Abstract
The present work, herein, studied the effects of corncob-derived xylooligosaccharides (CDXOS) and Lactobacillus plantarum CR1T5 (LP) integrated into fish diets (diet 1 (0—control), diet 2 (10 g kg−1 CDXOS), diet 3 (108 CFU g−1L. plantarum CR1T5), diet 4 (10 g kg−1 CDXOS +108 CFU g−1L. plantarum CR1T5)) on growth performance, innate immune parameters, and disease resistance of Nile tilapia (Oreochromis niloticus). Fingerlings, with average mean weight of 4.97 ± 0.04, were randomly distributed into 16 glass tanks (20 fish per tank) for 12 weeks. Growth performance, skin mucus, and serum immune parameters were evaluated at the conclusion of the experiment. Eight randomly selected fish were used for challenge test against Streptococcus agalactiae. The results indicated that fish fed CDXOS and LP had significantly improved final weight (FW), weight gain (WG), specific growth rate (SGR), and feed conversion ratio (FCR). However, no significant difference in survival rate was observed between specimens fed the supplemented diets and the control. Regarding skin mucus, the dietary inclusion of CDXOS and LP significantly increased lysozyme and peroxidase activities compared with the control (P < 0.05). Similarly, significant increases in serum lysozyme, peroxidase, alternative complement, phagocytosis, and respiratory burst activities were observed in the fish fed the supplemented diets. However, no significant differences were found in these parameters between fish fed CDXOS and LP diets. For the challenge test, diet 4 produced a higher relative percentage of survival (RPS) and resistance to S. agalactiae than fish from the other experimental groups (P < 0.05). The results suggested that CDXOS and L. plantarum CR1T5 are viable considerations for potential feed-additive sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the expansion of intensive aquaculture and increases in culture density, fish diseases have become a frequent dilemma [1,2,3,4,5,6,7,8]. The common way in which farmers deal with the outbreak of diseases in aquaculture is through antibiotics and/or chemotherapeutics [9, 10]. Nonetheless, their application, which is banned in some countries, may give rise to other problems, such as resistance to bacterial strains, environmental hazards, and difficulties with food safety [11, 12]. Of recent concern is the possible transfer of antimicrobial resistance genes and resistant bacteria from aquatic animals to humans, with side effects occurring in both humans and the aquatic environment [10]. These concerns have led to a search for natural strategies as alternatives to the use of antibiotics and chemotherapeutics in aquaculture [10, 13]. Among them, the inclusion of prebiotics and probiotics in farmed fish and shellfish diets has been assayed to enhance digestion, digestive enzymes, growth, and immune response [14,15,16,17,18,19,20,21].
A prebiotic is defined as a non-digestible compound that, through its metabolization by microorganisms in the gut, modulates the composition and/or activity of the gut microbiota, thus conferring a beneficial physiological effect on the host [22, 23]. It has well-established that prebiotics play a pivotal role in enhancing host’s health and well-being, when by-products are fermented by favorable microbiota [24,25,26]. In this sense, agricultural by-products are potential fiber sources, which, when integrated as functional ingredients in food products, can inhibit diseases related to the alterations in intestinal microflora [27]. As they are often discarded as wastes, or burned in the field after each crop, the further use of these by-products will help in their proper disposal, as well as to generate employment and provide farmers with an additional income [28]. Global corn production is approximately 1 billion metric tons [29], resulting in huge amounts of corncobs. Corncobs have long been used as a component for growing several industrially important bacteria and fungi, and in the production of pharmaceuticals and nutraceutically important enzymes [28]. The major elements of corncob by-product are xylooligosaccharides (XOS), xylitol, and xylose [30, 31]. Of these, XOS has proved to be a potential prebiotic [32] and has been considered a potential functional ingredient in the diet. Several properties that play a vital role in improving human health have been attributed to XOS, immunomodulatory, antioxidative, antidiabetic, and anti-cancer activities, as well as the ability to stimulate the proliferation of colonic bifidobacteria, calcium absorption, and lipid metabolism [33,34,35].
On the other hand, probiotics are defined as “live microorganisms which, when administered in adequate amount, confer a health benefit on the host” [36, 37]. The beneficial effects of dietary probiotics consumption have been demonstrated in aquaculture, including the promotion of growth, stimulation of the immune response, and enhanced disease resistance [14, 38,39,40,41]. Lactobacillus plantarum belongs to the genus Lactobacillus, which plays an important role in the fish intestine. Strains belonging to this species are able to release antibacterial compounds which inhibit the growth of harmful microorganisms [42, 43]. Dietary supplementations of L. plantarum have been reported to stimulate the immune response, enhance growth performance, and metabolic functions, compete for adhesion and for nutrition, as well as improve disease resistance in several fish species [44,45,46,47,48,49,50].
Synbiotics are defined as “mixtures of probiotics and prebiotics that beneficially affect the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract of the host” [51]. In the last decade, the application of synbiotics has been widely studied in fish and shellfish [15]. However, to the best of our knowledge, there are no previous studies regarding the use of corncob-derived xylooligosaccharide (CDXOS) and Lactobacillus plantarum on the growth performance, humoral immunity (both mucosal and seric), and disease resistance of Nile tilapia (O. niloticus); this situation has given rise to the present study.
Materials and Methods
Xylooligosaccharides Preparation
Raw Materials Preparation
Corncobs obtained from the experiment farm of the Faculty of Agriculture, Chiang Mai University (Thailand), were oven dried at 60 °C for 2 days before being crushed by hammer mill, and then filtered using 100-μm mesh size sieve, and stored at 4 °C until use.
Xylan Extraction
Xylan was isolated following the protocol of Chapla et al. [28] with some modifications as described in our previous publication [52]. The xylan obtained was used as substrate for enzymatic hydrolysis [53].
Enzymatic Hydrolysis
XOS was obtained by enzymatic hydrolysis of xylan after thoroughly mixing with 0.01 M potassium phosphate buffer at pH 6.5 (15% w/v). Then, 100 U/g of substrate of crude xylanase from Aspergillus niger (supplied by ASIA STAR CO., LTD.) was added and the incubation was carried out at 55 °C for 24 h [54]. The incubated samples were collected and then centrifuged at 10,000 rpm for 10 min. The supernatant was gathered and freeze-dried at − 40 °C in a freeze dryer (FreezeZone® Plus Labconco, USA), and the powder was kept at − 20 °C until further use.
Experimental Design
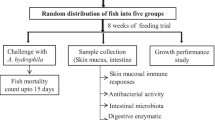
Nile tilapia fingerlings were bought from the Chiang Mai Pattahana Farm, Chiang Mai, Thailand. Upon arrival, the fish were placed in 3000-L tanks and allowed to acclimatize for 2 weeks; after which, they were randomly distributed into 16 glass tanks (150 L), stocked at a density of 20 fish tank−1. The fish were fed the experimental, twice a day (at 09.00 h and 17.00 h) to apparent satiation, for 12 weeks. Fifty percent of the water in each tank was exchanged daily to maintain water quality. The water quality parameters, temperature, pH, and dissolved oxygen, were monitored daily and maintained at 28.55 ± 0.81 °C, 7.90 ± 0.50, and 5.40 ± 0.35 mg l−1, respectively.
Lactobacillus plantarum CR1T5 was kindly provided by Dr. Saowanit Tongpim, (Department of Microbiology, Faculty of Science, Khon Kaen University, Thailand). A pure culture of L. plantarum CR1T5 was inoculated in MRS broth and incubated at 30 °C. After 15 h of incubation (30 °C), the bacterial cells were harvested, washed with 0.85% (w/v) NaCl, and resuspended in the same solution. The cell suspension density was adjusted spectrophotometrically to reach an optical density at 600 nm (OD600) of 0.2 to 1.8, using sterile 0.85% (w/v) NaCl. Various cell concentrations within the OD600 were observed, and thus a linear relationship between the viable cells, established through the spread-plate technique, and the OD600 was determined. The OD600 of each cell suspension was set up to a desired cell concentration (CFU mL−1) for further feed formulation experiment.
The selected L. plantarum CR1T5 dose (108 colony forming units, cfu g−1) replicated that of our previous studies [45, 55]. The L. plantarum CR1T5 added to the tested diets was prepared daily, following the protocol described by Irianto, Austin [56]. A basal diet [57] was supplemented with CDXOS and/or L. plantarum CR1T5 in which to prepare the experimental diets: 0 g kg−1 CDXOS and 0 L. plantarum CR1T5 (diet 1—control), 10 g kg−1 of CDXOS (diet 2), 108 cfu g−1L. plantarum CR1T5 (diet 3), and 10 g kg−1of CDXOS +108 cfu g−1L. plantarum CR1T5 (diet 4) (Table 1).
Growth Performance
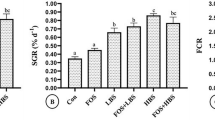
After 4-, 8-, and 12-week post feeding, the final weight, weight gain, specific growth rate, and feed conversion ratio were calculated according to the previously described formulae [48].
Sample Collection and Immune Response Analysis
Sample Collection
Within the same periods described above, four fish from each replication were used for innate immune response analysis, in which skin mucus was collected according to the method of Ross et al. [58]. Blood and serum were collected, as described in our previous studies [46, 59]. Leucocyte separation and collection from un-clotted blood was carried out according to Chung, Secombes [60] with modifications, as described in previous studies [46, 59].
Immune Parameters
Lysozyme Activity
The protocol described by Parry et al. [61] was followed for the determination of serum and mucus lysozyme activities, and expressed as μg ml−1.
Peroxidase Activity
Serum and mucus peroxidase activities were measured according to the methods of Quade, Roth [62] and Cordero et al. [63]. Briefly, 5 μL of serum or skin mucus was placed in flat bottomed, 96-well plates in triplicate. Then, 45 μL of Hank’s balanced salt solution (HBSS), without Ca+2 or Mg+2, and 100 μL of solution (40 ml of distilled water, 10 μL of H2O2 (30%—Sigma-Aldrich), and one tablet of 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich)) were added. Fifty microliters of 2 M H2SO4 was added once the reaction color changed, and the optical density was read at 450 nm via a plate reader (Synergy H1, BioTek, USA). Standard samples with no serum or skin mucus were considered as blanks, a single unit was defined as the amount producing an absorbance change of 1, and the activity was expressed as units of (U) mg−1 serum or mucus.
Phagocytosis Activity
The serum phagocytic activity was determined according to the method described by Yoshida and Kitao [64] with slight modifications, described in details in our previous studies [46, 59].
Respiratory Burst Activity
The respiratory burst activity of the Nile tilapia peripheral blood leucocytes was determined through the suggestions of Secombes [65], with slight modifications, as described in detail in our previous studies [46, 59].
Alternative Complement Pathway Activity
The serum alternative complement pathway activity (ACH50) was measured according to Yanno [66], as described in [46, 59].
Challenge Study
Streptococcus agalactiae source, preparation, and injection dose were as detailed in a previous publication [48]. Briefly, S. agalactiae was cultured in Tryptic Soy Broth and incubated at 37 °C for 24 h in a rotation shaker, at a speed of 110 rpm. The sub-culture was obtained from the stock, as follows: 5 mL of the stock solution was transferred into a 50-mL flask containing Tryptic Soy Broth, and incubated at 37 °C for 24 h. The sub-cultures within the present study were raised in duplicate, under similar conditions. Growth was evaluated by optical density of 560 nm and then confirmed by plate counting, in Tryptic Soy Agar.
After 12 weeks of feeding, eight randomly selected fish in each tank were injected intraperitoneally with 0.1 ml of 0.85% normal saline solution (NSS) containing 107 CFU ml−1 of S. agalactiae [67]. Dead fish from each tank were removed daily, and the mortality (%) in each treatment was computed 15 days, post-challenge. The relative percentage of survival (RPS) was then calculated through the following equation:
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. The mean values were considered significantly different, when P < 0.05. All statistical analysis was conducted using SAS Computer Program [68].
Results
Growth Performance
Statistically significant increases were recorded for the specific growth rate (SGR), weight gain (WG), and final weight (FW) within each supplemented diet, for 4, 8, and 12 weeks, as compared to the control group (P < 0.05; Table 2). Fish fed diet 4, a combination of CDXOS and L. plantarum CR1T5 (LP), had the highest FW, WG, and SGR values (Table 2), yet had the lowest feed conversion ratio (FCR). The highest FCR was found in fish from the control group (diet 1) (P < 0.05). However, there were no significant differences in any of the parameters in fish fed diet 2 or 3 (P > 0.05; Table 2). There were also no effect on the survival rate detected among fish receiving either the control, or any of the supplemented diets (Table 2).
Innate Immune Parameters
Dietary supplementations of CDXOS (diet 2), LP (diet 3), and the combination of CDXOS and LP (diet 4) each significantly enhanced skin mucus lysozyme and peroxidase activities (SMPA), compared with the control group, after each of the three feeding periods (P < 0.05; Table 3). The highest values were found in fish fed diet 4, followed by fish fed diet 3, and diet 2. However, no significant differences were detected in mucus parameters among fish fed the control and the supplemented diets after 4 weeks, or between fish fed diet 2 and 3 (P > 0.05; Table 3).
Differences were observed in serum lysozyme activity between control and the supplemented groups (Table 4), in which diets 2, 3, and 4 produced higher serum lysozyme activity than that of the control diet (P < 0.05; Table 4). The highest serum lysozyme activity (SL) was detected in fish fed diet 4, whereas no significant difference was observed between the CDXOS (diet 2) and LP (diet 3) supplemented diets (P > 0.05; Table 4). Similarly, alternative complement activity (ACH50), and phagocytosis activity (PI) increased in fish fed the supplemented diets, compared with the control; the highest values being generated by diet 4 (Table 4). No significant differences were detected in the values of fish fed the CDXOS (diet 2) and the LP (diet 3) diets, regarding seric lysozyme, complement activity, and phagocytosis (P > 0.05; Table 4). Higher levels of serum peroxidase activity resulted in fish fed the supplemented diets, versus those fed the control diet, though no significant differences were observed among the supplemented diets. Lastly, significant differences in respiratory burst activity were detected between fish fed supplemented diets and the control diet, but only at 4 weeks (Table 4).
Challenge Test
The challenge test using S. agalactiae was carried out after 12 weeks of feeding, and the survival rate was recorded over the following 15 days. Dead fish exhibited a loss of appetite, darkness, exophthalmia, fins basal hemorrhage, and pale liver, which are typical symptoms of Streptococcus infection. The results show that the survival rates of fish fed CDXOS (diet 2), 56.25%; LP (diet 3), 59.38%; and the combination of CDXOS + LP (diet 4), 71.88%, were significantly higher (P < 0.05) than that recorded for fish fed the control diet 31.25% (Fig. 1). Among the supplemented groups, fish fed diet 4 showed significantly higher RPS than those within other groups, as well as the greatest resistance to S. agalactiae (Fig. 1).
Survival rate of tilapia, O. niloticus fed different concentrations of dietary CDXOS and L. plantarum (n = 8, mean ± SD): diet 1 (control), diet 2 (10 g kg−1 CDXOS), diet 3 (108 CFU g−1L. plantarum), and diet 4 (10 g kg−1 CDXOS +108 CFU g−1L. plantarum) during 15 days post-challenge with S. agalactiae
Discussion
Because of today’s growing restrictions on the use of antibiotics as growth promoters, safe and natural feed additives are being enthusiastically investigated as alternatives to enhance growth performance and to protect against diseases [69]. A wide range of feed additives, like probiotics and prebiotics, which distribute have positive effects upon the host, have been applied in aquaculture, in which to control diseases, promote growth, and enhance the host’s immune response [70]. Such functional feed additives have gained great attention for their beneficial effects in enhancing production and the well-being of farmed fish, as well as for increasing overall resistance to diseases [71].
Dietary supplementation of corncob-derived xylooligosaccharides (CDXOS) and L. plantarum CR1T5 (LP), either alone or combined, produced significantly positive effects on final weight, weight gain, specific growth rate, and feed conversion ratio of Nile tilapia. To the best of our knowledge, this is the first investigation that has demonstrated such positive effect on growth performance of Nile tilapia. In line with the present study, the positive effects of XOS, Lactobacillus plantarum CR1T5, and other synbiotics were previously reported in European sea bass (Dicentrarchus labrax) [72, 73], blunt snout bream (Megalobrama amblycephala) [74], sea cucumber (Apostichopus japonicus) [75, 76], Nile tilapia (Oreochromis niloticus) [44,45,46, 48, 77], angelfish (Pterophyllum scalare) [78], snakehead (Channa striata) [79, 80], rockfish (Sebastes schlegeli) [81], Asian sea bass (Lates calcalifer) [82], and major carp (Cirrhinus mrigala) [83]. In contrast, Abid et al. [84] reported that a P. acidilactici and scFOS supplemented diet had no effect on the growth performance of Atlantic salmon. Similarly, probiotic or prebiotic supplemented diets had no effect on the growth or survival of Totoaba (Totoaba macdonaldi) [85]. The discrepancies in these findings may attributable to differences in species, experimental design, XOS form, and the method of administration [86, 87], as well as sampling strategy. Indeed, reports have confirmed that the effects of probiotics on fish depend on the dose and duration of the treatment, and the source, as well as on the species in question [88]. It has been further reported that the inclusion of pre- and probiotics in feed is associated with improved health status, improved prebiotic digestion, and an increase in probiotic survival and colonization, compared with individual pre- or probiotic applications [89,90,91,92]. These effects were most probably mediated by short-chain fatty acids, as by-products of fermentation of probiotic strains in the existence of prebiotics [81, 93]. Yu et al. [94] reported that acetate was the dominant short-chain fatty acid found to result from the fermentation process between xylooligosaccharides from corn cobs and L. plantarum. In addition to short-chain fatty acids, the dietary consumption of both pro- and prebiotics resulted in the formation of bioactive microbial metabolites, such as vitamins and biological peptides [95]. These, in turn, improved nutrient digestion and absorption in the host intestine and consequently had a positive effect on growth. As previously reported, this may be attributable to the favorable effects of XOS that help normalize gut microbiota, and enhance the gut digestive and absorptive capabilities of fish [96], and may have led to the improvement of feed utilization in the present study.
The innate immune system of farmed fish is considered a crucial defense system, which provides protection against opportunistic pathogens [97]. The present investigation indicated that the dietary inclusion of prebiotics, probiotics, and a synbiotic resulted in an increase in serum lysozyme, serum peroxidase, ACH50, phagocytic, and respiratory activities. Also, the synbiotic diet led to higher non-specific immune parameters than in the other groups. To the best of our knowledge, there exists no information regarding the effects of CDXOS, Lactobacillus plantarum CR1T5, or their combination on the innate immunological parameters of Nile tilapia. Nonetheless, in agreement with present results, Hoseinifar et al. [98] revealed that serum ACH50 and lysozyme activities significantly increased in rainbow trout fingerlings fed the pre-, pro-, and synbiotic diets. Recently, Kumar et al. [83] showed that the dietary inclusion of mannanoligosaccharide (MOS) and Bacillus subtilis as a synbiotic significantly stimulated the innate immunological parameters in major carp (Cirrhinus mrigala). Similarly, a significant improvement in the innate immune response was observed in rockfish (Sebastes schlegeli), fed Pediococcus acidilactici, galactooligosaccharide, and synbiotic additives [81]; snakehead (Channa striata), fed the prebiotics GOS and MOS in combination with Saccharomyces cerevisiae and L. acidophilus [80]; and Asian sea bass (Lates calcalifer) fed low molecular weight sodium alginate and Pediococcus acidilactici [82]. However, the dietary inclusion of Bacillus subtilis and chitosan [91] as well as B. subtilis and FOS [90] failed to stimulate immune parameters of cobia (Rachycentron canadum) and yellow croaker (Larimichthys crocea), respectively. The discrepancies of these findings may be due partly to species-specific, fish ages, and/or pre- or probiotic doses and types [99].
The mucosal immune system of fish consists of a unique array of specific and innate immune cells, which include lymphocytes, mast cells, macrophages, and granulocytes. It also contains molecules complement proteins, immunoglobulins, lysozyme, proteases, esterases, and antimicrobial peptides with antibacterial, anti-viral, and anti-fungal activities [100,101,102,103]. The present findings indicate that the dietary inclusion of prebiotic, probiotic, and synbiotic significantly increased skin mucus lysozyme and peroxidase activities in Nile tilapia. The fish that were fed the synbiotic diet obtained higher skin mucus immune parameters than the fish fed both components individually. The results were similar to previous findings involving the supplementation of heat-killed L. plantarum and β-glucan in red sea bream (Pagrus major) [104], Pediococcus acidilactici and galactooligosaccharide in common carp (Cyprinus carpio) [105], Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum in Nile tilapia (Oreochromis niloticus) [45], Pediococcus acidilactici and GOS in rainbow trout and rock fish [81], and low molecular weight sodium alginate and P. acidilactici in Asian sea bass (Lates calcalifer) [82]. The significant improvement observed in the immune response of fish in the present study may be attributed to the effects of CDXOS and L. plantarum CR1T5. Yu et al. [94] demonstrated that a combination of CDXOS and L. plantarum in an in vivo model could increase the number of lactobacilli and bifidobacteria in mouse feces, and reduce the viability of Enterococcus, Enterobacter, and Clostridia spp. Additionally, an in vitro antioxidant assay indicated that CDXOS fermented with L. plantarum possessed significant 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azino-bis, and superoxide anion radical-scavenging activities. However, the combination effects of CDXOS and L. plantarum CR1T5 merit further investigation.
It is well-known that nutritional manipulation is a useful means to enhance disease resistance in fish [106]. Synbiotic therapy is also regarded as an effective method of disease prevention [107]. The results of the present study showed that Nile tilapia fed prebiotic, probiotic, and synbiotic diets significantly increased resistance to Streptococcus agalactiae, of which the highest resistance was detected in fish fed the synbiotic diet. The benefit for the fish’s immune system provided by CDXOS and L. plantarum CR1T5 was demonstrated through the increased resistance to S. agalactiae infection in Nile tilapia through these supplements. Similar results, in which dietary supplementation with pre-, pro-, and synbiotic significantly increased disease resistance, were observed in hybrid tilapia (Oreochromis niloticus × O. aureus), against Aeromonas hydrophilla [108]; sea cucumber (Apostichopus japonicus), against Vibrio splendidus [76]; major carp (Cirrhinus mrigala), against Aeromonas hydrophila [83]; rockfish (Sebastes schlegeli), against Edwardsiella tarda [81]; and European sea bass (D. labrax), against V. anguillarum [73]. These significant increases in disease resistance may be due to the combined effects of CDXOS and L. plantarum CR1T5. A recent study showed that cell-free L. plantarum supernatant added to the corncob XOS presented strong antibacterial activities against Shigella flexneri and E. coli, compared with the activity of Staphylococcus aureus and Salmonella typhimurium. Culturing L. plantarum in MRS broth in the presence of XOS revealed inhibition zones. This indicated that the antagonistic activities of this strain originate from alternative or simultaneous acid and hydrogen peroxide (H2O2) inhibition. These results suggest that XOS could stimulate the proliferation of favorable microbiota and increase the production of the antimicrobial substances in the cell-free supernatant, which would include organic acids, H2O2, bacteriocins, and low molecular mass peptides [94, 109]. In conclusion, data from the present study suggest that CDXOS and Lactobacillus plantarum CR1T5, applied singularly or in combination, can be used as functional feed additives for better growth performance and S. agalactiae resistance in tilapia aquaculture.
References
Pulkkinen K, Suomalainen LR, Read AF, Ebert D, Rintamäki P, Valtonen ET (2010) Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proceedings Biological sciences 277(1681):593–600. https://doi.org/10.1098/rspb.2009.1659
Faggio C, Piccione G, Marafioti S, Arfuso F, Trischitta F, Fortino G, Fazio F (2014) Monthly variations of haematological parameters of Sparus aurata and Dicentrarchus labrax reared in Mediterranean land off-shore tanks. Cah Biol Mar 55:437–443
Fazio F, Marafioti S, Torre A, Sanfilippo M, Panzera M, Faggio C (2013) Haematological and serum protein profiles of Mugil cephalus: effect of two different habitats. Ichthyol Res 60(1):36–42. https://doi.org/10.1007/s10228-012-0303-1
Fazio F, Marafioti S, Arfuso F, Piccione G, Faggio C (2013) Influence of different salinity on haematological and biochemical parameters of the widely cultured mullet, Mugil cephalus. Mar Freshw Behav Phy 46(4):211–218. https://doi.org/10.1080/10236244.2013.817728
Faggio C, Piccione G, Marafioti S, Arfuso F, Fortino G, Fazio F (2014) Metabolic response to monthly variations of Sparus aurata reared in Mediterranean on-shore tanks. Turk J Fish Aquat Sc 14(2):567–574
Faggio C, Fedele G, Arfuso F, Panzera M, Fazio F (2014) Haematological and biochemical response of Mugil cephalus after acclimation to captivity. Cah Biol Mar 55:31–36
Fazio F, Marafioti S, Filiciotto F, Buscaino G, Panzera M, Faggio C (2013) Blood hemogram profiles of farmed onshore and offshore gilthead sea bream (Sparus aurata) from Sicily, Italy. Turk J Fish Aquat Sc 13(3):415–422
Fazio F, Faggio C, Marafioti S, Torre A, Sanfilippo M, Piccione G (2012) Comparative study of haematological profile on Gobius niger in two different habitat sites: Faro Lake and Tyrrhenian Sea. Cah Biol Mar 53(1):213–219
Done HY, Venkatesan AK, Halden RU (2015) Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J 17(3):513–524. https://doi.org/10.1208/s12248-015-9722-z
Santos L, Ramos F (2018) Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Ag 52(2):135–143. https://doi.org/10.1016/j.ijantimicag.2018.03.010
Newaj-Fyzul A, Al-Harbi AH, Austin B (2014) Review: developments in the use of probiotics for disease control in aquaculture. Aquacult 431:1–11. https://doi.org/10.1016/j.aquaculture.2013.08.026
Gatesoupe FJ (2010) Probiotics and other microbial manipulations in fish feeds: prospective health benefits. In: Bioactive foods in promoting health. Elsivier, In, pp 541–552. https://doi.org/10.1016/B978-0-12-374938-3.00032-3
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14(3):251–258. https://doi.org/10.1016/j.mib.2011.03.004
Akhter N, Wu B, Memon AM, Mohsin M (2015) Probiotics and prebiotics associated with aquaculture: a review. Fish Shellfish Immunol 45(2):733–741. https://doi.org/10.1016/j.fsi.2015.05.038
Huynh T-G, Shiu Y-L, Nguyen T-P, Truong Q-P, Chen J-C, Liu C-H (2017) Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: a review. Fish Shellfish Immunol 64:367–382. https://doi.org/10.1016/j.fsi.2017.03.035
Guardiola FA, Porcino C, Cerezuela R, Cuesta A, Faggio C, Esteban MA (2016) Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol 52:298–308. https://doi.org/10.1016/j.fsi.2016.03.152
Nath S, Matozzo V, Bhandari D, Faggio C (2018) Growth and liver histology of Channa punctatus exposed to a common biofertilizer. Nat Prod Res:1–8
Capillo G, Savoca S, Costa R, Sanfilippo M, Rizzo C, Lo Giudice A, Albergamo A, Rando R, Bartolomeo G, Spanò N (2018) New insights into the culture method and antibacterial potential of Gracilaria gracilis. Mar Drugs 16(12):492
Carbone D, Faggio C (2016) Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol 54:172–178. https://doi.org/10.1016/j.fsi.2016.04.011
Faggio C, Fazio F, Marafioti S, Arfuso F, Piccione G (2015) Oral administration of Gum Arabic: effects on haematological parameters and oxidative stress markers in Mugil cephalus. Iran J Fish Sci 14(1):60–72
Hoseinifar SH, Yousefi S, Capillo G, Paknejad H, Khalili M, Tabarraei A, Van Doan H, Spanò N, Faggio C (2018) Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol 83:232–237. https://doi.org/10.1016/j.fsi.2018.09.046
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Opinion: towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12(5):303–310. https://doi.org/10.1038/nrgastro.2015.47
Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, Tuohy K (2008) FAO technical meeting on prebiotics. J Clin Gastroenterol 42(Suppl 3 Pt 2):S156–S159. https://doi.org/10.1097/MCG.0b013e31817f184e
Choque Delgado GT, WMdSC T, MRM J, YMF M, Pastore GM (2011) The putative effects of prebiotics as immunomodulatory agents. Food Res Int 44(10):3167–3173. https://doi.org/10.1016/j.foodres.2011.07.032
Hoseinifar SH, Esteban MÁ, Cuesta A, Sun Y-Z (2015) Prebiotics and fish immune response: a review of current knowledge and future perspectives. Rev Fish Sci Aquac 23(4):315–328. https://doi.org/10.1080/23308249.2015.1052365
Song SK, Beck BR, Kim D, Park J, Kim J, Kim HD, Ringø E (2014) Prebiotics as immunostimulants in aquaculture: a review. Fish Shellfish Immunol 40(1):40–48. https://doi.org/10.1016/j.fsi.2014.06.016
Buruiana C-T, Gómez B, Vizireanu C, Garrote G (2017) Manufacture and evaluation of xylooligosaccharides from corn stover as emerging prebiotic candidates for human health. LWT-Food Sci Technol 77:449–459. https://doi.org/10.1016/j.lwt.2016.11.083
Chapla D, Pandit P, Shah A (2012) Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour Technol 115(supplement C):215–221. https://doi.org/10.1016/j.biortech.2011.10.083
Statista (2019) Worldwide production of grain in 2018/19, by type. https://www.statista.com/statistics/263977/world-grain-production-by-type/. Accessed 15 April 2019
Aachary AA, Prapulla SG (2009) Value addition to corncob: production and characterization of xylooligosaccharides from alkali pretreated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Bioresour Technol 100(2):991–995. https://doi.org/10.1016/j.biortech.2008.06.050
Sun J, Zhang Z, Xiao F, Jin X (2015) Production of xylooligosaccharides from corncobs using ultrasound-assisted enzymatic hydrolysis. Food Sci Biotechnol 24(6):2077–2081. https://doi.org/10.1007/s10068-015-0276-8
Salas-Veizaga DM, Villagomez R, Linares-Pastén JA, Carrasco C, Álvarez MT, Adlercreutz P, Nordberg Karlsson E (2017) Extraction of glucuronoarabinoxylan from quinoa stalks (Chenopodium quinoa Willd.) and evaluation of xylooligosaccharides produced by GH10 and GH11 xylanases. J Agric Food Chem 65(39):8663–8673. https://doi.org/10.1021/acs.jafc.7b01737
Carvalho AFA, Neto PO, da Silva DF, Pastore GM (2013) Xylo-oligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res Int 51(1):75–85. https://doi.org/10.1016/j.foodres.2012.11.021
Gullón P, Gullón B, Cardelle-Cobas A, Alonso JL, Pintado M, Gomes AM (2014) Effects of hemicellulose-derived saccharides on behavior of lactobacilli under simulated gastrointestinal conditions. Food Res Int 64:880–888. https://doi.org/10.1016/j.foodres.2014.08.043
Jagtap S, Deshmukh RA, Menon S, Das S (2017) Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour Technol 245:283–288. https://doi.org/10.1016/j.biortech.2017.08.174
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with liver lactic acid bacteria. Food and Agriculture Organization and World Health Organization Joint report. http://www.fao.org/3/a-a0512e.pdf. Accessed 15 January 2019
Dawood MAO, Koshio S (2016) Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquacult 454:243–251. https://doi.org/10.1016/j.aquaculture.2015.12.033
Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI (2016) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol 57:170–178. https://doi.org/10.1016/j.fsi.2016.08.038
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2016) Effects of dietary inactivated Pediococcus pentosaceus on growth performance, feed utilization and blood characteristics of red sea bream, Pagrus major juvenile. Aquac Nutr 22(4):923–932. https://doi.org/10.1111/anu.12314
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Calo-Mata P, Arlindo S, Boehme K, de Miguel T, Pascoal A, Barros-Velazquez J (2008) Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products. Food Bioprocess Tech 1:43–63. https://doi.org/10.1007/s11947-007-0021-2
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquacult 274(1):1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Yu L, Zhai Q, Zhu J, Zhang C, Li T, Liu X, Zhao J, Zhang H, Tian F, Chen W (2017) Dietary Lactobacillus plantarum supplementation enhances growth performance and alleviates aluminum toxicity in tilapia. Ecotoxicol Environ Saf 143:307–314
Van Doan H, Hoseinifar SH, Dawood MAO, Chitmanat C, Tayyamath K (2017) Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 70(Supplement C):87–94. https://doi.org/10.1016/j.fsi.2017.09.002
Van Doan H, Hoseinifar SH, Tapingkae W, Tongsiri S, Khamtavee P (2016) Combined administration of low molecular weight sodium alginate boosted immunomodulatory, disease resistance and growth enhancing effects of Lactobacillus plantarum in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 58:678–685. https://doi.org/10.1016/j.fsi.2016.10.013
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A, Bai SC (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 61(Supplement C):201–210. https://doi.org/10.1016/j.fsi.2016.12.035
Van Doan H, Hoseinifar SH, Khanongnuch C, Kanpiengjai A, Unban K, Van Kim V, Srichaiyo S (2018) Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquacult 491:94–100. https://doi.org/10.1016/j.aquaculture.2018.03.019
Beck BR, Kim D, Jeon J, Lee SM, Kim HK, Kim OJ, Lee JI, Suh BS, Do HK, Lee KH, Holzapfel WH, Hwang JY, Kwon MG, Song SK (2015) The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 42(1):177–183. https://doi.org/10.1016/j.fsi.2014.10.035
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34(2):660–666. https://doi.org/10.1016/j.fsi.2012.12.008
FAO/WHO (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. FAO and WHO, Rome Accessed 26 April 2019
Van Doan H, Hoseinifar SH, Faggio C, Chitmanat C, Mai NT, Jaturasitha S, Ringø E (2018) Effects of corncob derived xylooligosaccharide on innate immune response, disease resistance, and growth performance in Nile tilapia (Oreochromis niloticus) fingerlings. Aquacult 495:786–793. https://doi.org/10.1016/j.aquaculture.2018.06.068
Yoon KY, Woodams EE, Hang YD (2006) Enzymatic production of pentoses from the hemicellulose fraction of corn residues. LWT - Food Sci Technol 39(4):388–392. https://doi.org/10.1016/j.lwt.2005.02.005
Boonchuay P, Techapun C, Seesuriyachan P, Chaiyaso T (2014) Production of xylooligosaccharides from corncob using a crude thermostable endo-xylanase from Streptomyces thermovulgaris TISTR1948 and prebiotic properties. Food Sci Biotechnol 23(5):1515–1523. https://doi.org/10.1007/s10068-014-0207-0
Meidong R, Doolgindachbaporn S, Sakai K, Tongpim S (2017) Isolation and selection of lactic acid bacteria from Thai indigenous fermented foods for use as probiotics in tilapia fish Oreochromis niloticus. AACL Bioflux 10(2):455–463
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25(6):333–342. https://doi.org/10.1046/j.1365-2761.2002.00375.x
Van Doan H, Hoseinifar SH, Tapingkae W, Khamtavee P (2017) The effects of dietary kefir and low molecular weight sodium alginate on serum immune parameters, resistance against Streptococcus agalactiae and growth performance in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 62:139–146. https://doi.org/10.1016/j.fsi.2017.01.014
Ross NW, Firth KJ, Wang A, Burka JF, Johnson SC (2000) Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis Aquat Org 41(1):43–51. https://doi.org/10.3354/dao041043
Van Doan H, Tapingkae W, Moonmanee T, Seepai A (2016) Effects of low molecular weight sodium alginate on growth performance, immunity, and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 55:186–194. https://doi.org/10.1016/j.fsi.2016.05.034
Chung S, Secombes CJ (1988) Analysis of events occurring within teleost macrophages during the respiratory burst. Comp Biochem Physiol B 89(3):539–544. https://doi.org/10.1016/0305-0491(88)90171-X
Parry RM, Chandan RC, Shahani KM (1965) A rapid and sensitive assay of muramidase. Exp Biol Med (Maywood) 119(2):384–386. https://doi.org/10.3181/00379727-119-30188
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58(3–4):239–248
Cordero H, Cuesta A, Meseguer J, Esteban MA (2016) Changes in the levels of humoral immune activities after storage of gilthead seabream (Sparus aurata) skin mucus. Fish Shellfish Immunol 58:500–507. https://doi.org/10.1016/j.fsi.2016.09.059
Yoshida T, Kitao T (1991) The opsonic effect of specific immune serum on the phagocytic and chemiluminescent response in rainbow trout, Oncorhynchus mykiss phagocytes. Fish Pathol 26(1):29–33. https://doi.org/10.3147/jsfp.26.29
Secombes CJ (1990) Isolation of salmonid macrophage and analysis of their killing ability. Techniques in fish immunology. SOS Publication, New Jersey
Yanno T (1992) Assays of hemolitic complement activity. In: Stolen JS, Fletcher TC, Anderson DP, Kaatari SL, Roley AF (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 131–141
Wang B, Gan Z, Cai S, Wang Z, Yu D, Lin Z, Lu Y, Wu Z, Jian J (2016) Comprehensive identification and profiling of Nile tilapia (Oreochromis niloticus) microRNAs response to Streptococcus agalactiae infection through high-throughput sequencing. Fish Shellfish Immunol 54:93–106. https://doi.org/10.1016/j.fsi.2016.03.159
SAS (2003). SAS Institute Inc, SAS Campus Drive, Cary, NC USA 27513
Millet S, Maertens L (2011) The European ban on antibiotic growth promoters in animal feed: from challenges to opportunities. Vet J 187(2):143–144. https://doi.org/10.1016/j.tvjl.2010.05.001
Markowiak P, Śliżewska K (2018) The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog 10:21. https://doi.org/10.1186/s13099-018-0250-0
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Güroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish Shellfish Immunol 30(1):1–16. https://doi.org/10.1016/j.fsi.2010.08.009
Guerreiro I, Oliva-Teles A, Enes P (2015) Improved glucose and lipid metabolism in European sea bass (Dicentrarchus labrax) fed short-chain fructooligosaccharides and xylooligosaccharides. Aquacult 441:57–63. https://doi.org/10.1016/j.aquaculture.2015.02.015
Torrecillas S, Rivero-Ramírez F, Izquierdo MS, Caballero MJ, Makol A, Suarez-Bregua P, Fernández-Montero A, Rotllant J, Montero D (2018) Feeding European sea bass (Dicentrarchus labrax) juveniles with a functional synbiotic additive (mannan oligosaccharides and Pediococcus acidilactici): an effective tool to reduce low fishmeal and fish oil gut health effects? Fish Shellfish Immunol 81:10–20. https://doi.org/10.1016/j.fsi.2018.07.007
Abasubong KP, Liu WB, Zhang DD, Yuan XY, Xia SL, Xu X, Li XF (2018) Fishmeal replacement by rice protein concentrate with xylooligosaccharides supplement benefits the growth performance, antioxidant capability and immune responses against Aeromonas hydrophila in blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol In press 78:177–186. https://doi.org/10.1016/j.fsi.2018.04.044
Li C, Ren Y, Jiang S, Zhou S, Zhao J, Wang R, Li Y (2018) Effects of dietary supplementation of four strains of lactic acid bacteria on growth, immune-related response and genes expression of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol 74:69–75. https://doi.org/10.1016/j.fsi.2017.12.037
Wang X, Sun Y, Wang L, Li X, Qu K, Xu Y (2017) Synbiotic dietary supplement affects growth, immune responses and intestinal microbiota of Apostichopus japonicus. Fish Shellfish Immunol 68:232–242. https://doi.org/10.1016/j.fsi.2017.07.027
Hamdan AM, El-Sayed AF, Mahmoud MM (2016) Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). J Appl Microbiol 120(4):1061–1073. https://doi.org/10.1111/jam.13081
Azimirad M, Meshkini S, Ahmadifard N, Hoseinifar SH (2016) The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish Immunol 54:516–522
Munir MB, Hashim R, Chai YH, Marsh TL, Nor SAM (2016) Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings. Aquacult 460:59–68. https://doi.org/10.1016/j.aquaculture.2016.03.041
Munir MB, Hashim R, Nor SAM, Marsh TL (2018) Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol 75:99–108. https://doi.org/10.1016/j.fsi.2018.02.005
Rahimnejad S, Guardiola FA, Leclercq E, Ángeles Esteban M, Castex M, Sotoudeh E, Lee S-M (2018) Effects of dietary supplementation with Pediococcus acidilactici MA18/5M, galactooligosaccharide and their synbiotic on growth, innate immunity and disease resistance of rockfish (Sebastes schlegeli). Aquacult 482:36–44. https://doi.org/10.1016/j.aquaculture.2017.09.020
Ashouri G, Mahboobi Soofiani N, Hoseinifar SH, Jalali SAH, Morshedi V, Van Doan H, Torfi Mozanzadeh M (2018) Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5M on growth performance, haematological and innate immune responses of Asian sea bass (Lates calcalifer) juveniles. Fish Shellfish Immunol 79:34–41. https://doi.org/10.1016/j.fsi.2018.05.009
Kumar P, Jain KK, Sardar P (2018) Effects of dietary synbiotic on innate immunity, antioxidant activity and disease resistance of Cirrhinus mrigala juveniles. Fish Shellfish Immunol 80:124–132. https://doi.org/10.1016/j.fsi.2018.05.045
Abid A, Davies S, Waines P, Emery M, Castex M, Gioacchini G, Carnevali O, Bickerdike R, Romero J, Merrifield D (2013) Dietary synbiotic application modulates Atlantic salmon (Salmo salar) intestinal microbial communities and intestinal immunity. Fish Shellfish Immunol 35(6):1948–1956
González-Félix ML, Gatlin Iii DM, Urquidez-Bejarano P, de la Reé-Rodríguez C, Duarte-Rodríguez L, Sánchez F, Casas-Reyes A, Yamamoto FY, Ochoa-Leyva A, Perez-Velazquez M (2018) Effects of commercial dietary prebiotic and probiotic supplements on growth, innate immune responses, and intestinal microbiota and histology of Totoaba macdonaldi. Aquacult 491:13. https://doi.org/10.1016/j.aquaculture.2018.03.031
Hoseinifar SH, Khalili M, Rostami HK, Esteban MÁ (2013) Dietary galactooligosaccharide affects intestinal microbiota, stress resistance, and performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immunol 35(5):1416–1420
Hoseinifar SH, Zare P, Merrifield DL (2010) The effects of inulin on growth factors and survival of the Indian white shrimp larvae and postlarvae (Fenneropenaeus indicus). Aquac Res 41(9):e348–e352. https://doi.org/10.1111/j.1365-2109.2010.02485.x
Hai NV (2015) Research findings from the use of probiotics in tilapia aquaculture: a review. Fish Shellfish Immunol 45(2):592–597. https://doi.org/10.1016/j.fsi.2015.05.026
Cerezuela R, Guardiola FA, Meseguer J, Esteban MÁ (2012) Increases in immune parameters by inulin and Bacillus subtilis dietary administration to gilthead seabream (Sparus aurata L.) did not correlate with disease resistance to Photobacterium damselae. Fish Shellfish Immunol 32(6):1032–1040. https://doi.org/10.1016/j.fsi.2012.02.025
Ai Q, Xu H, Mai K, Xu W, Wang J, Zhang W (2011) Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquacult 317(1–4):155–161. https://doi.org/10.1016/j.aquaculture.2011.04.036
Geng X, Dong X-H, Tan B-P, Yang Q-H, Chi S-Y, Liu H-Y, Liu X-Q (2011) Effects of dietary chitosan and Bacillus subtilis on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Fish Shellfish Immunol 31(3):400–406. https://doi.org/10.1016/j.fsi.2011.06.006
Ye JD, Wang K, Li FD, Sun YZ (2011) Single or combined effects of fructo- and mannan oligosaccharide supplements and Bacillus clausii on the growth, feed utilization, body composition, digestive enzyme activity, innate immune response and lipid metabolism of the Japanese flounder Paralichthys olivaceus. Aquac Nutr 17(4):e902–e911. https://doi.org/10.1111/j.1365-2095.2011.00863.x
Hoseinifar SH, Mirvaghefi A, Amoozegar MA, Merrifield DL, Ringø E (2017) In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac Nutr 23(1):111–118. https://doi.org/10.1111/anu.12373
Yu X, Yin J, Li L, Luan C, Zhang J, Zhao C, Li S (2015) Prebiotic potential of xylooligosaccharides derived from corn cobs and their in vitro antioxidant activity when combined with Lactobacillus. J Microbiol Biotechnol 25(7):1084–1092. https://doi.org/10.4014/jmb.1501.01022
Stanton C, Ross RP, Fitzgerald GF, Van Sinderen D (2005) Fermented functional foods based on probiotics and their biogenic metabolites. Curr Opin Biotechnol 16(2):198–203. https://doi.org/10.1016/j.copbio.2005.02.008
Xu B, Wang Y, Li J, Lin Q (2009) Effect of prebiotic xylooligosaccharides on growth performances and digestive enzyme activities of allogynogenetic crucian carp (Carassius auratus gibelio). Fish Physiol Biochem 35(3):351–357. https://doi.org/10.1007/s10695-008-9248-8
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20(2):137–151. https://doi.org/10.1016/j.fsi.2004.09.006
Hoseinifar SH, Mirvaghefi A, Amoozegar MA, Sharifian M, Esteban MÁ (2015) Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout (Oncorhynchus mykiss) upon synbiotic feeding. Fish Shellfish Immunol 45(1):27–32. https://doi.org/10.1016/j.fsi.2015.03.029
Hoseinifar SH, Ringø E, Shenavar Masouleh A, Esteban MÁ (2016) Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: a review. Rev Aquacult 8(1):89–102. https://doi.org/10.1111/raq.12082
Burgos-Aceves MA, Cohen A, Smith Y, Faggio C (2016) Estrogen regulation of gene expression in the teleost fish immune system. Fish Shellfish Immunol 58:42–49. https://doi.org/10.1016/j.fsi.2016.09.006
Kabat AM, Pott J, Maloy KJ (2016) The mucosal immune system and its regulation by autophagy. Front Immunol 7(240). https://doi.org/10.3389/fimmu.2016.00240
Lauriano ER, Pergolizzi S, Capillo G, Kuciel M, Alesci A, Faggio C (2016) Immunohistochemical characterization of toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol 59:250–255. https://doi.org/10.1016/j.fsi.2016.11.003
Lazado CC, Caipang CMA (2014) Mucosal immunity and probiotics in fish. Fish Shellfish Immunol 39(1):78–89. https://doi.org/10.1016/j.fsi.2014.04.015
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2015) Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol 45(1):33–42. https://doi.org/10.1016/j.fsi.2015.01.033
Modanloo M, Soltanian S, Akhlaghi M, Hoseinifar SH (2017) The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol 70(supplement C):391–397. https://doi.org/10.1016/j.fsi.2017.09.032
Oliva-Teles A (2012) Nutrition and health of aquaculture fish. J Fish Dis 35(2):83–108. https://doi.org/10.1111/j.1365-2761.2011.01333.x
Cerezuela R, Meseguer J, Esteban MA (2011) Current knowledge in synbiotic use for fish aquaculture: a review. J Aquac Res Development S1(008). https://doi.org/10.4172/2155-9546.S1-008
Liu W, Wang W, Ran C, He S, Yang Y, Zhou Z (2017) Effects of dietary scFOS and lactobacilli on survival, growth, and disease resistance of hybrid tilapia. Aquacult 470:50–55. https://doi.org/10.1016/j.aquaculture.2016.12.013
Makras L, Triantafyllou V, Fayol-Messaoudi D, Adriany T, Zoumpopoulou G, Tsakalidou E, Servin A, De Vuyst L (2006) Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res Microbiol 157(3):241–247. https://doi.org/10.1016/j.resmic.2005.09.002
Acknowledgments
Thanks are also due to Assoc. Prof. Dr. Saowanit Tongpim, Assoc. Prof. Dr. Supamit Meckchay, and Assist. Prof. Dr. Chanagun Chitmanat for their kind assistance. Finally, the authors would like to thank the staff of the Central and Biotechnology Laboratories, Faculty of Agriculture, Chiang Mai University for their kind support during the data analysis process. The support of the Fundación Séneca de la Región de Murcia (grant number 19883/GERM/15) is also acknowledged.
Funding
This study received financial support from the Thai Research Fund (TRF) (Grant No. MRG5980127).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
The study was performed in accordance with the guidelines on use of animals for scientific purposes (Chiang Mai University).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van Doan, H., Hoseinifar, S.H., Tapingkae, W. et al. Boosted Growth Performance, Mucosal and Serum Immunity, and Disease Resistance Nile Tilapia (Oreochromis niloticus) Fingerlings Using Corncob-Derived Xylooligosaccharide and Lactobacillus plantarum CR1T5. Probiotics & Antimicro. Prot. 12, 400–411 (2020). https://doi.org/10.1007/s12602-019-09554-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09554-5