Abstract
The present study was conducted to investigate the effects of probiotic and encapsulated Lactobacillus bulgaricus on hematological and immunological factors after lead toxicity in rainbow trout (Oncorhynchus mykiss). Two hundred and forty fish weighing about 16 ± 3.8 g were divided randomly in to four groups including two groups which were fed by a diet containing ~ 108 CFU g−1Lactobacillus bulgaricus and encapsulated Lactobacillus bulgaricus bacteria and also the third group diet without Lactobacillus bulgaricus. After 45 days, in addition to probiotic (~ 108 CFU g−1), 500 μg kg of lead nitrate was added to the food of the three groups for 21 days. The fourth group (control) was first fed to the normal diet for 45 days then exposed to Pb. Blood samples were collected at days 45, 52, 59, and 66, and hematological and some immunological parameters were assessed. Results showed that hemoglobin, red blood cells, white blood cells, and lysozyme activity in the two probiotics groups were increased significantly up to 45 day (P < 0.05), but followed by a decreasing trend by adding Pb. Complement and bactericidal activity were enhanced significantly in the bulgaricus group (P < 0.05). Respiratory burst activity at day 45 in group bulgaricus had significant increase (P < 0.05) and decreased in all groups particularly after Pb exposure (P < 0.05). The achieved data shows that microencapsulation of probiotics with alginate-chitosan may be a suitable method to improve the fish condition against heavy metal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The progressively high rate of heavy metal pollution in aquatic environment due to several anthropogenic activities such as agricultural, industrial, and military operations and also geogenic (weathering process) activity and its harmful effects in the aquatic environment are of growing concern worldwide [9]. Generally, heavy metals are biologically nonessential and toxic to plants, animals, and humans as well as non-biodegradable forms of heavy metals can easily accumulate in soil [49], sediment, plant [4], and aquatic flora and fauna leading to biomagnifications in the food chain. The wastewaters which contain the heavy metal have detrimental effects on all forms of life upon direct discharge to the environment [1, 55]. Among dangerous heavy metals, lead (Pb) is one of the most important contaminant in the environment and causes numerous dysfunctions in living organisms. On the other hand, aquatic animals, particularly fish, are the main source of animal protein, hence are one of the major sources of heavy metal contamination in human body, so that intake of Pb as a long-lasting environmental pollutant causes central and peripheral nervous system damages, memory deterioration, diminished intellectual capacity, and skeletal disorder in humans especially children [9]. Methods for the elimination of heavy metals from water such as precipitation, flocculation, and ion exchange are sometimes expensive and are not effective at low metal accumulations. Researches have shown that probiotic bacteria can bind to many toxic compounds, for instance, cadmium and Pb, from water by Bifidobacterium longum 46, Lactobacillus fermentum ME3, and Bifidobacterium lactis Bb12 [24]. In other studies, researchers showed the absorption of aflatoxins, microcystin, and food-borne mutagens, within the gastrointestinal tract, by this means has reduced their uptake [24]. Lactic acid bacteria (LAB) are usually known as safe probiotic microorganisms. The WHO (World Health Organization) working group has defined probiotics as “live microorganisms which when administered in adequate amounts, confer a health benefit on the host” [26, 61].
In the aquaculture industry, the probiotic application is also widespread to control disease, improve water quality and decrease demands for using of antibiotics or disinfectants [2, 37]. However, some of the local gut bacteria can bind to harmful components, e.g., toxins, and prevent their adherence to the intestinal surface mucus layer, but probiotics have an important potential for inactivation of toxins by surface binding because of excessive adhesive properties of S-layer proteins in their cell wall [79].
Natural environment and a diversity of aquatic organisms can be affected by heavy metal pollution by overwhelming results on the ecological stability. They can diminish water and sediment quality and may unfavorably disturb fish health and other biological features due to their toxicity, persistence, and bioaccumulation in the food chains [55].
Conventional delivery methods in aquaculture, such as immersion, injection, diet supplements, and enrichment of live food, often deliver insufficient dosages and suffer from uncontrolled release and instability of bioactives (probiotics, vaccines) during processing and storage. Drug delivery is defined as approaches, formulations, and strategies to transport drugs or bioactive molecules to their site of action in the body. Novel drug delivery vehicles, such as biocompatible and biodegradable capsules, have been used to deliver specific nutrients, probiotics, genes, and chemicals in a constant and controllable method for human and veterinary uses. Such drug delivery vehicles can also be used to prevent the release of unwanted materials into the environment and extend the shelf life of the encapsulated materials and ensure a target-specific delivery of bioactives in a host [80]. Encapsulation has been considered to increase survival during storage and transit through the GI tract, protecting against acid and bile stress. Alginate is a commonly used biopolymer for microencapsulation, as it is nontoxic, easy, low-cost, and heat-resistant. Alginate in the presence of Ca2+ produces a mainly strong molecular framework, supporting cold-prepared, thermo-irreversible, and freeze-thaw-stable microcapsules [50]. The excellent mucoadhesion properties typical of hydrophilic polymers such as alginate are useful for enhancing the in situ delivery of bacteria along the GI tract [13].
Chitosan as a biocompatible, biodegradable, and nontoxic polymer is a naturally occurring and plentifully accessible biocompatible polysaccharide derived from chitin, a main constituent of the crustacean exoskeleton. It has also been noted for its chelating agent for detoxification of perilous waste application as a biomaterial given its immunostimulatory properties [36].
Microencapsulation is defined as a technology of packaging solid, liquid, or gaseous materials in small, sealed capsules that can release their contents at controlled rates under the influences of specific conditions. For better efficacy of probiotics in the gastrointestinal tract of carnivorous fish, microencapsulation procedure can be a simple, harmless, and improved method for encapsulation of live bacterial cells causing to the controlled discharge of metabolites [30].
The coating protects the active content from environmental stresses such as acidity, oxygen, and gastric conditions and can be used, for example, to help the content pass through the stomach [58].
Another advantage of microencapsulation is that persistence is increased during storage at high humidity, such as in sub-tropical countries like Iran, where part of this research was carried out with the aim to increase survival of probiotics during storage. In recent times, elimination of toxic metals via microbial biomass has been more attractive. In order to avoid toxin production and to eliminate, inactivate, or decrease their bioavailability in contaminated foods, several approaches have been practiced. Bioremoval treatments are of specific interest for economical reduction of bioavailability without losses of nutritional value among physical, chemical, and biological treatments for toxin degradation [79].
Bioremoval of heavy metals is an economically effective alternative which has been presented for conventional water refining methods since last decade of twentieth century. LABs can bind to cationic heavy metals such as Pb according to the microbial strain and pH. Very low removal is usually detected at pHs below 2–3, while at pHs above 3, a sharp increase in removal happens and maximum removal is often reached at pH 4–6. The effect of pH is a result of competition for negatively charged binding sites between cationic metals and protons [23]. Increasing the bacterial concentration enhances the binding of Pb; this may result from sorption of metals to dissolved organic acids that interfere with sorption to bacterial surface structures or creation of cell aggregates that decrease the surface area available for binding [25].
Pb binding by B. subtilis has been reported to start with a stoichiometric reaction between metallic cations and surface binding sites, followed by inorganic deposition of more metals [8]. In the other study, transmission electron micrographs of lyophilized B. longum and L. fermentum before and after Pb binding has been reported. Large deposits of Pb were obviously visible on the surface of both strains representing that Pb binding occurred at the surface of the bacteria [71].
Considering the potential of lactic acid bacteria in binding and removing heavy metals in vivo and in vitro conditions and the nonpathogenic nature of these bacteria [33, 44], the present study was conducted to investigate the effects of probiotic and encapsulated probiotic Lactobacillus bulgaricus on hematological and immunological factors by toxicity of Pb in rainbow trout (Oncorhynchus mykiss).
Materials and Method
Rearing Conditions
Two hundred and forty rainbow trout with an average weight of 16 ± 3.8 g were transferred to the laboratory of the Faculty of Veterinary Medicine of the Shahid Chamran University of Ahvaz and stored for 2 weeks under standard conditions for adaptability to laboratory conditions. During the adaptation period, the fish were fed twice daily with 3% of the body weight of the commercial diet (Biomar, France company). After the adaptation period, the fish were divided into four groups (60 fish in three replicates). The diet of first group contained ~ 108 CFU g−1 of L. bulgaricus (isolated from the digestive tract of Tor grypus) [41]; the second group was fed with a diet containing ~ 108 CFU g−1 encapsulated L. bulgaricus bacteria with alginate and chitosan; and the third group was just fed with common diet without L. bulgaricus and stored for 45 days. After completing this course, in addition to probiotic (~ 108 CFU g−1), 500 μg kg of lead nitrate was added to the food of the three groups for 21 days [41]. The fourth group (control or positive control group) was fed with common diet for 45 days firstly and then exposed to lead metal (500 μg kg−1 lead nitrate feed) until the end of the experiment period. Adding probiotic to commercial foods was carried out in accordance with the standard guidelines [54, 74]. Sampling was done on days 0, 45, 52, 59, and 66 (9 samples in each group at sampling times). The quality of water during the experiment period was at an acceptable and almost constant level. The physical and chemical parameters of water such as oxygen, temperature, pH, salinity, and electrical conductivity were measured daily and recorded. Water quality parameters such as ammonia nitrate were also evaluated on a weekly basis. The mean water temperature of the ponds during the breeding period was 15 ± 2.5 °C and the pH was 8.3 ± 0.2.
Preparation of the Suspension of Selected Bacteria
In this study, the probiotic L. bulgaricus isolated from the gut of T. grypus was used. The bacterium was isolated and identified using the 16S rRNA gene [41]. To prepare LABs and add them to fish food, the method recommended by Planas et al. [54] and Vine et al. [74] was used. In summary, each bacterium was cultured separately in MRS broth under anaerobic conditions. After the growth, the bacteria were isolated and rinsed with a centrifuge, and by the concentration of McFarland standard tubes, concentration was adjusted to 3 × 109 CFU ml−1 and then the desired concentration was sprayed for 1 g of food, by the concentration of ~ 108 CFU g−1. The foods were weighed in sterilized conditions, placed in sterile trays, and packed for 24 h under sterile conditions at the laboratory temperature. To ensure the number of live bacteria in the food, sampling and bacterial counting of the resulting food were performed. Only sterile physiological saline was sprayed on food of the control group [42].
Preparation of Bacteria for Microencapsulation
The bacterium was activated in 20 ml of MRS broth medium at 37 °C for 48 h, and then, the sample was cultured in MRS Agar medium for 48 h at 37 °C under anaerobic condition in the incubator. After growth, the specimens were washed with sterile physiological serum and centrifuged at 250g for 15 min and the sediment was suspended in a sterilized physiological serum using a standard McFarland tube with a concentration of 2.4 × 109 CFU ml−1.
Microencapsulation Process
According to Song et al. [69], 5 g calcium carbonate powder was added to 100 ml of distilled water and then sonicated for 15 min. The amount of 3.5 ml of calcium carbonate suspension was added to 55 mg of 2% alginate solution (Sigma–Aldrich viscosity 200–400 cP Lit−1) on a magnetic stirrer at 200 rpm and homogeneous for 30 min. The amount of 10 ml of the solution was mixed with 5 ml of microbial suspension at a concentration of 2.4 × 109 CFU ml−1 and homogenized at 150 rpm for 15 min. In each other, mix 35 ml of vegetable oil (rapeseed oil) with 0.5 g span 80 and stir it for 5 min at 100 rpm, then mix the solution with the above solution and mix it for 15 min. Ten milliliters of rapeseed oil and 0.5 ml of acetic acid were mixed, and drop by drop was added to the previous solution until the pH reached about 3.5 and stirred for 30 min at 200 rpm. Then, the phosphate buffer was used and samples were centrifuged for 5 min at 1000 rpm to separate the oil from the product. In the final step, at a rate of 15 ml of chitosan 0.4% (Sigma–Aldrich: low molecular weight) for 30 min, drop by drop was added at 800 rpm to the solution and then placed in a rotary position for 1 h. Ultimately, the encapsulated bacteria were collected by centrifugation at 1000g for 15 min.
Counting the Number of Bacteria Trapped in Microcapsules
One gram of microencapsulated samples was dissolved in 99 ml of 1% w/v sterile sodium citrate at pH 7.2 and for 20 min at room temperature, until microspheres were completely dissolved and bacteria were released; then by using MRS agar medium at 37 °C for 48 h, the number of bacteria was counted; this test was performed in three replicates.
Physical Particle Test
Determine the Size of the Particles and Their Distribution Patterns
Measurements of the microcapsules and their frequency using a scatter score 1 Qudix Korea particle diameter measuring apparatus were determined. For this purpose, microcapsules were dispersed in disinfected deionized water and the results were analyzed based on the mean diameter (VMD) of the particle ± standard error, dpeak, d10, d50, and d90, based on the mean diameter formulas.
Determination of Particle Morphology
To determine particle morphology and to see what they look like, electron microscopy and SEM techniques were used. Therefore, capsules were fixed by double-sided glue on cautery (SC 7620, England) and then coated with gold and palladium for 2 min. Observation of the capsule was carried out by electron microscopy (LEO 1455 VP, Germany) with the electron beam 10 kV.
Zeta Potential
In order to determine the zeta potential of the samples, the Nano-ZS zeta maker, made by Malvern, UK, was used.
Hematological Assays
Hemoglobin (Hb) measurement was determined by the cianometahemoglobin method. Packed cell volume (PCV) was determined by centrifuging micro-hematocrit in 10,000g for 10 min, according to the method that was used for mammals and birds [18]. Total red blood cell was calculated by Neubauer hemocytometer after diluting in Natt–Herrick solution [72]. Mean corpuscular volume (MCV), mean corpuscular hematocrit (MCH), and mean corpuscular hematocrit concentration (MCHC) were calculated. The blood sample was diluted with Natt–Herrick solution to determine total white blood cell (TWBC) by using Neubauer hemocytometer chamber; then, the Total WBC was calculated. For differential count of leukocytes, the blood smear on glass microscope slides was stained with Giemsa and 100 WBC were calculated, and the percentage of different types of leucocytes was determined following the method of Schaperclaus [65].
Immunological Responses of Treated Fish
Respiratory Burst Activity (NBT) Assay
Blood (0.1 ml) was placed in microtiter plate wells, and an equal amount of 0.2% nitroblue tetrazolium (NBT) solution was added to each well and incubated for 30 min at room temperature. A sample of the NBT blood cell suspension (0.1 ml) was added to a glass tube containing 2 ml N,N-dimethylformamide (Sigma, Germany) and centrifuged for 5 min at 1000g. The optical density (OD) of the supernatant was measured in a spectrophotometer (Biophotometer, Eppendorf, Germany) at 620 nm [20, 66].
Complement Activity Assay
The complement activity was assayed using rabbit red blood cells (RaRBC) as a target for the complement. RaRBCs were placed in 1.5% agarose (pH = 7.2), containing 75 μL MgCl2 1 M and 150 μL CaCl2 1 M in 100 ml phosphate-buffered saline (PBS, 0.1 M, pH = 7.0). The RaRBC suspension was washed with PBS by centrifugation at 750g for 5 min, and the cell concentration was adjusted to 1 × 108 cell ml−1. Twelve milliliters of agarose containing RaRBC was dispensed into a plate and incubated at 4 °C, and holes were punched (3 mm in diameter). Subsequently, each hole was filled with 20 μl of serum sample and incubated at room temperature for 24 h, after that the diameter of lysis was measured [6, 46].
Lysozyme Activity Assay
A turbidometric assay using lyophilized Micrococcus lysodeikticus (Sigma–Aldrich) was used to determine lysozyme activity in serum [67]. For this, 135 μl of M. lysodeikticus at a concentration of 0.2 mg ml−1 (w/v) in 0.02 M sodium phosphate buffer (SPB), pH 5.8 (Sigma–Aldrich), was added to 15 μl of the serum sample. As a negative control, serum was replaced with PBS. Results were expressed in units of lysozyme per milliliter of serum. A unit of lysozyme activity was defined as the amount of serum causing a reduction of 0.001 min at 450 nm at 22 °C.
Serum Bactericidal Activity
Serum bactericidal activity was determined using a previously described method by Kajita et al. [29]. Sera samples were diluted three times with 0.1% gelatin-veronal buffer (GVBC2) (pH 7.5, containing 0.5 mM Mg2+ and 0.15 mM Ca2+). Aeromonas hydrophila was suspended in the same buffer to make a concentration of 1 × 105 CFU ml−1. The diluted sera and bacteria were mixed at 1:1 ratio, incubated for 90 min at 25 °C, and shaken. Control tubes containing bacterial suspension in the same buffer were also incubated for 90 min at 25 °C. The numbers of viable bacteria were then calculated by counting the colonies from the resultant mixture incubated on TSA plates in duplicate after a 24-h incubation. The bactericidal activity of test serum was expressed as a percentage of colony-forming units in the test group to that in the control group.
Data Analyzing
For data analysis, SPSS software version 22 was used. The effect of probiotic on hematology and immunity was evaluated in five treatments by two-way ANOVA and 95% confidence intervals (ANOVA). To assess the significance of the mean differences, the supplementary Duncan test was used at a significance level of 0.05%. Graph drawing was also done in the Excel software environment (version 2010).
Results
Evaluating Characteristic of Encapsulated Bacteria
The initial number of live bacteria before microencapsulation was 2.4 × 109 CFU ml−1. According to data, the number of bacteria trapped in the capsule in the method of the emulsion was 2.21 ± 0.11 × 108.
Observation with SEM showed that the capsule appearance is largely in the form of spherical and elliptical. The mean capsule diameter (VMD) in the emulsion method was 3.45 μm (Fig. 1 and 2).
Diameter of 90% (d90) of particles was equal or less than 5.32 μm. The diameter of 50% of particles (d50) was equal or less than 3.22 μm and for 10% of the particles (d10); it was equal to or less than 245 nm. The dpeak for emulsification method was 13.6 μm. Zeta potential value in this study was calculated by emulsion method (36 + 2 mv) (solution pH = 3.5) (Fig. 3).
The results of two-way ANOVA of hematological and non-specific immune response parameters of fish fed with different diets have been presented in Table 1 and Figs. 4, 5, 6, and 7.
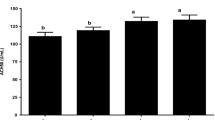
Lysozyme activity (unit ml−1) in the blood of rainbow trout. A diet containing ~ 108 CFU/g of Lactobacillus bulgaricus; ~ 108 CFU/g encapsulated Lactobacillus bulgaricus; control: basic diet. Fourth group, exposed to lead metal (500 μg/kg/21-day lead nitrate feed). Data represent the mean ± SD of a triplicate set of fish. Different capital superscripts denote a significant difference within columns (P < 0.05). Different lowercase superscripts denote significant differences between values in each row (P < 0.05)
Hematological Parameters
The number of red blood cells in the encapsulated group changed significantly at day 45. After that, it decreased to 1.06 ± .32 in day 52 (P < 0.05). Its downward trend continued and reached to 0.89 ± .11 and then 0.88 ± .08 in days 59 and 66, respectively. Red blood cells in the bulgaricus group increased on day 45, but followed by a decreasing trend by adding Pb to the diet and fell dramatically to 0.64 ± .20 in day 66 (P < 0.05). The white blood cells in the encapsulated probiotic group increased significantly at day 45 and 52 (P < 0.05), while the most number of WBC in the bulgaricus group was seen in day 52 (53.20 ± 14.60). After that, its value had a significant decrease and reached to 30.80 ± 7.94 (day 59). Also, the number of WBC in the bulgaricus group in day 66 was the lowest compared with other groups.
In the bulgaricus and encapsulated bulgaricus groups, the rate of hematocrit showed a significant increase compared to day 0 until the days 59 and 52, respectively (P < 0.05). In the encapsulated bulgaricus group, PCV rate had a decrease in day 59, but it rose to 40.80 ± 8.16 in day 66 (P < 0.05), while the bulgaricus group had a stable upward trend until day 59 and then it decreased dramatically in day 66 (P < 0.05).
Level of Hb in the encapsulated probiotic group was higher than with other treatments after 66 days (P < 0.05). Hemoglobin increased in the bulgaricus group until day 45 and continued to increase in days 52 and 59 (P < 0.05), and after that, it fell to 4.80 ± 0.71 in day 66 (Table 2).
Lysozyme Activity
The serum lysozyme activity in the group fed with L. bulgaricus increased at 45 dpf (days post-feeding) compared to the day 0; interestingly, no significant changes were observed after adding Pb (P > 0.05). L. bulgaricus–encapsulated-fed group displayed the highest level of lysozyme activity at 45 dpf (P < 0.05); however, by adding the Pb to the diet, level of lysozyme activity decreased and showed the lowest amount at 59 and 66 dpf (Fig. 4). In the control group, the activity of lysozyme was at least and equal to zero in other groups. In the control group containing Pb, the most activity of lysozyme was observed at 52 dpf and then significantly decreased.
Complement Activity
Complement activity in the L. bulgaricus group at 66 dpf (days of post-feeding), also in the group fed with the encapsulated L. bulgaricus and the control group at 45 dpf, increased significantly (P < 0.05), although the level of complement activity in the control group with Pb was not significantly different in different days (P > 0.05) (Fig. 5).
Serum Bactericidal Activity
Serum bactericidal activity increased in two probiotic-treated groups compared with the control and control + Pb groups in sampling intervals (P < 0.05). Though in the group of fish fed with the diet containing encapsulated L. bulgaricus, serum bactericidal activity after exposing with lead metal in different sampling intervals did not improve (P > 0.05) (Fig. 6).
Respiratory Burst Activity Assay
NBT reduction was seen in all groups particularly after Pb exposure (P < 0.05). The activity of NBT in the bulgaricus group increased significantly on day 45, and then by adding Pb, there was a significant decrease, but the minimum level of oxide anion production upon stimulation with probiotics was seen on day 66 in the group of fish fed with the diet containing non-encapsulated bulgaricus (Fig. 7).
Discussion
Hematological elements such as the percentage volume of erythrocytes and the total and differential leucocyte count in the blood of fish are used as a sign of their physiological condition, and their study can be helpful in the checking of pathologies and management of different stresses in fish farming [11, 62].
The Lactobacillus strain approved for this study succeeded in improving certain hematological and immunological parameters compared with the control fish. Munir et al. [45] investigated three commercial prebiotics (β-glucan, GOS or galacto-oligosaccharide, MOS or Mannan-oligosaccharide); and two probiotics—Saccharomyces cerevisiae, Lactobacillus acidophilus—on diet of Channa striata fingerlings. During the study, supplementation with dietary prebiotics and probiotics led to significant (P < 0.05) improvement in the red blood cells, white blood cells, packed cell volume, hemoglobin concentration, and serum protein level and lysozyme activities. In current training, the probiotic dietary exposure to Pb induced a tolerable reduction in the red blood cells; therefore, the LAB supplementations were effective in attenuating the negative effects of Pb on blood factors compared with the control group. This finding might be attributed to the fact that the use of probiotics increased the blood indices as a result of hemopoietic stimulation. Few studies have reported almost the similar findings in other fish species using different bacterial strains as probiotics. Dotta et al. [17] reported increased RBC count and Hb level in Nile tilapia using Lactobacillus plantarum as probiotic supplementation. Krishnaveni and Thambidurai [34] also found increased MCHC and MCH in the Catla catla supplemented with probiotics and yeast.
Toxicity exposure to substances, for instance, heavy metals, usually induces the lysis of erythrocytes in aquatic animals, leading to the depletion in hemoglobin and hematocrit values besides the deformed erythrocytes and anemia [38]. Various studies have reported a decrease in RBC count, hematocrit value, and hemoglobin concentration in fish exposed to various toxicants [7]. Researchers showed that because of the high affinity of Pb with RBC, it can increase the osmotic and mechanical susceptibility of them giving rise to reduced deformability and a shortened lifespan. High concentrations of Pb in the blood also impair heme synthesis, consequently inhibiting hemoglobin synthesis and anemia [73]. Jacob et al. [28] suggested that Pb exposure results in damage to the blood system by interference in heme and hemoglobin syntheses and alteration of erythrocyte morphology, which lead to anemia and depleted hematocrit.
Absorbed metal is transported through the bloodstream to the liver then metabolized and excreted by producing the metal-binding proteins such as metallothioneins, so fish blood can be an important accumulation section [32]. Mazon and Fernandes [40] reported a significant copper accumulation in the blood of the prochilodontidae, Prochilodus scrofa, exposed to excessive levels of copper.
Bhakta et al. [10] formerly isolated Pb-absorbing LAB from heavy metal–contaminated environments. Particularly, the rate of Pb absorption by LAB strains reported in previous studies is not consistent, likely due to species-specific differences in binding conditions and bacterial structures [43]. The binding depends on several factors like the microbial strain, pH, and bacterial concentration. So the minimum removal is generally observed at pHs below 2–3, while at pHs above 3, the amount of removal is increased and maximum removal regularly arrives at pH 4–6. The effect of pH is a result of competition for negatively charged binding sites between cationic metals and protons. Increasing the bacterial concentration has a direct correlation to binding of both cadmium and lead, due to sorption of metals to dissolved organic acids that interfere with sorption to bacterial surface structures or formation of cell aggregates that decrease the external region accessible for binding [79].
ALAD (δ-aminolevulinic acid dehydratase) is an enzyme that plays vital roles in the heme synthesis systems and has also been reported to be one of the most sensitive enzymes to Pb toxicity [3]. The inhibition of ALAD activity by Pb exposure can cause an unusual accumulation of δ-aminolevulinic acid, which induces oxidative stress and causes dysfunctions in the hematologic system [64].
Zhai et al. [77] revealed that the dietary supplementation of L. plantarum provided significant protective effects against waterborne Pb exposure in the Nile tilapia; also, it was effective in promoting growth performance, preventing Pb-induced death, decreasing tissue Pb accumulation, alleviating oxidative stress, and recovering Pb toxicity–related biomarkers in fish.
Probiotics can provide protection against pathogens by overcoming the negative consequences of antibiotics and have a various stimulatory sequel on the immune system of fish, notably, the effect on the immune cells, antibodies, acid phosphatase, lysozyme, and antimicrobial peptides [47]. Several studies have been conducted on probiotic immunization. Rengpipat et al. [56] showed that Bacillus sp. (strain S11) can be protective against diseases by activating the Penaeus monodon immunity systems. In other research by Marteua and Ramboud [39], the stimulus of immune response is enhanced by antibody and macrophage activity.
In this study, the results of counting WBC revealed that although the white blood cells in the encapsulated probiotic group did not change significantly at different days even after adding Pb, the decreasing trend was seen in the non-encapsulated probiotic group. Irianto and Austin [27] disclosed that the feeding of Gram-positive and Gram-negative probiotic bacteria remarkably increased the number of erythrocyte within 2 weeks of feeding experiment. Moreover, the increased WBC count helps in the non-specific immunity by way of neutrophils and macrophages. In the other words, probiotics correlate with the mononuclear phagocytic cells (monocytes and macrophages) and polymorphonuclear leukocytes (neutrophils) and NK cells to raise innate immune reactions [27, 35]. Also, probiotics robustly motivate the proliferation of lymphocytes (both B and T cells) and more immunoglobulin construction in fish [2, 52].
Immunostimulants such as probiotics are the specific biological compounds that stimulate non-specific host defense mechanisms and enhance the disease resistance and growth of the hosts [68]. Probiotics are responsible for the enhancement of the natural complement activity of the fish [51, 60]. An increased complement activity was recognized on O. mykiss from the fourth week of feeding heat-inactivated probiotics (Pdp 11 or 51M6) [14]. The highest complement activity in our study was recorded in the L. bulgaricus supplemented group on day 66 (3 weeks after exposing to Pb). Also, the probiotic group showed a higher level of complement activity than the control group after 45 days of the experiment. It may be due to probiotic bacteria’s ability to stimulate complement receptor expression. The activation of the alternative pathway of the complement system in the LAB group and neutralizing effect of probiotics with the mechanisms that mentioned may be attributed to the supplemented probiotics, confirming the benefit for the non-specific innate immunity of this fish. Pirarat et al. [53] reported the microencapsulation of probiotic Lactobacillus rhamnosus GG and studied its effect against Streptococcus agalactiae in tilapia. The combined addition of the probiotic, B. subtilus, and micro-algae in a fish meal could advance the development and acclimatization of the useful bacterium in the gut region [12].
Lysozyme is an important defense molecule of the fish innate immune system [63] and, therefore, has a bactericidal activity. Lysozyme activity of serum or plasma is also found to be greatly affected by the immune status of fish [57]. Our results also suggest the increased immune status as well as lesser stress in the fish group supplemented with probiotic and encapsulated probiotic, which make the role of probiotic more evident in promoting the immune response of the fish particularly after exposing to Pb. Previous studies have reported increased lysozyme activity due to use of probiotics in different species of fish [22, 70, 75]. In the present study, fish fed with supplemented diets showed a stimulation in serum lysozyme activity than those fed the basal diet and group fed with encapsulated L. bulgaricus (group B) had higher serum lysozyme activities than those fed with the basal diet and other dietary groups after 45 days post-feeding. Then, with the addition of Pb to the diet, the probiotic recipient group showed a high rate of lysozyme activity, while the microencapsulated group continued to reduce after exposure to Pb. The higher serum lysozyme is in consistent with lower mortality rate in probiotic-treated fish, suggesting that resistance against bacterial pathogens in probiotic-fed fish due to possible increase of the bacterial destruction by lysozyme. Many species of Lactobacillus spp. were also able to enhance the lysozyme activity by oral administration in different fishes [21, 51], which is in agreement with our results’ increased serum lysozyme activity observed in the L. bulgaricus fed trout. In fact, the effect of encapsulation can be attributed to several reasons, such as micro-encapsulation method, the lack of opening of many microspheres, pH condition of the digestive tract, the establishment deficiency of the large number of microspheres in the intestinal, different dose in the feed, and the length of the studies.
Respiratory bursts are produced by phagocytes in order to attack invasive pathogens during phagocytosis and have been widely used to evaluate the defense ability against pathogens; actually, superoxide anion along with hydroxyl radicals and nitric oxides are induced reactive oxygen species, which are related to enhance microbial killing capacity of macrophages [15]. Data from the present study showed the L. bulgaricus–fed groups had higher respiratory burst activity at 45 days post-feeding when compared with the other groups. These results were in agreement with previous reports by Balcazar and Rojas–Luna [5], showing a higher respiratory burst in rainbow trout fed L. lactis and L. mesenteroides. Salinas et al. [59] reported that the respiratory burst activity of teleost fish (Sparus aurata L.) improved in vitro by the addition of heat-inactivated L. lactis. The associated outcome was also detected by Nikoskelainen et al. [48], who described that rainbow trout fed L. rhamnosus (8 × 104 CFU g−1) for 2 weeks showed a significant increase in respiratory burst activity compared with the control group. In another investigation, Aeromonas salmonicida and Yersiniaruckeri were seen to increase the phagocytic activity, respiratory burst beside serum, and gut mucosal lysozyme activity [31]. Administration of a lactic acid bacterium L. rhamnosus (strain ATCC 53103) stimulates respiratory burst activity in rainbow trout Oncorhynchus mykiss [48]. Also, in the current study, after exposure to Pb on the 59th day, NBT activity increased significantly in the microencapsulated group; the presence of alginate–chitosan as immune stimulants can also be related to this finding. The findings of respiratory burst activity following the probiotic treatment in fish are often contradictory, while some studies indicated probiotics did not have any significant impact on this non-specific defense mechanism of fish [16, 67]. Several in vitro and in vivo studies showed a significant increase in respiratory burst activity by various probiotics in many aquatic animals including fish. Probiotics like B. subtilis and certain members of the LAB group can stimulate respiratory burst activity in fish [78]. Heat-inactivated L. delbrueckii and B. subtilis under in vitro condition also enhanced the activity of head kidney leucocytes of gilt-head sea bream [59]. This study further confirmed that probiotics might be responsible for degrading free radical production by host phagocytic cells, and the variances observed in superoxide anion production between the probiotic groups may be associated with colonization ability on the intestinal mucus.
The outcomes of investigators expressed that B. subtilis confer significant protective effects against Pb toxicity by preventing alterations in the levels of bioaccumulation, superoxide dismutase, catalase, and glutathione. B. subtilis also assists in the recovery of blood δ-aminolevulinic acid dehydratase, lysozyme, and IgM levels while regulating the expression of immune-related genes including IL-10, lysozyme, TNFα, IgM, and Hsp70 after 60 days of Pb exposure. Their results suggest that administration of B. subtilis (109 CFU g−1) has the potential to combat Pb toxicity in Carassius auratus gibelio [76]. The L. bulgaricus in this study is the critical bacteria that can effectively attenuate Pb accumulation by several ways. Pb binding by L. bulgaricus has been reported to start with a stoichiometric response among metallic cations and surface binding locations, followed by inorganic deposition of more metal [8].
The data from the current experiment shows that although the probiotic strain employed in this training is acid and bile tolerant and can be survived through the gastrointestinal tract and possibly remains both in the intestinal and other mucosal surface of the fish so it stimulated immune response without encapsulation, microencapsulation of probiotics with alginate–chitosan may be a suitable method to improve the physiological condition of fish when being exposed to heavy metal in the diet because of chelating effect of chitosan in addition to probiotic bacteria. Also that is probably for cell walls of Gram-positive bacteria those effective metal chelators due to their high peptidoglycan and teichoic acid content [43]. Therefore, LAB, including lactobacilli, is predicted to express a number of different ligands capable of binding heavy metal cations [19]. However, further study on rainbow trout should be conducted to clearly elucidate which microencapsulation method may be stronger against heavy metal over using the free form.
References
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40(3):997–1026. https://doi.org/10.1016/j.procbio.2004.04.008
Al-Dohail MA, Hashim R, Aliyu-Paiko M (2009) Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac Res 40(14):1642–1652. https://doi.org/10.1111/j.1365-2109.2009.02265.x
Alexander B, Checkoway H, Costa-Mallen P, Faustman E, Woods J, Kelsey K, van Netten C, Costa L (1998) Interaction of blood lead and delta-aminolevulinic acid dehydratase genotype on markers of heme synthesis and sperm production in lead smelter workers. Environ Health Perspect 106:213–216
Ashraf MA, Maah MJ, Yusoff I (2011) Heavy metals accumulation in plants growing in ex tin mining catchment. Int J Environ Sci Technol 8(2):401–416. https://doi.org/10.1007/BF03326227
Balcázar JL, De Blas I, Ruiz-Zarzuela I, Vendrell D, Gironés O, Muzquiz JL (2007) Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunol Med Microbiol 51(1):185–193. https://doi.org/10.1111/j.1574-695X.2007.00294.x
Barta O (1993) Veterinary clinical immunology laboratory. Bar-Lab Incorporated, USA
Benifey TJ, Biron M (2000) Acute stress response in triploid rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Aquaculture 184:167–176. https://doi.org/10.1016/S0044-8486(99)00314-2
Beveridge TJ, Murray RG (1980) Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol 141(2):876–887
Bhakta JN, Munekage Y, Ohnishi K, Jana BB (2012a) Isolation and identification of cadmium-and lead-resistant lactic acid bacteria for application as metal removing probiotic. Int J Environ Sci Technol 9(3):433–440. https://doi.org/10.1007/s13762-012-0049-3
Bhakta JN, Ohnishi K, Munekage Y, Iwasaki K, Wei M-Q (2012b) Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J Appl Microbiol 112(6):1193–1206. https://doi.org/10.1111/j.1365-2672.2012.05284.x
Carvalho CS, Fernandes MN (2006) Effect of temperature on copper toxicity and hematological responses in the neotropical fish Prochilodus scrofa at low and high pH. Aquaculture 251:109–117. https://doi.org/10.1016/j.aquaculture.2005.05.018
Cerezuela R, Guardiola FA, González P, Meseguer J, Esteban MÁ (2012) Effects of dietary Bacillus subtilis, Tetraselmis chuii, and Phaeodactylum tricornutum, singularly or in combination, on the immune response and disease resistance of sea bream (Sparus aurata L.). Fish Shellfish Immunol 33(2):342–349. https://doi.org/10.1016/j.fsi.2012.05.004
Chen S, Cao Y, Ferguson LR, Shu Q, Garg S (2013) Evaluation of mucoadhesive coatings of chitosan and thiolated chitosan for the colonic delivery of microencapsulated probiotic bacteria. J Microencapsul 30:103–115. https://doi.org/10.3109/02652048.2012.700959
Choi SH, Yoon TJ (2008) Non-specific immune response of rainbow trout (Oncorhynchus mykiss) by dietary heat-inactivated potential probiotics. Immune Netw 8:67–74
Dalmo RA, Ingebrigtsen K, Bøgwald J (1997) Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J Fish Dis 20(4):241–273. https://doi.org/10.1046/j.1365-2761.1997.00302.x
Díaz-Rosales P, Arijo S, Chabrillón M, Alarcón F, Tapia-Paniagua S, Martínez-Manzanares E, Balebona MC, Moriñigo MA (2009) Effects of two closely related probiotics on respiratory burst activity of Senegalese sole (Solea senegalensis, Kaup) phagocytes, and protection against Photobacterium damselae subsp. Piscicida. Aquaculture 293:16–21. https://doi.org/10.1016/j.aquaculture.2009.03.050
Dotta BT, Buckner CA, Cameron D, Lafrenie RM, Persinger MA (2011) Biophoton emissions from cell cultures: biochemical evidence for the plasma membrane as the primary source. Gen Physiol Biophys 30(3):301–309. https://doi.org/10.4149/gpb_2011_03_301
Feldman BF, Zinkl JG, Jain NC (2000) Schalm’s veterinary hematology, 5th edn. Lippincott Williams and Wilkins, New York, pp 1120–1124
Garcia EF, Luciano WA, Xavier DE, da Costa WC, de Sousa Oliveira K, Franco OL, de Morais Júnior MA, Lucena BT, Picão RC, Magnani M, Saarela M (2016) Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus strains. Front Microbiol 7:1371. https://doi.org/10.3389/fmicb.2016.01371
Geng X, Dong XH, Tan BP, Yang QH, Chi SY, Liu HY, Liu XQ (2012) Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac Nutr 18(1):46–55. https://doi.org/10.1111/j.1365-2095.2011.00875.x
Gonçalves AT, Maita M, Futami K, Endo M, Katagiri T (2011) Effects of a probiotic bacterial Lactobacillus rhamnosus dietary supplement on the crowding stress response of juvenile Nile tilapia Oreochromis niloticus. Fish Sci 77(4):633–642. https://doi.org/10.1007/s12562-011-0367-2
Gupta A, Gupta P, Dhawan A (2014) Dietary supplementation of probiotics affects growth, immune response and disease resistance of Cyprinus carpio fry. Fish Shellfish Immunol 41(2):113–119. https://doi.org/10.1016/j.fsi.2014.08.023
Halttunen T (2008) Removal of cadmium, lead and arsenic from water by lactic acid bacteria. Dissertation, University of Turku
Halttunen T, Salminen S, Tahvonen R (2007) Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int J Food Microbiol 114:30–35. https://doi.org/10.1016/j.ijfoodmicro.2006.10.040
Harvey RW, Leckie JO (1985) Sorption of lead to two gram-negative marine bacteria in seawater. Mar Chem 15(4):333–344. https://doi.org/10.1016/0304-4203(85)90044-1
Hotel ACP, Cordoba A (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 5(1):1–10
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25(6):333–342. https://doi.org/10.1046/j.1365-2761.2002.00375.x
Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE (2000) The effect of low-level blood lead on hematologic parameters in children. Environ Res 82(2):150–159. https://doi.org/10.1006/enrs.1999.4011
Kajita Y, Sakai M, Atsuta S, Kobayashi M (1990) The immunomodulatory effects of levamisole on rainbow trout, Oncorhynchus mykiss. Fish Pathol 25(2):93–98. https://doi.org/10.3147/jsfp.25.93
Kanmani RS, Kumar N, Yuvaraj KA, Paari V, Pattukumar V, Arul (2011) Cryopreservation and microencapsulation of a probiotic in alginate-chitosan capsules improve survival in simulated gastrointestinal conditions. Biotechnol Bioprocess Eng 16:1106–1114. https://doi.org/10.1007/s12257-011-0068-9
Kim DH, Austin B (2006) Cytokine expression in leucocytes and gut cells of rainbow trout, Oncorhynchus mykiss Walbaum, induced by probiotics. Vet Immunol Immunopathol 114(3–4):297–304. https://doi.org/10.1016/j.vetimm.2006.08.015
Kim JH, Kang JC (2017) Toxic effects on bioaccumulation and hematological parameters of juvenile rockfish Sebastes schlegelii exposed to dietary lead (Pb) and ascorbic acid. Chemosphere 176:131–140. https://doi.org/10.1016/j.chemosphere.2017.02.097
Kinoshita H, Sohma Y, Ohtake F, Ishida M, Kawai Y, Kitazawa H, Saito T, Kimura K (2013) Biosorption of heavy metals by lactic acid bacteria and identification of mercury binding protein. Res Microbiol 164(7):701–709. https://doi.org/10.1016/j.resmic.2013.04.004
Krishnaveni R, Thambidurai S (2013) Industrial method of cotton fabric finishing with chitosan–ZnO composite for anti-bacterial and thermal stability. Ind Crop Prod 47:160–167. https://doi.org/10.1016/j.indcrop.2013.03.007
Kumar R, Mukherjee SC, Ranjan R, Nayak SK (2008) Enhanced innate immune parameters in Labeo rohita (ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunol 24(2):168–172. https://doi.org/10.1016/j.fsi.2007.10.008
Lasko CL, Hurst MP (1999) An investigation into the use of chitosan for the removal of soluble silver from industrial wastewater. Environ Sci Technol 33(20):3622–3626. https://doi.org/10.1021/es980443r
Ma CW, Cho YS, Oh KH (2009) Removal of pathogenic bacteria and nitrogens by Lactobacillus spp. JK-8 and JK-11. Aquaculture 287(3):266–270. https://doi.org/10.1016/j.aquaculture.2008.10.061
Maheswaran R, Devapanl A, Muralidharan S, Velmurugan B, Ignaeimuthu S (2008) Haematological studies of fresh water fish, Clarias batradrus (L) exposed to mercuric chloride. Int J Integr Biol 2:49–54
Marteua P, Ramboud JC (1993) Potential of using lactic acid bacteria for therapy and immunomodulation in man. FEMS Microbiol Lett 12(1–3):207–220. https://doi.org/10.1016/0168-6445(93)90064-G
Mazon A-F, Fernandes M-N (1999) Toxicity and differential tissue accumulation of copper in the tropical freshwater fish, Prochilodus scrofa (Prochilodontidae). Bull Environ Contam Toxicol 63:797–804. https://doi.org/10.1007/s001289901049
Mohammadian T, Alishahi M, Tabandeh M-R, Ghorbanpoor M, Gharibi D, Tollabi M, Rohanizade S (2016) Probiotic effects of Lactobacillus plantarum and L. delbrueckii subsp. bulguricus on some immune-related parameters in Barbus grypus. Aquac Int 24(1):225–242. https://doi.org/10.1007/s10499-015-9921-8
Mohammadian T, Alishahi M, Tabandeh M-R, Ghorbanpoor M, Gharibi D (2017) Effect of Lactobacillus plantarum and Lactobacillus delbrueckii subsp. bulgaricus on growth performance, gut microbial flora and digestive enzymes activities in Tor grypus (Karaman, 1971). Iran J Fish Sci 16(1):296–317
Monachese M, Burton JP, Reid G (2012) Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics. Appl Environ Microbiol 78(18):6397–6404. https://doi.org/10.1128/AEM.01665-12
Mrvčić J, Stanzer D, Bacun-druzina V, Stehlik-Tomas V (2009) Copper binding by lactic acid bacteria (LAB). Biosci Microflora 28(1):1–6. https://doi.org/10.12938/bifidus.28.1
Munir MB, Hashim R, Nor SAM, Marsh TL (2018) Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol 75:99–108. https://doi.org/10.1016/j.fsi.2018.02.005
NavinChandran M, Iyapparaj P, Moovendhan S, Ramasubburayan R, Prakash S, Immanuel G, Palavesam A (2014) Influence of probiotic bacterium Bacillus cereus isolated from the gut of wild shrimp Penaeus monodon in turn as a potent growth promoter and immune enhancer in P. monodon. Fish Shellfish Immunol 36(1):38–45. https://doi.org/10.1016/j.fsi.2013.10.004
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Nikoskelainen S, Ouwehand A, Bylund G, Salminen S, Lilius EM (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol 15(5):443–452. https://doi.org/10.1016/S1050-4648(03)00023-8
Nwachukwu MA, Feng H, Alinnor J (2010) Assessment of heavy metal pollution in soil and their implications within and around mechanic villages. Int J Environ Sci Technol 7(2):347–358. https://doi.org/10.1007/BF03326144
Ouwerx C, Velings N, Mestdagh MM, Axelos MV (1998) Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym Gels Networks 6:393–408. https://doi.org/10.1016/S0966-7822(98)00035-5
Panigrahi A, Kiron V, Satoh S, Hirono I, Kobayashi T, Sugita H, Puangkaew J, Aoki T (2007) Immune modulation and expression of cytokine genes in rainbow trout Oncorhynchus mykiss upon probiotic feeding. Dev Comp Immunol 31(4):372–382. https://doi.org/10.1016/j.dci.2006.07.004
Picchietti S, Fausto AM, Randelli E, Carnevali O, Taddei AR, Buonocore F, Scapigliati G, Abelli L (2009) Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol 26(3):368–376. https://doi.org/10.1016/j.fsi.2008.10.008
Pirarat K, Pinpimai C, Rodkhum N, Chansue EL, Ooi T, Katagiri M, Maita (2015) Viability and morphological evaluation of alginate-encapsulated Lactobacillus rhamnosus GG under simulated tilapia gastrointestinal conditions and its effect on growth performance, intestinal morphology and protection against Streptococcus agalactiae. Anim Feed Sci Technol 207:93–103. https://doi.org/10.1016/j.anifeedsci.2015.03.002
Planas M, Vázquez JA, Marqués J, Pérez-Lomba R, González M, Murado M (2004) Enhancement of rotifer (Brachionus plicatilis) growth by using terrestrial lactic acid bacteria. Aquaculture 240(1–4):313–329. https://doi.org/10.1016/j.aquaculture.2004.12.008
Rayes AA (2012) Field studies on the removal of lead, cadmium, and copper by the use of probiotic lactic acid bacteria from the water for culturing marine tilapia T. spilurus. N Y Sci J 5(11):74–82
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P (2000) Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 191(4):271–288. https://doi.org/10.1016/S0044-8486(00)00440-3
Røed KH, Fevolden SE, Fjalestad KT (2002) Disease resistance and immune characteristics in rainbow trout (Oncorhynchus mykiss) selected for lysozyme activity. Aquaculture 209(1):91–101. https://doi.org/10.1016/S0044-8486(01)00810-9
Rokka S, Rantamäki P (2010) Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur Food Res Technol 231(1):1–12. https://doi.org/10.1007/s00217-010-1246-2
Salinas I, Díaz-Rosales P, Cuesta A, Meseguer J, Chabrillón M, Morinigo MA, Esteban MA (2006) Effect of heat-inactivated fish and non fish derived probiotics on the innate immune parameters of a teleost fish (Sparus auratus L.). Vet Immunol Immunopathol 111(3–4):279–286. https://doi.org/10.1016/j.vetimm.2006.01.020
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 25(1–2):114–123. https://doi.org/10.1016/j.fsi.2008.03.011
Salminen S, Ouwehand A, Benno Y, Lee Y-K (1999) Probiotics: how should they be defined? Trends Food Sci Technol 10(3):107–110. https://doi.org/10.1016/S0924-2244(99)00027-8
Sampath K, James R, Ali KA (1998) Effects of copper and zinc on blood parameters and prediction of their recovery in Oreochromis mossambicus (pisces). Indian J Fish 45(2):129–139
Saurabh S, Sahoo PK (2008) Lysozyme: an important defense molecule of the fish innate immune system. Aquac Res 39(3):223–239. https://doi.org/10.1111/j.1365-2109.2007.01883.x
Saxena G, Flora S (2004) Lead-induced oxidative stress and hematological alterations and their response to combined administration of calcium disodium EDTA with a thiol chelator in rats. J Biochem Mol Toxicol 18:221–233. https://doi.org/10.1002/jbt.20027
Schäperclaus W (1992) Causes, development and prevention of fish disease. In: Schaperclaus W, Kulow H, Schreckenback K (eds) Fish disease, 5th edn. AA Balkema Publisher, Rotterdam, pp 3–42
Secombes CJ (1990) Isolation of salmonid macrophages and analysis of their killing activity. Tech Fish Immunol 1:137–154
Sharifuzzaman S, Austin B (2009) Influence of probiotic feeding duration on disease resistance and immune parameters in rainbow trout. Fish Shellfish Immunol 27(3):440–445. https://doi.org/10.1016/j.fsi.2009.06.010
Skjermo J, Storseth TR, Hansen K, Handa A, Oie G (2006) Evaluation of β-(1→ 3, 1→6)-glucans and high-M alginate used as an immunostimulatory dietary supplement during first feeding and weaning of Atlantic cod (Gadus morhua L.). Aquaculture 261(3):1088–1101. https://doi.org/10.1016/j.aquaculture.2006.07.035
Song H, Yu W, Gao M, Liu X, Ma X (2013) Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr Polym 96(1):181–189. https://doi.org/10.1016/j.carbpol.2013.03.068
Sun YZ, Yang HL, Ma RL, Lin WY (2009) Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol 29:803–809. https://doi.org/10.1016/j.fsi.2010.07.018
Teemu H, Seppo S, Jussi M, Raija T, Kalle L (2008) Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int J Food Microbiol 125(2):170–175. https://doi.org/10.1016/j.ijfoodmicro.2008.03.041
Thrall MA (2004) Veterinary hematology and clinical chemistry. Lippincott Whiliams & Wilkins, New York, pp 277–288
Toplan S, Ozcelik D, Gulyasar T, Akyolcu MC (2004) Changes in hemorheological parameters due to lead exposure in female rats. J Trace Elem Med Biol 18:179–182. https://doi.org/10.1016/j.jtemb.2004.02.006
Vine NG, Leukes WD, Kaiser H, Daya S, Baxter J, Hecht T (2004) Competition for attachment of aquaculture candidate probiotic and pathogenic bacteria on fish intestinal mucus. J Fish Dis 27(6):319–326. https://doi.org/10.1111/j.1365-2761.2004.00542.x
Won SH, Kim YR, Kim EY, Sungchul BC, Kong IS (2013) Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus). Aquaculture 376–379. https://doi.org/10.1016/j.aquaculture.2012.11.009
Yin Y, Zhang P, Yue X, Du X, Li W, Yin Y, Yi C, Li Y (2018) Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: bioaccumulation, antioxidant responses and immune responses. Ecotoxicol Environ Saf 161:755–762. https://doi.org/10.1016/j.ecoenv.2018.06.056
Zhai Q, Wang H, Tian F, Zhao J, Zhang H, Chen W (2017) Dietary Lactobacillus plantarum supplementation decreases tissue lead accumulation and alleviates lead toxicity in Nile tilapia (Oreochromis niloticus). Aquac Res 48(9):5094–5103. https://doi.org/10.1111/are.13326
Zhou X, Tian Z, Wang Y, Li W (2010) Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36:501–509. https://doi.org/10.1007/s10695-009-9320-z
Zoghi A, Khosravi-Darani K, Sohrabvandi S (2014) Surface binding of toxins and heavy metals by probiotics. Mini-Rev Med Chem 14(1):84–98
Zuidam NJ, Shimoni E (2010) Overview of microencapsulates for use in food products or processes and methods to take them. In: Zuidam NJ, Nedovic V (eds) Encapsulation Technologies for Active Food Ingredients and Food Processing. Springer-Verlag, New York, NY, pp 3–29
Funding
This investigation was funded by a Grant from the Shahid Chamran University of Ahvaz Research Council (Grant No. 27176, 1395.3.2). The authors of this study followed instructions of the university in Iran, and performed trials based on Ethical Guideline of laboratory animals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadian, T., Dezfuly, Z.T., Motlagh, R.G. et al. Effect of Encapsulated Lactobacillus bulgaricus on Innate Immune System and Hematological Parameters in Rainbow Trout (Oncorhynchus mykiss), Post-Administration of Pb. Probiotics & Antimicro. Prot. 12, 375–388 (2020). https://doi.org/10.1007/s12602-019-09544-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09544-7