Abstract
The effect of prebiotic xylooligosaccharides (XOS) on the growth performance and digestive enzyme activities of the allogynogenetic crucian carp, Carassius auratus gibelio, was investigated. XOS was added to fish basal semi-purified diets at three concentrations by dry feed weight: diet 1, 50 mg kg−1; diet 2, 100 mg kg−1; diet 3, 200 mg kg−1, respectively. Twelve aquaria (n = 20) with three replicates for each treatment group (diets 1–3) and control treated without XOS were used. Weights of all collected carp from each aquarium were determined at the initial phase and at the end of the experiment, and the carp survival was also determined by counting the individuals in each aquarium. After 45 days, there were significant differences (P < 0.05) in the relative gain rate (RGR), and daily weight gain (DWG) of diets 1–3 were compared with the control. However, the survival rate was not affected (P > 0.05) by the dietary treatments. For enzymatic analysis, dissection produced a crude mixture of intestine and hepatopancreas of each segment to measure. The protease activity in the intestine and hepatopancreas content of fish in diet 2 (487.37 ± 20.58 U g−1 and 20.52 ± 1.93 U g−1) were significantly different (P < 0.05) from that in the control (428.13 ± 23.26 U g−1 and 12.81 ± 1.52 U g−1) and diet 3 (428.00 ± 23.78 U g−1 and 14.04 ± 1.59 U g−1). Amylase activity in the intestine was significantly higher for diet 2 compared to diet 1 and the control. As for amylase in the hepatopancreas, assays showed higher activity in diet 2 (P < 0.05) compared to the rest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing economic and social concerns of decreasing the use of antibiotics and other chemicals used in fish farming have encouraged more environmentally friendly approaches for increasing growth (Verschuere et al. 2000). Therefore, the need for alternative techniques is increasing, and the contribution of prebiotics such as xylooligosaccharides (XOS) may be considerable. XOS, fructooligosaccharides (FOS), inulin, and other related carbohydrates have received considerable attention because of the health benefits they are believed to confer on the host (Mussatto and Mancilha 2007; Cerezuela et al. 2008). These so-called prebiotic carbohydrates are defined as "nondigestible food ingredient(s) that beneficially affect host health by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon" (Gibson and Roberfroid 1995). The biological functions of these prebiotics are not fully understood (Boehm et al. 2005). However, there is evidence that they play an important role in generating a colonic microflora that comprises predominantly bifidobacteria in young mammals (Houdijk et al. 1998; Erney et al. 2000; Costalos et al. 2008). Moreover, due to the decrease of the intestinal pH caused by their fermentation, oligosaccharides added as prebiotics provoke an increase of animal growth and the availability of minerals (Crittenden and Playne 1996; Spring 1999; Rivero-Urgell and Santamaria-Orleans 2001; Valancony et al. 2001; Olsen et al. 2001; Mussatto and Mancilha 2007). Thus, the popularity of oligosaccharide as a food and feed ingredient has strongly increased, mainly in the last few years.
Xylooligosaccharides (XOS) are xylose-based oligomers and have some specific characteristics that are driving research efforts to develop applications in fields related to the food and feed industries. Like other oligosaccharides, XOS are non-digestible and act as prebiotics promoting the growth of beneficial bifidobacteria in the colon of animals (Crittenden and Playne 1996; Torrecillas et al. 2007). Besides their effects in the large bowel, a range of additional biological activities such as antioxidant activity and a protective effect against lipid peroxidation were reported for XOS (Meyer 2004; Tuohy et al. 2005; Mussatto and Mancilha 2007). This study was designed to evaluate the application of XOS as a feed additive in the diet of allogynogenetic crucian carp, one of the most valuable freshwater fish species cultured in China.
Materials and methods
Diets and experimental design
Four treatments were carried out with allogynogenetic crucian carp (Carassius auratus gibelio). Twelve aquaria were used with three replicates by batch for three experimental and one control batch. The ingredients and chemical composition of the basal diets used in the experiment were according to Lovell (1989, 1998). The basal diet formulation and proximate composition are shown in Table 1.
The XOS was incorporated in diet 1 at 50 mg kg−1 of dry feed weight. Diet 2 contained XOS at 100 mg kg−1 of dry feed weight, and diet 3 was supplemented with 200 mg kg−1. The control batch received the basal diet. These ingredients and the XOS at definite concentration were mixed, extruded, and air-dried at room temperature. The diets were kept at −20°C until used. All ingredients and chemicals used were purchased from Sangon and East China Pharmaceuticals Company, Shanghai, China, and Longli Biotechnology Co., Shandong, China.
Healthy juveniles of the allogynogenetic crucian carp provided by the Fish Hatchery of Hangzhou, China, were acclimated in two concrete tanks (each measuring 400 × 150 × 100 cm) and were fed with basal feed twice daily for 2 weeks. Then healthy carp were distributed into 12 aquaria with an initial stocking density of 20 crucian carp per aquarium in the Laboratory of Aquaculture for 45 days of culture. All carp had similar initial weights (16.88–17.56 g). The experiment was conducted as a completely randomized design with four treatments (diets 1–3 and control).
Crucian carp were fed three times daily at 6:00, 12:00, and 18:00. Every day the food remaining in the aquaria was cleaned by siphoning before the second daily feeding. The survival was surveyed daily. Every third day, each aquarium was partially cleaned, including the crucian carp feces, and the water was partially changed (about 50%).
The aquaria were supplied with running fresh water that had been filtered through a special cotton filter (flow rate: 1 l min−1), then passed successively through a tungsten heater and degassing column packed with plastic rings (Zhenhua Electric Industrial Co., Ltd., China). The temperature range of the aquarium was 24–26°C. The water temperature was maintained by an air-conditioning apparatus installed in the laboratory at 28°C. The photoperiod was 12 h light and 12 h dark. The temperature and dissolved oxygen of water were measured daily, and analyses of total ammonium, nitrite, and pH levels were performed weekly using the Hach kit model DREL 2400 (Hach Company, Loveland, CO). Dissolved oxygen was maintained above 6 mg l−1 by aeration.
Sampling and analytical methods
The proximate composition including crude protein, crude fat, crude ash, gross energy, and moisture of basal diets was determined according to Zhang and Zhu (1998). Crude protein was determined using the Kjeltec Analyzer Unit (2300, Sweden), and crude fat was determined using the Soxtec Auto Extraction Unit (2050, Sweden). Gross energy was determined with an adiabatic bomb calorimeter (PARR 1281, USA). Weights of all crucian carp were determined at the beginning (initial weight) and at the end (final weight) of the experiment. At the same time, crucian carp survival was determined by counting the individuals in each aquarium. The relative gain rate (%) (RGR) was determined as follows: [mean final weight (g) − mean initial weight (g)]/mean initial weight (g) × 100%, and the daily weight gain (DWG) was calculated as: [mean final weight (g) − mean initial weight (g)]/45 days.
For enzymatic analysis, six allogynogenetic crucian carp fasted for 24 h were collected from each aquarium at the end of the trial and anesthetized in diluted MS-222 (ethyl 3-aminobenzoate methanesulfonate, Tricaine; Sigma) (1:2,500) in order to study the effect of XOS based on digestive enzyme activities. Intestine and hepatopancreas were dissected at 4°C and rinsed with cold distilled water according to the method of Huang et al. (1996, 1999). Their content was extracted and diluted 1/10 (w/v) and homogenized separately in PBS at pH 7.5 (1 g/10 ml) using a hand-held glass homogenizer at 4°C. The homogenate was then centrifuged at 4°C at 15,000 × g for 15 min. The supernatant was then stored at 4°C prior to analysis. All enzymatic assays were conducted within 24 h after extraction. Protease activity was measured by the method of Lowry et al. (1951) using Folin-phenol reagent, and amylase activity was quantified using a solution to reveal non-hydrolyzed starch (Jiang 1982; Worthington 1993). Enzyme activities including protease and amylase were both expressed as U g−1 intestine/hepatopancreas content.
Statistical analysis
Statistical analysis using one-way analysis of variance (ANOVA; Statistical Analysis System, SAS, version 6.03) and t-test was performed to find significant differences between the experiments (diets 1–3) and control. A significance level of P < 0.05 was used.
Results
Growth performances
Total ammonium (0–0.2 mg l−1), nitrite (0–0.1 mg l−1), and pH (7.0–7.4) were stable, and there was no obvious effect of XOS on the water quality in the present trial. Initial and final weights of allogynogenetic crucian carp given the four diets are shown in Table 2. Survival rate was not affected by the dietary treatments after 45 days of culture. At the beginning, no significant difference was observed in the initial weight between diets 1–3 and control (P > 0.05). There were significant differences (P < 0.05) in the RGR and DWG of diets 1–3 compared with the control. However, there were no remarkable differences (P > 0.05) in final weight between diet 1 and diet 2. As for RGR, diet 2 showed the highest value (0.34 ± 0.01%) compared to the others, and there were also no significant differences (P > 0.05) in RGR between diet 1 and diet 3. Fish fed on diet 2 displayed a higher weight (P < 0.05) DWG (0.1313 ± 0.0084 g day−1) compared to the fish that received diet 3 (0.1011 ± 0.0088 g day−1) (Table 2). Nevertheless, there was no significant difference (P > 0.05) in DWG of diet 1 compared with diet 2 and diet 3.
Enzyme activity
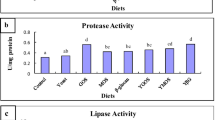
After 45 days of culture, the protease activity in the intestinal content of crucian carp on diet 2 was significantly different (P < 0.05) with that of the control and diet 3 fish, but there was no significant difference (P > 0.05) between diet 2 and diet 1 (Fig. 1a). Amylase activity was significantly higher for diet 2 compared to diet 1 and the control (Fig. 1b); however, there was no significant difference between diet 2 and diet 3 (Fig. 1b).
Specific activity of protease (a) and amylase (b) in intestinal content of allogynogenetic crucian carp fed a basal diet (control) and three diets containing different concentrations XOS (diets 1–3) at the end of the 45 days of culture. Means with different superscripts were significantly different (P < 0.05)
Data for specific activity of protease and amylase in the hepatopancreas content of allogynogenetic crucian carp fed a basal diet (control) and three diets containing different concentrations of XOS (diets 1–3) at the end of the 45 days of culture are illustrated in Fig. 2. The protease activity in hepatopancreas content was remarkably higher (P < 0.05) in diet 2 (20.52 ± 1.93 U g−1) compared with diet 3 (14.04 ± 1.59 U g−1) and the control (12.81 ± 1.52 U g−1) (Fig. 2a). However, there was no difference (P > 0.05) between diet 3 and the control, although the average value of protease activity in diet 3 presented an increasing trend (Fig. 2a). As for amylase in hepatopancreas content, assays showed higher activity in diet 2 (P < 0.05) as compared to the rest. However, although the amylase in diet 1 and diet 3 added with XOS had a relatively higher activity, there was no significant difference (P > 0.05) compared to that of the control (Fig. 2b).
Specific activity of protease (a) and amylase (b) in hepatopancreas content of allogynogenetic crucian carp fed a basal diet (control) and three diets containing different concentrations XOS (diets 1–3) at the end of the 45 days of culture. Means with different superscripts were significantly different (P < 0.05)
Discussion
To our knowledge, there are some data available concerning the in vivo use of XOS in rats (Yuan et al. 2005) and pigs (Moura et al. 2007). Olsen et al. (2001) observed that a diet supplemented with 15% inulin caused harmful effects in Arctic charr (Salvelinus alpinus L.). However, their previous studies (Ringø et al. 1998; Ringø and Olsen 1999) showed that dietary fatty acids and carbohydrates altered the bacterial flora of the gastrointestinal tract of fish. Similar findings were obtained by Li and Gatlin (2004, 2005), who investigated the effect of commercial prebiotics Grobiotic™ AE and Grobiotic™ supplemented in diets of hybrid striped bass (Morone chrysops × M. saxatilis) growth and showed that the prebiotic promoted the growth performance. The results of this present study clearly indicated that diet supplemented with XOS at a suitable concentration (50 and 100 mg kg−1) could improve the growth performance of allogynogenetic crucian carp (Table 2). The beneficial influence of XOS on growth was possibly due to an alteration of the intestinal microflora. However, a detailed study of intestinal microflora was needed. Lactic acid bacteria had been considered beneficial residents of the fish intestinal ecosystem because of producing bacteriocins and thus positively affecting the host’s microflora (Ringø et al. 1998; Irianto and Austin 2002; Ringø et al. 2006). Some reports have shown that XOS, as well as other prebiotic ingredients, may promote the maintenance of lactic acid-producing bacteria (Mussatto and Mancilha 2007; Moura et al. 2007). However, the hypothesis needed to be tested by further studies in allogynogenetic crucian carp.
Fish fed the diet supplemented with 100 mg kg−1 (diet 2) XOS had consistently better growth performances throughout the feeding trial compared to fish fed the other diets with 50 mg kg−1 and 200 mg kg−1 XOS (diet 1 and diet 3, respectively). It indicated that there was no positive correlation between the amount of XOS and the growth performance of carp according to the present study. Furthermore, no significant difference in final weight was observed between diet 3 treated with 200 mg kg−1 XOS and the control. Thus, the highest concentration of XOS might have affected digestibility of other nutrients and therefore accounted for the less improved performance of diet 3.
The fish gut microbiota played a role in host health, and the establishment of a normal gut flora could be regarded as complementary to the establishment of digestive enzymes (Ringø and Gatesoupe 1998). Thus, the better enzyme activities of groups treated with prebiotic XOS might be associated with the manipulation of the carp's gut flora towards a potentially more beneficial microbial community. The addition of prebiotic XOS with a certain content improved digestive enzymes, including protease and amylase, which might consequently explain the better growth performances observed with the same supplemented diets. The different concentrations of XOS could affect the enzyme activities differently, and diet 2 induced a stronger stimulation than the others. This stimulation of amylase activities was correlated with the growth performance and therefore might explain this increase. As for protease, it had the same result as the previous one.
In summary, the results of the study presented here show the effect of prebiotic XOS on the growth performance of carp, and also on digestive enzymes, including protease and amylase. In our research, use of a 100 mg kg−1 supplement of prebiotic XOS in the carp diet was recommended based on results of this study. The successful use of XOS in carp culture will require knowledge of such gut flora-function relationships. This work could be extended to include the use of many different prebiotics in carp ponds.
References
Boehm G, Stahl B, Jelinek J, Knol J, Minielli V, Moro GE (2005) Prebiotic carbohydrates in human milk and formulas. Acta Paediatr 94(suppl 449):18–21. doi:10.1080/08035320510043493
Cerezuela R, Cuesta A, Meseguer J, Esteban MA (2008) Effects of inulin on gilthead seabream (Sparus aurata L.) innate immune parameters. Fish Shellfish Immunol 24:663–668. doi:10.1016/j.fsi.2007.10.002
Costalos C, Kapiki A, Apostolou M, Papathoma E (2008) The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum Dev 84:45–49. doi:10.1016/j.earlhumdev.2007.03.001
Crittenden R, Playne M (1996) Production, properties, and applications of food-grade oligosaccharides. Trends Food Sci Technol 7:353–361. doi:10.1016/S0924-2244(96)10038-8
Erney RM, Malone WT, Skelding MB, Marcon AA, Kleman-Leyer KM (2000) Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr 30:131–133. doi:10.1097/00005176-200002000-00016
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 125:1401–1412
Houdijk JGM, Bosch MW, Verstegen MWA, Berenpas HJ (1998) Effects of dietary oligosaccharides on the growth performance and faecal characteristics of young growing pigs. Anim Feed Sci Technol 71:5–48. doi:10.1016/S0377-8401(97)00138-7
Huang F, Yan A, Wang X (1996) Studies on trypsin in silver carp and bighead carp. J Fish China 20:68–71
Huang F, Yan AS, Zhang GR, Zou GW (1999) The protease and amylase of Hypophthalmichthy molitrix and Aristichys nobilis. J Fish Sci 6:14–17
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25:633–642. doi:10.1046/j.1365-2761.2002.00422.x
Jiang CK (1982) Activity measuring for implemental enzyme. Science and Technology Press, Shanghai
Li P, Gatlin DM (2004) Dietary brewers yeast and the prebiotic Grobiotic™ AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops × M. saxatilis) to Streptococcus iniae infection. Aquaculture 231:445–456. doi:10.1016/j.aquaculture.2003.08.021
Li P, Gatlin DM (2005) Evaluation of the prebiotic GrobioticTM A and brewers yeast as dietary supplements for sub-adult hybrid striped bass (Morone chrysops × M. saxatilis) challenged in situ with Mycobacterium marinum. Aquaculture 248:197–205. doi:10.1016/j.aquaculture.2005.03.005
Lovell RT (1989) Nutrition and Feeding of Fish. Van Nostrand Reinhold Publishers, New York, USA
Lovell RT (1998) Nutrition and Feeding of Fish, 2nd edn. Kluwer Academic Publishers, Boston, USA
Lowry OH, Rosebrough NJ, Farr AL (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Meyer PD (2004) Nondigestible oligosaccharides as dietary fiber. J AOAC Int 87:718–726
Moura P, Marques S, Alves L, Freire JPB, Cunha LF, Esteves MP (2007) Effect of xylo-oligosaccharides from corn cobs autohydrolysis on the intestinal microbiota of piglets after weaning. Livest Sci 108:244–248. doi:10.1016/j.livsci.2007.01.053
Mussatto SI, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohydr Polym 68:587–597. doi:10.1016/j.carbpol.2006.12.011
Olsen RE, Myklebust R, Kryvi H, Mayhew TM, Ringø E (2001) Damaging effect of dietary inulin on intestinal enterocytes in Arctic charr (Salvelinus alpinus L.). Aquac Res 32:931–934. doi:10.1046/j.1365-2109.2001.00626.x
Ringø E, Gatesoupe FJ (1998) Lactic acid bacteria in fish: a review. Aquaculture 160:177–203. doi:10.1016/S0044-8486(97)00299-8
Ringø E, Olsen RE (1999) The effect of diet on aerobic bacterial flora associated with intestine of Arctic charr, Salvelinus alpinus L. J Appl Microbiol 86:22–28. doi:10.1046/j.1365-2672.1999.00631.x
Ringø E, Bendiksen HR, Gausen SJ, Sundsfjord A, Olsen RE (1998) The effect of dietary fatty acids on lactic acid bacteria associated with the epithelial mucosa and from faecalia of Arctic charr, Salvelinus alpinus L. J Appl Microbiol 85:855–864. doi:10.1046/j.1365-2672.1998.00595.x
Ringø E, Sperstad S, Myklebust R, Refstie S, Krogdahl A (2006) Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.). Aquaculture 261:829–841. doi:10.1016/j.aquaculture.2006.06.030
Rivero-Urgell M, Santamaria-Orleans A (2001) Oligosaccharides: application in infant food. Early Hum Dev 65:S43–S52. doi:10.1016/S0378-3782(01)00202-X
Spring P (1999) Modes of action of dietary mannan oligosaccharide as a growth enhancer. Zootech Int 22:34–36
Torrecillas S, Makol A, Jcaballero M, Montero D, Robaina L, Real F et al (2007) Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol 23:969–981. doi:10.1016/j.fsi.2007.03.007
Tuohy KM, Rouzaud GCM, Brueck WM, Gibson GR (2005) Modulation of the human gut microflora towards improved health using prebiotics-assessment of efficacy. Curr Pharm Des 11:75–90. doi:10.2174/1381612053382331
Valancony H, Humbert F, Rukelibuga J, Bougon M, Balaine L, Lalande F (2001) Comparison of some substitutes for antibiotic additives in diets for turkey poults: effects on production and on resistance to salmonella colonization. Sci Tech Avicoles 35:25–34
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671. doi:10.1128/MMBR.64.4.655-671.2000
Worthington V (1993) Worthington enzyme manual. Enzymes and Related Biochemicals. Worthington Chemical, New Jersey, USA
Yuan X, Wang J, Yao H (2005) Feruloyl oligosaccharides stimulate the growth of Bifidobacterium bifidum. Anaerobe 11:225–229. doi:10.1016/j.anaerobe.2005.02.002
Zhang LY, Zhu FH (1998) Feed Mensuration. College of Laiyang Agriculture Publishers, Qingdao, China
Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 30700620) and the Doctor Foundation of Shandong Agricultural University (no. 23174). We also acknowledge valuable help provided by Dr. X.X. Zhou and my colleagues for their many helpful suggestions. Acknowledgements are also made to L.Y. Hua for help with diet analysis. Thanks are due to all involved workers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, B., Wang, Y., Li, J. et al. Effect of prebiotic xylooligosaccharides on growth performances and digestive enzyme activities of allogynogenetic crucian carp (Carassius auratus gibelio). Fish Physiol Biochem 35, 351–357 (2009). https://doi.org/10.1007/s10695-008-9248-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9248-8