibited an absorption band near 891 cm−1 (Chen et al. 2006). The two absorption bands near 843.2 and 893.8 cm−1 indicated that the sugar units were linked by both α-glycosidic bond and β-glycosidic bond. The band at 1093.2 cm−1 spectrum of the sample under study were due to C–O vibrations. The absorption at 1154.6 cm−1 was due to C–O ester groups. The absorption band at 1247.0 cm−1 was attributed to the C–O stretch in carboxyl group while the absorption between 1400.9 and 1466.2 cm−1 is due to –CH deformation modes. 1747.3 cm−1 represented the ester bond C=O. CH2 absorption occured at 2854 cm−1. The band in the region of 2924.9 cm−1 was attributed to C–H stretching vibration which was regarded as characteristic absorption of polysaccharides.
In general, the peaks which indicated the presence of protein were observed with absorption at around 1640 cm−1 (amide I) and 1530 cm−1 (amide II). The presence of these bands in the spectra resulted from proteins which were covalently bound to polysaccharides or may resulted from protein impurities (Synytsya et al. 2009). Amide II band which was due to bending vibrations of N–H groups and was used for estimation of protein content in DCR crude polysaccharides. Therefore, the protein content in DCR crude polysaccharides was low which confirmed the above mentioned finding.
Hydrolysis of crude polysaccharides by artificial human gastric juice
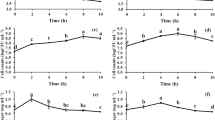
The degree of hydrolysis of crude polysaccharides as a function of time was determined after incubation with artificial human gastric juice and the results are as shown in Fig. 1. According to the requirement of a prebiotic, the product should not be hydrolyzed by gastric acid to enable the product to reach the intestine to facilitate the proliferation of probiotics. The degrees of hydrolysis after 6 h of incubation at pH 5, 4, 3, 2 and 1 were 6.97, 8.11, 8.85, 9.63 and 12.03% respectively. Similar to FOS, DCR crude polysaccharides was also resistant to human gastric juice (88%) and its degree of hydrolysis had a significantly negative correlation with its pH. For example, after incubation at pH 5 and 1 for 6 h, the degree of hydrolysis of crude polysaccharides were 6.97 and 12.03% respectively. Degree of hydrolysis as observed in this study increased with decreased pH from 5 to 1. At low pH, the degree of hydrolysis is higher than at high pH. The reason for this was probably due to the glycosidic bond being more easily ruptured at low pH and resulted in partial hydrolysis of the polysaccharides. Incubation time also affected the degree of hydrolysis as a longer duration of incubation was conducive for more polysaccharides to be degraded to mono- and di-saccharides in acidic conditions (Wang et al. 2015).
The digestibility of polysaccharides during the first two hours was high especially at pH 1 and 2. According to Wichienchot et al. (2010), food is usually retained in the stomach for about 2 h where gastric juice at pH 2-4 is secreted. Based on the results of the digestibility study, it can be deduced that when coconut crude polysaccharides are consumed, about 88% of the product at pH 1 reached the intestine after 2 h. This percentage is lower compared to FOS which is about 98%. Food generally passes through the human digestive tract within 4 h. Hence there is a possibility that most of DCR crude polysaccharides reached the intestine and then utilized by resident probiotics.
Proliferation of probiotics on DCR crude polysaccharides
Results on proliferation of probiotics on DCR crude polysaccharides are presented in Table 2. The two tested LAB could utilize DCR crude polysaccharides as the proliferation of Lb. casei Shirota and Lb. bulgaricus in all conditions studied was higher compared to that of control. proliferation of Lb. casei Shirota significantly increased with increased concentrations of glucose, inulin and DCR crude polysaccharides from 0.25 to 1.0%. The proliferation of the probiotics could be due to the solubility of the polysaccharides since good water soluble carbohydrates could be utilized readily, rapidly and completely by probiotics (Montagne et al. 2003). Moreover, it has been reported that the monomeric composition, polymerization degree and type of glycosidic linkages could affect the growth of probiotics (Hernandez–Hernandez et al. 2012).
Proliferation of Lb. bulgaricus was the highest in carbohydrate-free MRS supplemented with glucose in all concentrations. DCR crude polysaccharides enhanced the proliferation of Lb. bulgaricus as evidence by increased bacterial population from 5.81 to 6.12 log CFU mL−1 at 0.25–1% concentration. However proliferation was slightly lower compared to media supplemented with inulin at 1%. Proliferation of Lb. bulgaricus in media supplemented with 0.25% of DCR crude polysaccharides and inulin showed no significant different (P > 0.05). The proliferation of the Lb. bulgaricus in all media at 0.5 and 1% was significantly different (P < 0.05). The proliferation of Lb. bulgaricus in the media supplemented with inulin shows slightly lower proliferation after 48 h as compared to DCR crude polysaccharides and glucose. This finding is in accordance with the report by Wichienchot et al. (2010) who claimed inulin enhanced the growth of probiotic strains but had slightly lower activity as compared to glucose. Hence proliferation of probiotic bacteria on DCR crude polysaccharides was comparable to commercial carbohydrate such as inulin.
There were no significant difference (P > 0.05) in the proliferation of E. coli in carbohydrate-free LB medium supplemented with 0.25%, glucose and DCR crude polysaccharides while the proliferation in all media supplemented with 0.5 and 1% carbohydrates were significantly different (P < 0.05). This finding concur with that of Al-Sheraji et al. (2012a, b).
Proliferation of probiotics in DCR crude polysaccharides could possibly be explained by one of the following. The sugar content of polysaccharide prebiotics could affect the proliferation of probiotics. The molecular weight of DCR crude polysaccharides was another important factor which could influence accessibility of a carbohydrate prebiotic to probiotics. It has been generally accepted that the proliferation of probiotics was far more superior on non-digestible carbohydrate with low molecular weight. The composition of monosaccharides could also influence the effect of carbohydrate on probiotic proliferation. Under normal circumstances a carbohydrate prebiotic contained fructose, glucose, galactose and xylose (Manning and Gibson 2004). DCR crude polysaccharides also contained glucose and fructose which facilitated probiotic proliferation. Another factor which contributed towards different prebiotic effects was the different molar ratio of monosaccharides. Finally, solubility could also be an important factor as good water-soluble carbohydrates were more readily, rapidly and completely utilized by probiotics (Montagne et al. 2003).
A requirement to qualify as a prebiotic is the ability of the product to selectively stimulate the proliferation of beneficial intestinal bacteria and at the same time suppress the proliferation of pathogenic bacteria. In this study it was observed that the proliferation of E. coli varies according to the supplementation of media with different carbohydrates. Lb. casei Shirota showed a much proliferation rate compared to E. coli. It is evident from this study that crude polysaccharides could suppress the proliferation of E. coli. However, Lb. bulgaricus showed results similar to that of E. coli especially with 1% concentration of inulin and DCR crude polysaccharides.
Acidifying activity of probiotics on DCR crude polysaccharides
Figure 2 showed the acidifying activity of Lb. casei Shirota, Lb. bulgaricus cultured in three carbohydrate-free media each supplemented with inulin, glucose and DCR crude polysaccharides compared to that of E. coli ATCC 1129. Results showed that the proliferation of the above mentioned probiotics varied depending on the types and concentrations of prebiotics used in the basal media. The acidifying activity of the probiotic strains increased with the increased concentration of prebiotics. For Lb. casei Shirota acid production in the media supplemented with DCR and inulin are the same in all concentrations of carbohydrates. Minimum pH of ~4 was observed 48 h following incubation for all media supplemented with 1% of carbohydrates. On the other hand, types of media and the concentration of carbohydrate influenced the production of acid by Lb. bulgaricus where minimum pH of ~4.8 was observed in the media supplemented with 1% glucose. Acidifying activity of E. coli increased with increased concentration to 1% of carbohydrates; however the level of acid production are the same in all the media. The types of media do not have any significant influence on the acidifying activities. Although the acidifying activity of E. coli is increased up to a certain level in vitro, the low pH in the intestine is however due to production of lactic acid and acetic acid by LAB which restrict or inhibit the growth of E. coli and many other pathogenic bacteria (Cruz-Guerrero et al. 2014; Nadour et al. 2015).
From the above data, the acidifying activity increase with the increase in the number of bacteria and the increase concentration of carbohydrate. The reduced pH could be due to the production of organic acid from the microbial fermentation of carbohydrates. This minimum pH values not only reflect the acidifying activity of the tested bacterial strain but also the utilization of the carbohydrates by the specific strain. In this respect polysaccharides were metabolized by the bacteria to produced some short-chain fatty acids which led to the drop of pH in the media. Results from this study indicated that an appropriate prebiotics should be selected for each probiotic strain for increased acidifying activities. It was reported that prebiotic effect of polysaccharides may be due to the structural features such as molecular weight, monosaccharide composition and chain conformation. However, the exact mechanism underlying the prebiotic effect exerted by polysaccharides is still not fully understood (He et al. 2015; Wang et al. 2015).
Conclusion
This study demonstrated that DCR crude polysaccharides were resistant to artificial gastric juice and not only stimulated the proliferation of the tested probiotics but also increased their acidifying activity in vitro. Thus, the ability of DCR crude polysaccharides to be metabolized by Lactobacillus casei Shirota and Lactobacillus bulgaricus, supporting its potential prebiotic activity. Although the capability of probiotics to utilize certain carbohydrates in vitro gave some indication whether the probiotic strain may succeed or not under given conditions, a final conclusion could only be achieved based on in vivo experiments.
References
Albalasmeh A, Berhe AA, Ghezzehei T (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohyd Polym 97:253–261
Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA (2012a) Fermentation and non-digestibility of Mangifera pajang fibrous pulp and its polysaccharides. J Funct Foods 4:933–940
Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA (2012b) Purification, characterization and antioxidant activity of polysaccharides extracted from the fibrous pulp of Mangifera pajang fruits. LWT-Food Sci Technol 48:291–296
AOAC (1997) Methods of analysis of association of official agricultural chemists, 17th edn. Association of Official Analytical Chemists, Washington, pp 684–697
Ares G, Giménez A, Gámbaro A (2009) Consumer-perceived healthiness and willingness to try functional milk desserts. Influence of ingredient, ingredient name and health claim. Food Qual Prefer 20:50–56
Azmi AFMN, Mustafa S, Hashim DM, Manap YA (2012) Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules 17:1635–1651
Bielecka M, Biedrzycka E, Majkowska A (2002) Selection of probiotics and prebiotics for synbiotics and confirmation of their in vivo effectiveness. Food Res Int 35:125–131
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–254
Cai W, Gu X, Tang J (2008) Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohyd Polym 71:403–410
Cencic A, Chingwaru W (2010) The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2:611–625
Charalampopoulos D, Rastall RA (2012) Prebiotics in foods. Curr Opin Biotechnol 23:187–191
Chen H-L, Cheng H-C, Liu Y-J, Liu S-Y, Wu W-T (2006) Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition 22:1112–1119
Cruz-Guerrero A, Hernández-Sánchez H, Rodríguez-Serrano G, Gómez-Ruiz L, García-Garibay M, Figueroa-González I (2014) Commercial probiotic bacteria and prebiotic carbohydrates: a fundamental study on prebiotics uptake, antimicrobials production and inhibition of pathogens. J Sci Food Agric 11:2246–2252
Dubois M, Gilles KA, Hhamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Forssten SD, Sindelar CW, Ouwehand AC (2011) Probiotics from an industrial perspective. Anaerobe 17:410–413
Graham H, Gron Rydberg MB, Aman P (1988) Extraction of soluble fiber. J Agric Food Chem 36:494–497
Grigelmo-Miguel N, Martin-Belloso O (1999) Characterization of dietary fiber from orange juice extraction. Food Res Int 35:355–361
Havilah ED, Morris WR, Woolnough J (1977) A microcolorimetric method for determination of ammonia in Kjeldahl digests with a manual spectrophotometer. Lab Pract 26:545–547
He Z, Wang X, Li G, Zhao Y, Zhang J, Niu C, Zhang L, Zhang X, Ying D, Li S (2015) Antioxidant activity of prebiotic ginseng polysaccharides combined with potential probiotic Lactobacillus plantarum C88. Int J Food Sci Technol 50:1673–1682
Hernandez-Hernandez O, Muthaiyan A, Moreno FJ, Montilla A, Sanz ML, Ricke SC (2012) Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol 30:355–361
Hu B, Gong QH, Wang Y, Ma YM, Li JB, Yu WG (2006) Prebiotic effect s of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 12:260–266
Huebner J, Wehling RL, Hutkins RW (2007) Functional activity of commercial prebiotics. Int Dairy J 17:770–775
Huebner J, Wehling RL, Parkhurst A, Hutkins RW (2008) Effect of processing conditions on the prebiotic activity of commercial prebiotics. Int Dairy J 18:287–293
Khuwijitjaru P, Watsanit K, Adachi S (2012) Carbohydrate content and composition of product from subcritical water treatment of coconut meal. J Ind Eng Chem 18:225–229
Korakli M, Ganzle MG, Vogel RF (2002) Metabolism by bifidobacteria and lactic acid bacteria of polysaccharides from wheat and rye, and exopolysaccharides produced by Lactobacillus sanfranciscensis. J Appl Microbiol 92:958–965
Li D, Kim JMÃZJ, Zhou J (2008) Prebiotic effectiveness of inulin extracted from edible burdock. Anaerobe 14:29–34
Manning TS, Gibson GR (2004) Prebiotics. Best Pract Res Clin Gastroenterol 18:287–298
Montagne L, Pluske JR, Hampson DJ (2003) A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol 108:95–117
Nadour M, Laroche C, Pierre G, Delattre C, Moulti-Mati F, Michaud P (2015) Structural characterization and biological activities of polysaccharides from olive mill wastewater. Appl Biochem Biotechnol 177:431–445
Ng SP, Tan CP, Lai OM, Long K, Mirhosseini H (2010) Extraction and characterization of dietary fiber from coconut residue. J Food Agric Environ 8:172–177
Parkar SG, Redgate EL, Wibisono R, Luo X, Koh ETH (2010) Gut health benefits of kiwifruit pectins: comparison with commercial functional polysaccharides. J Funct Foods 2:210–218
Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A (2010) Prebiotic effects: metabolic and health benefits. Br J Nutr 104:S1–S63
Robertson J, Ryden P, Louise Botham R, Reading S, Gibson G, Ring SG (2001) Structural properties of diet-derived polysaccharides and their influence on butyrate production during fermentation. LWT-Food Sci Technol 34:567–573
Su P, Henriksson A, Mitchell H (2007) Selected prebiotics support the growth of probiotic mono-cultures in vitro. Anaerobe 13:134–139
Synytsya A, Mickova K, Synytsya A, Jablonsky I, Spevacek J, Erban V (2009) Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohyd Polym 76:548–556
Thetsrimuang C, Khammuang S, Chiablaem K, Srisomsap C, Sarnthima R (2011) Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chem 128:634–639
Thongsook T, Chaijamrus S (2014) Modification of physiochemical properties of copra meal by dilute acid hydrolysis. Int J Food Sci Technol 49:1461–1469
Wang X, Huang M, Yang F, Sun H, Zhou X, Guo Y, Wang X, Zhang M (2015) Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohyd Polym 125:232–240
Wichienchot S, Jatupornpipat M, Rastall RA (2010) Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem 120:850–857
Yalegama LLWC, Chavan JK (2006) Studies on utilization of coconut flour as a source of tropical agricultural research. Trop Agric Res 18:9
Acknowledgements
The authors are gratefully acknowledge for the financial support under the Research University Grant Scheme (RUGS) (Project No: 05-02-12-2141RU) from Universiti Putra Malaysia, Serdang, Selangor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
SI. 1
Monosaccharides compositions of coconut’s crude polysaccharides (Peak 1 at RT 3.955: solvent, peak 2 at RT 6.302: glucose and peak 3 at RT 6.916: fructose). (DOC 55 kb)
SI. 2
FTIR spectra of crude polysaccharides from defatted coconut residue. (DOCX 138 kb)
Rights and permissions
About this article
Cite this article
Mohd Nor, N.N., Abbasiliasi, S., Marikkar, M.N. et al. Defatted coconut residue crude polysaccharides as potential prebiotics: study of their effects on proliferation and acidifying activity of probiotics in vitro. J Food Sci Technol 54, 164–173 (2017). https://doi.org/10.1007/s13197-016-2448-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2448-9