Abstract
The use of probiotic bacteria in novel foods to provide beneficial health effects is today of increasing interest in the food industry. The process stability of probiotics is, however, not always optimal. Microencapsulation technology can be used to maintain the viability of probiotic bacteria during food product processing and storage. Both true microcapsules with coating as well as microspheres where the bacteria are evenly spread in the coating material are discussed. It is important that encapsulation keeps the probiotics active through the gastrointestinal tract and releases them in their target organ. The survival of microencapsulated cells in simulated gastric conditions is therefore also reviewed. Polysaccharides like alginate, gellan, κ-carrageenan and starch are the most commonly used materials in microencapsulation of bifidobacteria and lactobacilli. Techniques commonly applied for probiotic microencapsulation are emulsion, extrusion, spray drying, and adhesion to starch. Bead stability can be improved by using different coating materials, e.g. chitosan. Future challenges in the field include recognition of new potent applications, selection of appropriate techniques, materials and bacterial strains, and minimizing the extra costs incurred by microencapsulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are defined as live microbial feed supplements that have beneficial effects on the host by improving its intestinal microbial balance [1], or as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [2]. Probiotics can be used both for animals as well as for human beings [1]. This review concentrates on their human use. To benefit human health, a probiotic must have good technological properties, survive through the upper gastrointestinal tract, and be able to function in the gut environment [3]. The safety of probiotics is of prime importance, covering aspects such as non-pathogenicity, antibiotic resistance specifications, and the strain’s human origin [4]. However, the specificity of action and ability to remain viable at the target is more important than the origin in probiotics for human consumption [2]. Probiotics have been commonly added to fermented dairy products, but nowadays also to other kinds of food. There is a lack of intervention studies to establish the doses required for probiotic effects, and they are most probably highly dependent on the probiotic strain used. The concentration of probiotics in commercial dairy products is usually in the range of 108–109 cfu/mL. This is above the recommendations of Kurmann and Rasic (105–107 bifidobacteria/mL at the date of consumption) [5] and also above the minimum level suggested by the International Dairy Federation (at least 107 CFU/g in the product to the date of minimum durability) [6].
The most commonly applied probiotic strains reported in the literature belong to the Bifidobacterium and Lactobacillus genera. Bifidobacteria are gram-positive, strictly anaerobic, and grow at pH 4.5–8.5. They are usually found in large intestine of humans. Lactobacilli are a heterogenous group of gram-positive, microaerobic or anaerobic species that vary widely in growth and metabolic characteristics. They have been traditionally used for fermented food, especially dairy products.
Probiotics employ different mechanisms for affecting human health. They normalize the intestinal microbiota and possess metabolic effects as well as immunomodulation potential [7]. Table 1 summarizes various health benefits from the consumption of probiotics. Often, however, the number of viable probiotic bacteria capable of delivering their targeted beneficial effect is too low. Many factors such as acidity, oxygen content, and concentration of lactic and acetic acids affect the survival of probiotics in food and in the gastrointestinal tract of the host. Several methods have been used to enhance the viability of probiotics, including selection of resistant strains, stress adaptation, incorporation of micronutrients, and microencapsulation. A list of lactobacilli and bifidobacteria used in microencapsulation studies is presented in Table 2.
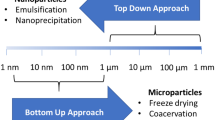
Microencapsulation is defined as the technology for packaging solids, liquids or gaseous materials in miniature, sealed capsules that can release their contents at controlled rates under specific conditions [59]. A microcapsule consists of a semipermeable, spherical, thin and strong membrane surrounding a solid or liquid core, with a diameter varying from a few microns to 1 mm [60]. Beads without coating can also be considered as microencapsules in a broad sense. Coating protects the active content from environmental stresses such as acidity, oxygen and gastric conditions, and can be used, for example, to help the content pass through the stomach. Besides enhancing the viability of bacteria, microencapsulation facilitates handling of cells and allows a controlled dosage.
This article reviews the present technology and the future prospects and challenges of microencapsulation of probiotics applied in functional foods.
Materials used for microencapsulation of probiotics
Materials commonly used in encapsulation of probiotic bacteria include polysaccharides originating from seaweed (κ-carrageenan, alginate), other plants (starch and its derivatives, gum Arabic), or bacteria (gellan, xanthan), and animal proteins (milk, gelatin).
κ-Carrageenan is composed of repeating d-galactose-4-sulphate units and 3,6-anhydro-d-galactose joined by alternating α1 → 3 and β1 → 4 glycosidic linkages. Gelation is dependent on a change in temperature, and the beads are formed after dropping a mixture of polymer and cells into a KCl or CaCl2 solution. Carrageenan is commonly used as a food additive, but there is new evidence that it induces inflammation and intestinal neoplasia [61].
Alginic acid is composed of 1 → 4 linked β-d-mannuronic and α-l-guluronic acids. On addition to divalent cations like calcium, interfacial polymerization is instantaneous, with precipitation of calcium alginate followed by a gradual gelation of the interior. Alginates are widely used in laboratory-scale microencapsulation. The capsules are very porous, however, and allow diffusion of water in and out of the matrix.
Starch consists of d-glucose units joint together with glycosidic bonds. Since some bifidobacteria are able to adhere to starch granules, it may be possible to use starch from various sources like maize, potato, barley or oat in microencapsulation technology [10]. The entrapment of probiotics in starch polymers renders gel-like structures. Resistant starch also works as a prebiotic in the human intestine [3]. Moreover, certain fibre preparations are found to have potential in protecting probiotic cells during processing and storage in food matrices [55].
A cellulose derivative polymer, cellulose acetate phthalate (CAP) has proven effective in microencapsulation of probiotics by both emulsion and spray-drying techniques [60]. CAP is physiologically inert and widely used as an enteric coating material.
Gum Arabic is exuded from the stems and branches of acacia trees. Gum Arabic is composed of highly branched arrangement of simple sugars and 2% protein. Its ability to act as an emulsifier is an important aspect for spray drying [62].
Polymers of bacterial origin, such as exopolysaccharides produced by lactic acid bacteria, have potential for using in bacterial encapsulation [63].
Gellan gum is an anionic polysaccharide derived from Sphingomonas elodea and is widely used in the food industry. The repeating unit of the polymer is a tetrasaccharide that consists of two residues of d-glucose and one of each residue of l-rhamnose and d-glucuronic acid. The units are connected with each other using an α1 → 3 glycosidic bond. Xanthan is an exopolysaccharide derived from the plant-pathogenic bacterium Xanthomonas campestris [64]. It is composed of glucose, mannose, and glucuronic acid. A combination of gellan and xanthan has been used in a number of microencapsulation studies [8, 23, 54].
Other probiotic encapsulation materials include milk proteins, which can be divided into caseins and whey proteins [18, 58]. Whey proteins are easily heat-denatured, which affects aggregation and reduction in emulsion stability. Caseins and caseinates are not as heat-sensitive as whey proteins and also show superior surface-active properties. Skim milk and lactose, often in combination with milk proteins, are widely used as protective agents in spray drying, especially with pharmaceuticals [53, 56]. Precrystallization of the lactose solution is necessary prior to spray drying to avoid caking problems [62].
Gelatin is frequently used in the food and pharmaceutical industries [65, 66]. It is a protein derived by partial hydrolysis of collagen of animal origin. Gelatin has a very special structure and versatile functional properties, and forms a solution of high viscosity in water, which sets to a gel on cooling. Like starch it does not form beads but could still be considered as material for microencapsulation.
Polycations, like chitosan or poly-l-lysine strengthen the alginate gel structure, and they can be used in coating of microcapsules [15, 47, 60, 67–69]. Chitosan is a positively charged polyamide that forms a semipermeable membrane around a negatively charged polymer [68].
Methods for microencapsulation
The techniques most commonly used in microencapsulation of probiotics are emulsion, extrusion and spray drying. The size of the obtained microcapsules is important because it influences the sensory properties of foods. Alginate beads made by the emulsion technique vary in size from 20 μm to 2 mm, and by the extrusion technique from 2 to 4 mm [54, 70] (Tables 3 and 4). With new extrusion technology, a bead size of 150 μm can be reached. Extrusion results in more uniformly shaped microcapsules than achieved by the emulsion technique [54]. Spray drying has been found to produce capsule sizes between 5 and 80 μm (Table 5). The average size of the starch granules which function as the adhesion base for bifidobacteria is reported to be 50 μm [3]. For encapsulation, probiotic bacteria are grown in their optimal culture conditions, after which they are centrifuged and used in suspension form or as freeze-dried powder. The use of freeze-dried powder is probably a more practical solution in the food industry because of the stability of the freeze-dried product during transport and storage.
Complex coacervation is a fluid–fluid phase separation of an aqueous polymeric solution. In the process, a change in pH results in formation of the shell by the polymer complex. Microcapsules are dried by freeze drying or spray drying. Complex coacervation is mainly used for microencapsulation of oils but it can also be used for probiotics [28, 78].
Emulsion technique
Sheu and Marshall [71] developed a method to entrap bacteria using a water/oil system. The encapsulation material, e.g. sodium alginate, is first mixed with the bacterial cells and the mixture is suspended in an oil bath containing Tween 80 as emulsifier. The emulsion is then broken by adding CaCl2, and the formed microcapsules are collected by centrifugation. Other materials, such as κ-carrageenan with KCl as the emulsion breaker or genipin cross-linked gelatin, can also be used to encapsulate probiotics by the emulsion technique [9, 33, 34]. Studies applying this technique for probiotic microencapsulation are listed in Table 3.
Reaction time affects the formation of microcapsules and, on the other hand, the survival of microorganisms [51]. The size of the calcium alginate beads has been observed to decrease as the concentration of sodium lauryl sulphate and Tween 80 increases [43, 44]. It is also possible to control the size of the microcapsules by utilizing a microporous glass membrane in the emulsification method [50].
Extrusion technique
Studies using the extrusion technique for probiotic microencapsulation are listed in Table 4. A hydrocolloid solution is first prepared, probiotics are added, and the solution is dripped through a syringe needle or nozzle. The droplets are allowed to fall into a hardening solution.
In this technique, alginate, κ-carrageenan, κ-carrageenan plus locust bean gum, xanthan plus gellan, alginate plus corn starch, and whey proteins have been used as wall materials for microencapsulation of lactobacilli and bifidobacteria [14, 36, 54, 57, 58].
The size of the microcapsules is affected by the nozzle size. Also, the diameter of the obtained alginate beads is found to increase as the concentration of sodium alginate increases [32], but the alginate concentration does not significantly influence the numbers of free cells [67]. A mixture of gellan and xanthan has better technological properties than alginate, κ-carrageenan, or locust bean gums [8], but the shape and size of the gellan and xanthan gum capsules has been found to vary [23, 24].
Adhesion to starch
A variety of starches and modified starches have been tested to entrap probiotic bacteria. For example, a calcium-induced alginate polymer containing Hi-Maize™ starch as a filler material has been used to encapsulate probiotics [26, 27]. Hylon VII maize starch granules have a high ratio of surface area to mass and also a good binding capacity. Low pH and protease have been found to inhibit adhesion of bifidobacteria to starch, so it is unlikely that adhesion is maintained during passage through the stomach [10]. Another starch-based microencapsulation method is to use starch granules combined with amylose coating [38, 79].
Spray drying of probiotics
Spray drying is a commonly used method of encapsulation in the food industry [18]. Spray drying involves atomization of an emulsion or a suspension of probiotics and carrier material into a drying gas, resulting in rapid evaporation of water. The capsules are obtained as dry powder. The spray-drying process is controlled by means of the product feed, gas flow and temperature [45, 62]. Besides polysaccharides, proteins can also be used as carriers; skim milk has proved to be a better wall material than gelatin, soluble starch and gum arabic, for instance [20–22]. However, lactose and milk powders containing lactose tend to stick to the walls of the dryer during processing if the temperature rises above the glass transition temperature [62]. Table 5 presents the coating materials and temperatures used for probiotic microencapsulation reported in the literature. Yet, despite the many advantages of the spray-drying method, the high temperatures needed to facilitate water evaporation lower the viability of the probiotics and reduce their activity in the final product.
Matrix encapsulation
A group of encapsulation technologies is referred to as matrix encapsulation because the microcapsules lack a core/shell structure but have a number of particles located at their surface. Still, the obtained properties are often sufficient to achieve the desired delayed release of the ingredient [78]. Encapsulation by MicroMAX® technology, for instance, using proteins, lipids and carbohydrates provides protection for probiotics during spray drying and storage, as well as during transit through the stomach [19]. In spray chilling, the atomization step is similar to spray drying, but the solidification of gel particles is based on the injection of cold air into the vessel. Spray chilling is a cheap technology that can be used to generate smaller beads [80]. Freeze drying of probiotic bacteria, where the frozen material is dried in a vacuum, is also widely used in industry.
Coating of microcapsules
Coating the microcapsules produced by different technologies with an additional film can prevent their exposure to oxygen during storage as well as improve their stability at low pH. Possible coating materials include chitosan, poly-l-lysine, alginate, starch, gum and gelatin [15, 17, 47, 67–69].
Chitosan-coated alginate beads are reported to provide better protection in simulated gastric conditions than poly-l-lysine or alginate coating [15]. Low-molecular weight chitosan has been found to show better control of cell release than high-molecular weight chitosan [68] and to result in more spherical beads without changing their size. Moreover, in a study on chitosan-coated alginate beads, beads coated with high-molecular weight chitosan partly collapsed [47]. Coating of microcapsules with alginate produces a uniform 1–2-μm thin exterior layer and has been found to improve the survival of bifidobacteria [9]. Coating the beads with poly-l-lysine and alginate is reported to limit Lactococcus lactis release but also to reduce the acidifying activity of the culture [67]. In a study by Reid et al. [58], beads produced with a commercial whey protein isolate and soaked in a milk-based solution were big in size, and they were not perfect spheres.
A two-step process involving emulsion and spray drying has been used to produce multiphase microcapsules [18, 73, 77]. In spray-drying, aqueous two-phase systems of soluble polymers can be utilized to design double-encapsulated ingredients in a single step, as bacterial cells tend to concentrate in one of the polymer phases [80].
Survival of microencapsulated probiotics
The survival of probiotic bacteria during processing, storage, and in gastric conditions is highly dependent on the strain used. Stability of the strain is thus one of the main criterias in selecting suitable probiotics. Further, food matrix environment has to be taken into account when selecting the materials for the microencapsulation.
Methods for studying survival rates
Survival rates of probiotic bacteria after various treatments have generally been studied by the plate count method, which is easy to carry out. Cultivability on plate, however, does not always tell the whole truth about the viability of bacteria. A non-cultivable population might still be metabolically active and provide the desired health-promoting effect in its target [81, 82]. The studies of microencapsulation of probiotics, however, do usually not contain any confirmation of health effects caused by encapsulated bacteria.
Thus, the use of commercial live/dead kits and flow cytometry can provide more information about the metabolic status of processed bacteria [38]. These methods are based on the use of fluorescent staining of nucleic acids, which distinguishes live bacteria with intact cytoplasmic membranes from dead bacteria with compromised membranes.
Moreover, the particle size distribution of the obtained microcapsules can be analysed by a light microscope, a scanning electron microscope or a laser diffractometer.
Survival of bacteria during encapsulation and drying
Thermal and osmotic resistance of lactic acid bacteria is species-dependent [20, 29, 35, 83]. The survival of probiotics after spray drying also depends on the kinds and concentrations of the carriers used as well as on the outlet temperature of the spray dryer [20, 53, 56]. Bacterial membranes are the main site of injury during spray drying [56]. Removal of water may damage cell membranes and associated proteins, because water is important in stabilizing biological molecules.
Typical survival rates in the spray-drying and freeze-drying processes are in the range of 70–85%. Although a survival rate may be acceptable, the prolonged storage stability of the product is often low. The presence of deoxidant and desiccant has been found to improve cell survival [20–22]. Sugars are known to protect dehydrated biomaterials, and it has been suggested that they act as water substitutes and replace water molecules around proteins and polar residues of membrane phospholipids. Sugars are also able to form hydrogen bonds with the proteins when water is removed and prevent protein denaturation [56]. It has been reported that disaccharides were effective in protecting both bacterial membranes and proteins during drying [84]. Cells from fresh cultures are reported to survive better than cells from freeze-dried cultures during encapsulation by emulsion and spray drying. The use of milk fat as the dispersing medium lowers the survival rates [18]. Studies also show that incorporation of soluble fibre from gum acacia (gum arabic) in a milk-based medium during storage prior to spray drying increases the viability of Lactobacillus paracasei [52], whereas a modified waxy maize starch coating does not improve the viability of Bifidobacterium cells [45].
In freeze drying, the drying media can have a greater effect on the stability of probiotics than microencapsulation itself [58]. Cryoprotectants can be added to maintain probiotic viability. Sultana et al. [40] reported that glycerol improved probiotic survival in freezing 100-fold. However, in long-term storage, the addition of a cryoprotectant or prebiotic has not been found to enhance the viability of microencapsulated cells [35, 56]. Wheat dextrin and polydextrose have proven promising fibre carriers to protect Lactobacillus rhamnosus during freeze drying [55]. Other results show that alginate offers better protection for probiotic bacteria than whey protein during freeze drying [85]. Micronization using a spiral jet mill reduces both the viability and heat resistance of freeze-dried bacteria, but the mortality rate is considered acceptable [83].
Addition of Hi-Maize starch to alginate has been found to result in a higher number of live bacteria in microcapsules [40]. Increasing the alginate concentration and capsule size also increases the survival of probiotics in heat treatment [49]. Chen et al. [13] reported that in heat treatment, the best protection for Bifidobacterium bifidum was provided by 2% sodium alginate combined with 1% gellan gum. The prebiotic effect of peptides was also confirmed. Another study observed that the use of non-fat milk in the extrusion process increases the number of viable cells [76].
Stability of microencapsulated probiotics in food
Table 6 summarizes specific applications of microencapsulated probiotics in foods.
Probiotics often have low viability in dairy products due to the high concentration of lactic and acetic acid, low pH, and the presence of hydrogen peroxide and oxygen. The viability of acid-sensitive bifidobacteria in yogurt can be increased by microencapsulation, but this effect depends on the strain [25]. Studies have reported that in yogurt, a high amount of bifidobacteria encapsulated in κ-carrageenan [33], gellan-xanthan [8], or alginate and Hi-maize [42], and some of cells encapsulated in whey protein [18], remain viable, whereas the number of free bacteria decrease significantly. Alginate has been found to offer better protection for probiotic bacteria in yogurt than whey protein [85]. Zhou et al. [68] reported that cells in an encapsulated state were relatively metabolically inert and that chitosan coating decreased the cell release rate during the early stages of fermentation. Of the prebiotics tested in a study by Capela et al. [35], raftilose best retained the viability of freeze-dried bacteria in fresh yogurt. Commercial yogurt products containing encapsulated lactic acid bacteria are already available in Korea (Doctor-Capsule, Bingrae Co., Kyunggi-do, Korea [32]) and in Taiwan (Kaung-Chuan Inc, [86]).
Encapsulation of bacteria in alginate has been found to improve survival rates by one log when compared to free cell counts when stored in skim milk for 24 h [25]. Microencapsulation of Lactobacillus bulgaricus with alginate and a chitosan coating can offer an effective means of maintaining their survival during storage in skim milk [47].
Encapsulation significantly improved the survival of probiotics in ice cream [30]. Entrapping in alginate is reported to increase the survival of lactobacilli by 40% during freezing of ice milk [71]. A study by Kebary et al. [87] showed that bifidobacteria microencapsulated in alginate survived freezing of ice milk better than when encapsulated in κ-carrageenan. The addition of glycerol and mannitol in preparing the alginate beads increased the survival rate, whereas glucose had no effect.
A study on probiotic spray-dried powder found that L. paracasei culture, spray-dried with skim milk, remained stable in Cheddar cheese during storage for at least seven weeks [53]. In other studies, however, microencapsulation did not increase the survival of probiotics during Cheddar cheese maturation [41] or during storage of feta cheese [27].
When probiotics are added to non-dairy products, factors like water activity, oxygen tension and temperature become important to their stability [3]. In spray drying, the highest moisture has been observed at the lowest outlet temperatures [37, 53, 56]. The water activity of spray-dried emulsions containing cellulose phthalate, or milk fat and whey protein film, has been found to vary from 0.107 to 0.230, which is within the values recommended for good stability of dried cultures [18, 29, 37, 77]. Other results show that water activity varied from 0.2 to 0.3 in microcapsules containing oil, caseinate, fructooligosaccharides, and dried glucose syrup or resistant starch [19]. Also, water activities between <0.03 and 0.17 have been observed for freeze-dried bacterial powders with fibres as carriers [55]. Lahtinen et al. [38] suggested that amylase coating of starch capsules is not sufficient to keep the water activity inside the capsules low when stored in oat drinks.
In mayonnaise, alginate-encapsulated bifidobacteria have been shown to survive better than free bacteria. After 10 weeks’ storage, mayonnaise with encapsulated probiotics was free of yeast and mould, while the controls were not, probably because of the antibacterial effect of the probiotics. The texture of the mayonnaise also improved with encapsulated probiotics [16].
A study on probiotics in fermented dry sausage reported that microencapsulation significantly improved the recovery of Bifidobacterium longum, but reduced the inhibitory action of probiotics against Escherichia coli O157:H7 [36]. On the other hand, microencapsulation has been found to enhance the survival of Bifidobacterium lactis in the traditional African fermented food mahewu when stored aerobically for three weeks. In another fermented food, amasi, the difference was less noticeable [24].
O’Riordan et al. [45] studied microencapsulation of probiotics as an approach to improve their viability during storage, finding that starch coating did not increase the viability of bifidobacteria in muesli or in a dry malted beverage powder. Another study reported that microentrapment with a milk-based solution or with whey proteins protected probiotic cells during the production of biscuit dough, but not during the storage of biscuits. During storage, the water activity in biscuits was too high to allow stable viability in the presence of oxygen [58]. L. rhamnosus have been found to survive well in chocolate-coated breakfast cereals when wheat dextrin and polydextrose carriers were used [55].
Reid et al. [58] observed microentrapment and a matrix based on milk enabled a high survival rate of L. rhamnosus in vegetable juice after two weeks’ storage when compared to free cells in a whey protein matrix. Viable counts dropped in frozen cranberry juice (pH 2.3), but again a matrix based on milk provided the best survival rate. Furthermore, oat flour with 20% β-glucan has been shown to have the best stability to fresh L. rhamnosus in apple juice, whereas freeze-dried cells survived poorly. L. rhamnosus with fibres as carriers did not change the pH of juice during three months’ storage [55]. Other studies show that encapsulation of bifidobacteria with potato starch does not enhance their culturability when stored in non-fermented or fermented oat drinks, whereas a lipid-matrix (cocoa butter) with polydextrose did result in improved protection against storage stress [38].
Sensory quality of foods with microencapsulated probiotics
Microencapsulation has certain consequences for the sensory quality of foods. Particle size influences the texture of foods, but particles with a diameter below 10 μm should not affect the mouthfeel properties of most foods [45]. The size of probiotic bacteria is typically 1–4 μm. The shape of the capsules, on the other hand, determines their flow properties, which is an important factor for industrial processes [23]. Moreover, in spray drying, the outlet temperature may affect the colour of the capsules due to a Maillard reaction [37].
The organic acid profile of fermented dairy products reflects the metabolic activity of the added bacterial cultures. Additional amounts of acetic and propionic acids produced by the probiotic organisms may cause reduced consumer acceptability of a product. Adhikari et al. [34] observed that encapsulation lowered the acetic acid content in yogurt significantly if bifidobacteria were added to the product before fermentation. The lactic acid content was dependent on the strains used [34]. Encapsulated probiotic bacteria have also been reported to lower the pH in yogurt during storage less than free bacteria [26].
The starch and sodium alginate used in the capsular matrix may have an influence on the mouthfeel of the product. Kailasapathy [26] observed that while encapsulated bacteria did not affect the colour, flavour or aftertaste of yogurts, their smoothness showed significant differences. The yogurts with encapsulated probiotics were considered more undesirable by a sensory panel [26]. Production of exopolysaccharide by probiotics and using starch as a filler polymer has been found to help maintain the stability of the yogurt gel [26] as well as to increase the water-holding capacity in feta cheese [27]. An Irish study found that the composition of Cheddar cheese was not affected by the addition of L. paracasei spray-dried with skim milk. Its sensory quality also reached the scores required for commercial-grade Cheddar cheese in Ireland [53].
McMaster et al. [24] detected no grittiness or texture change in the African fermented foods mahewu and amasi containing B. lactis. Microencapsulation actually helped to maintain the traditional flavour of amasi. Similarly, in sausage, microcapsules had no effect on texture or sensory quality in another study [72].
Survival under simulated physiological conditions
The general aim of microencapsulation is, firstly, to protect probiotic bacteria in foods and in the passage through the stomach, since free cells usually do not survive in gastric conditions, and secondly, to release the probiotics in their target, the gut. The survival of probiotic bacteria depends on the strain [28, 39, 47], and the type of food ingested also affects the survival rate of probiotics in the gastric environment [17].
The survival of probiotics is commonly studied under simulated physiological conditions. Simulated gastric juice typically consists of pepsin and sodium chloride adjusted to pH 1–3 with HCl. A simulated intestinal solution consists of bovine or porcine bile and pancreatin at pH 7.4–7.5 [9, 13, 18, 21, 49].
Several studies have shown that microencapsulation of bacteria with alginate or whey proteins protects them against acid stress, allowing the cells to survive in the stomach and be delivered in the intestines [18, 44, 47, 54, 69, 76]. An optimal capsule combination reported in the literature for probiotic survival in gastric conditions is 3% sodium alginate, 1% pancreatic digested casein and 3% fructooligosaccharides [75, 76]. Also caseinate and fructooligosaccharides with either dried glucose syrup or resistant starch are found to provide protection [19]. It has also been reported that the diameter of alginate microcapsules decreases in simulated stomach exposure [39]. Various results indicate that increasing the alginate concentration and capsule size enhances the survival of probiotics [32, 46, 49], whereas the CaCl2 concentration [46] and the initial cell numbers do not affect bacterial death rates [32].
L. rhamnosus and bifidobacteria encapsulated with starch have been shown to survive passage through the human gastrointestinal tract [3, 21], whereas Hi-Maize starch encapsulation did not protect Lactobacillus acidophilus or Bifidobacterium infantis from high acid conditions in a study by Sultana et al. [40]. Bifidobacteria encapsulated with gellan and xanthan, gum arabic and gelatin, and skim milk in simulated gastric juice have been found to survive somewhat better than free cells [8, 21, 24]. Also, the surface characteristics of microporous glass membrane microcapsules protect probiotic cells even in highly acidic conditions [50].
Studies also show that coating of microcapsules enhances the survival of probiotics in simulated gastric conditions [9, 12]. With chitosan coating, the higher the molecular weight of the chitosan, the better the survival of probiotic cells in simulated gastric juice [11]. An in vivo study of the survival of Saccharomyces boulardii yeast in alginate microspheres with and without chitosan coating indicated that 13.3% of the uncoated and 9% of coated yeast ingested were viable in rat faeces, whereas only 2% of free cells survived [88].
The release of probiotics from microcapsules at their target is essential for their colonization in colon. Exposure to simulated intestinal juice at alcalic pH solubilizes the alginate and releases the cells [9, 49]. Similarly, whey protein and MicroMAX® capsules as well as capsules of chitosan-coated alginate or skim milk mixed with alginate were completely dissolved and probiotics released when put in simulated intestinal juice pH 7.4 [18, 19, 47, 76].
Future challenges
Microencapsulation of probiotic bacteria in foods on an industrial scale faces technological, microbiological, and financial challenges, and also questions linked to consumer behaviour. More research data on appropriate technologies, carrier matrices, and bacterial strains is still required in order to promote surviving of bacteria under heat, osmotic and oxygen stresses as well as digestive stress [89]. Probiotic-containing food supplies are considered as functional food, and their market is continuously growing in several countries. The extra costs incurred by microencapsulation have to be realistically estimated so that they can be minimized. Future development efforts must also take into account the growing consumer interest in healthy food as well as in ecological aspects.
Selection of appropriate techniques, materials, and bacterial strains
The main challenge in applying microencapsulation of probiotics to new foods to meet consumer interests has to do with finding the appropriate microencapsulation technique, safe and effective encapsulating materials, and potent bacterial strains. Microencapsulation is expected to extend the shelf life of probiotics at room temperature in various food matrices, increase their heat resistance, improve their compression and shear stress resistance, and enhance their acid tolerance [90]. Environmentally conscious consumers also expect the applied technology to be nature-friendly and avoid the use of hazardous chemicals. Also, aqueous coating systems should be preferred to prevent harmful effects from organic compounds.
A wide variety of potential microencapsulation techniques are already available nowadays. The use of supercritical carbon dioxide is an interesting recent approach [92]. As to the basic techniques, the weak points of the spray drying, low survival rates and low stability during storage are tried to overcome by seeking strains that tolerate elevated temperatures, optimizing processing parameters, selecting appropriate drying medium, and using a stabilizing precondition treatment. Effective thermoprotectants are known to enhance the survival of probiotics in spray drying [56, 91]. Extrusion and emulsion techniques avoid using high temperatures during encapsulation process. By both methods high survival rates of bacteria are achieved. Because the emulsion techniques are easier to scale up and the size of the beads is smaller, these techniques probably have potential to develop into large-scale technology. For instance, the microporous glass membrane emulsification technique is both simple and easy to scale up [50]. The costs of the emulsion techniques are, however, increased by the use of vegetable oil [70].
The selected microencapsulation technique determines the materials used. This means, for instance, evaluating the thermal conductivity properties of food-grade biopolymers and lipids [61]. Probiotic/prebiotic combinations may be among the most important interests in the future. New carrier materials of natural origin, such as shellac and fruit polysaccharides, are also being tested [93, 94]. Special attention needs to be paid to their safety to create consumer confidence, and all raw materials must naturally be of food-grade quality. The fact that most food companies in Europe do not use ingredients derived from genetically modified organisms may restrict the use of some products, e.g. maize-derived starches. Bovine spongiform encephalopathy (BSE) and foot-and-mouth disease epidemics have reduced consumer confidence in the use of materials of animal origin, e.g. gelatin [95].
Several criteria are proposed for selecting a preferable probiotic strain for use in health foods [2, 3]. Some of these, like the probiotic’s tolerance to acid, human gastric juice and bile, are facilitated by microencapsulation. Other important concerns include the adherence of the strain to epithelial surfaces and persistence in the human gastrointestinal tract, antagonistic activity against pathogens, antimutagenic and anticarcinogenic properties, and immunostimulation. Often the persistence in the human gastrointestinal tract is tested in vitro in the connection of the microencapsulation experiments. More research is needed to confirm that the in vivo health effects of bacteria retain in microencapsulation processes.
The technology also has to consider the large size of the microbial cells (typically 1–4 μm) and particles of the freeze-dried culture (even exceeding 100 μm), which demands a large capsule size that influences the textural and sensorial properties of foods. On the other hand, larger microcapsules have been proven to give better protection to bacteria [54].
Altogether more than 1000 patents have been issued concerning various microencapsulation processes [78]. Interesting alternatives include gelation of proteins at high pressure (EP 0750854) or by heat (US5601760, [96]), as well as a film-forming protein-carbohydrate-oil emulsion (WO2005030229-A1, [19]).
Recognition of new applications
Until lately, probiotic microencapsulation techniques have mostly been applied to dairy products such as yogurt, milk, dry dairy beverages, frozen desserts and cheese. The selection is now expanding to fruit juices, muesli bars and cookies (Table 6). Probiotics have recently been added, for instance, to chocolate products [97]. Still, product development for new areas is very challenging due to the harshness of the product environment to probiotics. Developing new products, in which food matrix supports microbial survival in the gastrointestinal tract in combination with encapsulation, may prove to be successful. Adding probiotics to food systems is also seen as a means to restore initially food-associated microflora destroyed during processing, in sterilization, pasteurization disinfection, washing, etc. [98]. Microencapsulation can help to promote this objective.
Microencapsulation technology is not yet fully developed and requires additional experimental work. Indeed, new technical innovations are continuously being introduced. Companies using microencapsulation technology hold various expectations for new, high-volume products—such as carbonless paper was in the paper industry. The food industry also needs further expertise to be able to estimate the most promising commercial applications for the future.
Costs of microencapsulation
There are at least two reasons for the higher costs caused by encapsulation. The first has to do with product development. New, potent bacterial strains may call for novel encapsulation procedures, and the development of products takes both time and financial resources. Secondly, the microencapsulation phase adds costs to food processing. Since the margins in food ingredients are relatively low, encapsulated end products will have higher prices. The effect may vary greatly depending on the used technique and the volume of the product. Spray chilling, rarely reported for probiotics, is considered the least expensive encapsulation technology [78]. Encapsulation of probiotics using natural biopolymers is often difficult to scale up, and the processing costs are high [60, 78, 99]. Polysaccharides, e.g. alginate, and proteins are expensive to use in spray drying because of their low solubility in water [78]. Milk proteins are more costly than carbohydrates [62], but whey proteins are often available as a by-product of the dairy industry.
On the other hand, cost savings can be derived from easier manufacture of products, lower wastage of bacterial material and better health impact of the product. Brownlie [95] estimates that the price of encapsulated probiotic bacteria may be two or three times that of non-encapsulated probiotics. Nevertheless, despite the extra costs, microencapsulation has profit-making potential, e.g., in markets for higher-value products, in products where microencapsulation is absolutely necessary, and in markets where scale economies can be applied [95].
Conclusions
The use of probiotics in the food industry is currently expanding from dairy products to other categories such as juices, energy bars, and chocolate products. In these new products, the environment for probiotic survival is even more challenging than in dairy foods. Microencapsulation has proven one of the most potent methods for maintaining high viability and stability of probiotic bacteria, as it protects probiotics both during food processing and storage as well as in gastric conditions. Besides the polysaccharides traditionally used as a matrix in microencapsulation, starches, gelatin, and milk proteins can also be employed as bead material. New materials are being tested for carrier matrices. One of the major interests in the future concerns the use of probiotic/prebiotic combinations. Another question concerns the coating of capsules, which not only enhances the stability of cells but also increases the capsule size.
Techniques for encapsulation are developing, and new industrial-scale methods are being made available. Emulsion technology, in particular, shows many promising applications. Consumer health issues and environmental consciousness deserve special attention in the design of future carrier matrices and technology. Further research on these issues will benefit the development of novel functional food products.
References
Fuller R (1989) J Appl Bacteriol 66:365–378
FAO/WHO (2006) Probiotics in food. Health and nutritional properties and guidelines for evaluation, FAO Food and Nutrition Paper No. 85. World Health Organization and Food and Agriculture Organization of the United Nations, Rome
Mattila-Sandholm T, Myllärinen P, Crittenden R, Mogensen G, Fonden R, Saarela M (2002) Int Dairy J 12:173–182
Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T (2000) J Biotechnol 84:197–215
Kurmann JA, Rašić JLJ (1991) In: Robinson RK (ed) Therapeutic properties of fermented milks, Elsevier Applied Science, London, pp 117–157
Ouwehand AC, Salminen SJ (1998) Int Dairy J 8:749–758
Parvez S, Malik KA, Ah Kang S, Kim HY (2006) J Appl Microbiol 100:1171–1185
Sun W, Griffiths MW (2000) Int J Food Microbiol 61:17–25
Annan NT, Borza AD, Hansen LT (2008) Food Res Int 41:184–193
Crittenden R, Laitila A, Forssell P, Matto J, Saarela M, Mattila-Sandholm T, Myllärinen P (2001) Appl Environ Microbiol 67:3469–3475
Liserre AM, Re MI, Franco BDGM (2007) Food Biotechnol 21:1–16
Guerin D, Vuillemard JC, Subirade M (2003) J Food Prot 66:2076–2084
Chen MJ, Chen KN, Kuo YT (2007) Biotechnol Bioeng 98:411–419
Dinakar P, Mistry VV (1994) J Dairy Sci 77:2854–2864
Krasaekoopt W, Bhandari B, Deeth H (2004) Int Dairy J 14:737–743
Khalil AH, Mansour EH (1998) J Food Sci 63:702–705
Jung JK, Kil JH, Kim SK, Jeon JT, Park KY (2007) J Food Sci Nutr 12:58–63
Picot A, Lacroix C (2004) Int Dairy J 14:505–515
Crittenden R, Weerakkody R, Sanguansri L, Augustin MA (2006) Appl Environ Microbiol 72:2280–2282
Lian WC, Hsiao HC, Chou CC (2002) Int J Food Microbiol 74:79–86
Lian W, Hsiao H, Chou C (2003) Int J Food Microbiol 86:293–301
Hsiao H, Lian W, Chou C (2004) J Sci Food Agric 84:134–139
McMaster LD, Kokott SA, Slatter P (2005) World J Microbiol Biotechnol 21:723–728
McMaster LD, Kokott SA, Reid SJ, Abratt VR (2005) Int J Food Microbiol 102:231–237
Talwalkar A, Kailasapathy K (2003) Aust J Dairy Tech 58:36–39
Kailasapathy K (2006) LWT 39:1221–1227
Kailasapathy K, Masondole L (2005) Aust J Dairy Tech 60:252–258
Oliveira AC, Moretti TS, Boschini C, Baliero JCC, Freitas O, Favaro-Trindade CS (2007) J Microencapsul 24:673–681
Favaro-Trindade CS, Grosso CR (2002) J Microencapsul 19:485–494
Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH (2008) Food Chem 111:50–55
Ding WK, Shah NP (2007) J Food Sci 72:M446–M450
Lee KY, Heo TR (2000) Appl Environ Microbiol 66:869–873
Adhikari K, Mustapha A, Grun IU, Fernando L (2000) J Dairy Sci 83:1946–1951
Adhikari K, Gruen IU, Mustapha A, Fernando LN (2002) J Food Qual 25:435–451
Capela P, Hay TKC, Shah NP (2006) Food Res Int 39:203–211
Muthukumarasamy P, Holley RA (2007) Food Microbiol 24:82–88
Su L, Lin C, Chen M (2007) Int J Dairy Technol 60:49–54
Lahtinen SJ, Ouwehand AC, Salminen SJ, Forssell P, Myllärinen P (2007) Lett Appl Microbiol 44:500–505
Martoni C, Bhathena J, Urbanska AM, Prakash S (2008) Appl Microbiol Biotechnol 81:225–233
Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K (2000) Int J Food Microbiol 62:47–55
Godward G, Kailasapathy K (2003) Milchwissenschaft 58:624–627
Godward G, Kailasapathy K (2003) Milchwissenschaft 58:396–399
Shah NP, Ravula RR (2000) Aust J Dairy Technol 55:139–144
Shah NP, Ravula RR (2004) Dairy Industries Internat 69:31–32
O’Riordan K, Andrews D, Buckle K, Conway P (2001) J Appl Microbiol 91:1059–1066
Chandramouli V, Kailasapathy K, Peiris P, Jones M (2004) J Microbiol Methods 56:27–35
Lee JS, Cha DS, Park HJ (2004) J Agric Food Chem 52:7300–7305
Li XY, Chen XG, Cha DS, Park HJ, Liu CS (2008) J Microencapsul 1–10
Mandal S, Puniya AK, Singh K (2006) Int Dairy J 16:1190–1195
Song SH, Cho YH, Jiyong P (2003) J Food Sci 68:195–200
Yáñez-Fernández J, Ramos-Ramírez EG, Salazar-Montoya JA (2008) Eur Food Res Technol 226:957–966
Desmond C, Ross RP, O’Callaghan E, Fitzgerald G, Stanton C (2002) J Appl Microbiol 93:1003–1011
Gardiner GE, Bouchier P, O’Sullivan E, Kelly J, Kevin Collins J, Fitzgerald G, Paul Ross R, Stanton C (2002) Int Dairy J 12:749–756
Muthukumarasamy P, Allan WP, Holley RA (2006) J Food Sci 71:M20–M24
Saarela M, Virkajärvi I, Nohynek L, Vaari A, Matto J (2006) Int J Food Microbiol 112:171–178
Ananta E, Volkert M, Knorr D (2005) Int Dairy J 15:399–409
Reid AA, Vuillemard JC, Britten M, Arcand Y, Farnworth E, Champagne CP (2005) J Microencapsul 22:603–619
Reid AA, Champagne CP, Gardner N, Fustier P, Vuillemard JC (2007) J Food Sci 72:M31–M37
Shahidi F, Han XQ (1993) Crit Rev Food Sci Nutr 33:501–547
Anal AK, Singh H (2007) Trends Food Sci Technol 18:240–251
Bhattacharyya S, Borthakur A, Dudeja PK, Tobacman JK (2008) J Nutr 138:469–475
Vega C, Roos YH (2006) J Dairy Sci 89:383–401
Mozzi F, Gerbino E, Font de Valdez G, Torino MI (2009) J Appl Microbiol 107:56–64
Nisperos-Carriedo MO (1994) In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (ed) Edible coatings and films to improve food quality, Technomic Publishing Company, Lancaster
Young S, Wong M, Tabata Y, Mikos AG (2005) J Controlled Release 109:256–274
Baziwane D, He Q (2003) Food Rev Int 19:423–435
Champagne CP, Gaudy C, Poncelet D, Neufeld RJ (1992) Appl Environ Microbiol 58:1429–1434
Zhou Y, Martins E, Groboillot A, Champagne CP, Neufeld RJ (1998) J Appl Microbiol 84:342–348
Graff S, Chaumeil JC, Boy P, Lai-Kuen R, Charrueau C (2008) Biol Pharm Bull 31:266–272
Krasaekoopt W, Bhandari B, Deeth H (2003) Int Dairy J 13:3–13
Sheu TY, Marshall RT (1993) J Food Sci 58:557–561
Muthukumarasamy P, Holley RA (2006) Int J Food Microbiol 111:164–169
Picot A, Lacroix C (2003) Lait 83:237–250
Chen K, Chen M, Liu J, Lin C, Chiu H (2005) J Food Sci 70:M260–M266
Chen K, Chen M, Lin C (2006) J Food Eng 76:313–320
Ross GR, Gusils C, Gonzalez SN (2008) Biol Pharm Bull 31:2121–2125
Picot A, Lacroix C (2003) Int Dairy J 13:455–462
Gouin S (2004) Trends Food Sci Technol 15:330–347
Myllärinen P (2002) VTT-Publications, 473: pp 125
Champagne CP, Fustier P (2007) Curr Opin Biotechnol 18:184–190
Amor KB, Breeuwer P, Verbaarschot P, Rombouts FM, Akkermans ADL, De Vos WM, Abee T (2002) Appl Environ Microbiol 68:5209–5216
Lahtinen SJ, Ahokoski H, Reinikainen JP, Gueimonde M, Nurmi J, Ouwehand AC, Salminen SJ (2008) Lett Appl Microbiol 46:693–698
Picot A, Lacroix C (2003) J Food Sci 68:2693–2700
Leslie S, Israeli E, Lighthart B, Crowe J, Crowe L (1995) Appl Environ Microbiol 61:3592–3597
Kailasapathy K, Sureeta BS (2004) Aust J Dairy Technol 59:204–208
Chen M, Chen K (2007) In: Lakkis JM (ed) Encapsulation and controlled release technologies in food systems, 1st edn, Blackwell Publishing, Ames, pp 83–112
Kebary KMK, Hussein SA, Badawi RM (1998) Egypt J Dairy Sci 26:319–337
Graff S, Hussain S, Chaumeil JC, Charrueau C (2008) Pharm Res 25:1290–1296
Ross RP, Desmond C, Fitzgerald GF, Stanton C (2005) J Appl Microbiol 98:1410–1417
Siuta-Cruce P, Goulet J (2001) Nutraceuticals now Winter 2001 issue—Health and Wellness with Probiotics
Saarela MH (2007) AgroFOOD Industry Hi-tech 18:19–21
Thantsha MS, Cloete TE, Moolman FS, Labuschagne PW (2009) Int J Food Microbiol 129:88–92
Stummer S, Salar-Behzadi S, Viernstein H (2009) Sci Pharm 77:231
Ying DY, Parkar S, Luo XX, Seelye R, Sharpe JC, Saunders J, Schroeder R (2006) XIV international workshop on bioencapsulation & COST 865 Meeting, Lausanne, Switzerland, 5–7 October, 2006, p 57
Brownlie K (2007) In: Lakkis JM (ed) Encapsulation and controlled release technologies in food systems, 1st edn, Blackwell Publishing, Ames, pp 213–233
Lee SJ, Rosenberg M (2000) LWT 33:80–89
Barnhoorn R (2008) World of Food Ingredients 52:54–55
Siuta-Cruce P, Goulet J (2001) Food Technol 55:36–42
Doleyres Y, Lacroix C (2005) Int Dairy J 15:973–988
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rokka, S., Rantamäki, P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur Food Res Technol 231, 1–12 (2010). https://doi.org/10.1007/s00217-010-1246-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1246-2