Abstract
Probiotics have evolved as a successful heavy-metal removal approach in aquaculture. In this study, probiotic Lactobacillus plantarum encapsulated with alginate/chitosan nanoparticles were evaluated for lead toxicity mitigation, growth, and hematological development and modification in the intestinal enzyme activity in rainbow trout (Oncorhynchus mykiss). Six hundred juvenile fish (15±1.2 g; Mean±SD) were divided into five groups in triplicates as follows: first and second groups (G1 and G2) were fed with encapsulated and free Lactobacillus plantarum (108 CFU g-1) respectively. The third group (G3) was fed with diets containing alginate/chitosan free probiotic, and the control groups (G4 and G5) were fed with basal diet. After 8 weeks, lead acetate salt (500 μg g-1 of feed) was used for 3 weeks in all groups except G5. Growth, hemato-immunological indices, expression of metallothionein gene in liver as well as intestinal enzymes activity were evaluated at day zero, and weeks 8, 9, 10, and 11. Results showed that almost all growth indices and some immunological significantly (P<0.05) improved in G1 compare to control group. Hematological parameters, intestinal enzymes activity, and resistance against bacterial challenge as well as metallothionein gene expression elevated in G1 and G2 compared to the other groups (P<0.05). Hence, based on the results of this experiment, it can be concluded that using microencapsulated L. plantarum with alginate/chitosan in the diet not only reduced lead destructive toxicity, but also improved the growth and hemato-immunogical indices and resistance against bacterial challenge in rainbow trout, so this method is recommended to remove lead from the polluted ecosystems
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic environments are at high risk of contamination with various pollutants, including heavy metals (Rahman et al. 2012). Heavy metal contamination has become a serious problem due to its chemical stability, biodegradability, and ability to bioaccumulate in living organisms (Emenike et al. 2022; Das et al. 2022). Although these metals are naturally present in the environment, their levels are increased by human activities such as the rapid growth of industries, urbanization, and the increase in wastewater (Chen et al. 2022). Metals, with their complex structure, disrupt the normal activity of cells, and this leads to different physiological and biochemical responses in the body of living organisms (El-Greisy and El- Gamal 2015). Lead is one of the most important heavy metal pollutants in aquatic ecosystems. In aqueous environment, most of the lead is deposited in sediments and some of it remains soluble in water. When the pH of water suddenly drops, large amounts of precipitated lead are released and dissolved in water. Thus, lead-containing sediments are a very dangerous source for aquatic organisms (Chen et al. 2022; Lee et al. 2019). The mechanism of lead toxicity has been attributed to direct damage to mitochondria, disturbance in calcium homeostasis, competition for zinc-binding sites, disruption of intracellular sulfhydryl homeostasis, and induction of oxidative stress (Shi et al. 2014). Therapeutic and preventive strategies for the control of heavy metal poisoning have been reported in several studies However, these approaches are not necessarily enough considering a vast area and the amount of pollution, meanwhile most of these strategies have implementation problems (Bhattacharya 2019).
Probiotics positively affected fish health and can reduce the pollutant side effects in fish (Kirillova et al. 2017). The heavy metals elimination from an organism using probiotics has been successfully applied in the past few decades and recently termed as “gut remediation” (Liu et al. 2019). The beneficial effects of probiotics directly depend on their presence in the hindgut. Due to probiotics’ sensitivity to various conditions such as acidic conditions, bile salts, bacteriophages, and enzymes in the gastrointestinal tract, probiotics need methods to increase their bioavailability in target organs (Abumourad et al. 2013). Microencapsulation is a process in which probiotic cells are incorporated into an encapsulating matrix or membrane to provide a microenvironment favorable to the encapsulated microorganism (Antunes et al. 2013; Hooshyar et al. 2020). In recent years, several studies have been conducted in the field of probiotic microcoating using various materials such as alginate, starch, xanthan-gelan, chitosan, gelatin, which enhancing probiotic bacteria’s bioavailability (Homayouni et al. 2008; Nezamdoost et al. 2023). Some novel researches reported that probiotics can reduce adverse effect of lead toxicity in fish and microencapsulation of probiotics can improve their probiotic characterizations (Kirillova et al. 2017). Then in this study the effect of lactobacillus plantarum (none encapsulation) and nano/microencapsulated with alginate/chitosan on growth indices, hemato-immunological and intestinal enzymes parameters of rainbow trout (Oncorhynchus mykiss) after poisoning with Lead was evaluated.
Materials and method
Experimental design
Six hundred apparently healthy rainbow trout with average weight of 15 ± 1.2 g were obtained from trout farms without any disease in the last three years and transferred to the wet laboratory of the Faculty of Veterinary Medicine of the Shahid Chamran University of Ahvaz and stored for 2 weeks under standard conditions (15°C, pH=7.8–8.1) for adaptability to laboratory conditions. During adaptation and experiment period chemo-physical parameters of water maintained in optimum condition by means of aeration pumps, biofilters and frequent water changes (100% daily) for all groups. The health status of the investigated fish was examined by taking wet mount samples from the skin and gills for external parasitic diseases and bacteriological sampling from the brain and kidney for bacterial diseases. During the adaptation period, the fish were fed twice daily with 3% of the body weight of the commercial diet (Biomar, France company). After the adaptation period, the fish were randomly divided into five equal groups in triplicates (40 fish in each replicate). The diet of the first group (G1) contained 108 CFU g−1 of L. plantarum encapsulated with alginate and chitosan; the second group (G2) was fed with a diet containing 108 CFU g−1 L. plantarum; the third group (G3) was fed with diet incorporated with alginate and chitosan (the same amount of encapsulated group without probiotic); the fourth group (G4) was fed with basal feed (negative control) and the fifth group (G5) was fed with diet incorporated with lead without any enrichment (positive control). After 8 weeks, all groups (except G4= negative control) were exposed to lead in the form of acetate salt (500 μg/gr) for three weeks (Alves and Wood 2006). Fish were sampled at day zero and at the end of 8th, 9th, 10th, and 11th weeks of experiment. By the end of the period, remained fish challenge with Streptococcus iniae and cumulative mortality compered among the groups.
Preparation of the suspension of probiotic
The probiotic L. plantarum, which was identified using the 16S rRNA gene, isolated from the gut of native fish, Tor grypus (Mohammadian et al. 2016). The bacterium was isolated and identified using the 16S rRNA gene (Mohammadian 2016). L. plantarum was cultured in MRS broth under anaerobic conditions. After the growth, the bacteria were rinsed with a centrifuge (1500 rpm, 15 min), and its concentration adjusted by McFarland standard tubes, and plated count method. Probiotic concentration was adjusted to, 2.4 ×109 CFU ml-1 and final concentration of probiotics in the feed was adjusted to 108 CFU g-1. Sterile PBS was sprayed on food of the control group (Vine et al. 2006).
Microencapsulation process
Internal emulsion method was used according to (Song et al. 2013). First, calcium carbonate (Merck,102066) suspension was slowly added to sodium alginate solution, then the resulting solution was slowly mixed with bacterial suspension (2.4 ×109). In the other beaker, olive oil (Sigma: O1514) with 1.5% Span 80 (Sigma: 85548), then mix the contents of the first and the second beaker with an electric mixer for 15 minutes. In the third beaker, olive oil was mixed with 5% acetic acid and added dropwise to the above solution to reach a pH of 3.5. The oil was separated from the solution by adding phosphate buffer and centrifuging the product. In the next step, 15 ml of 0.4% chitosan solution was added to the solution and stirred thoroughly for one hour. The resulting solution was finally centrifuged at 4,000 rpm for 15 min (Song et al. 2013).
Characteristics of microparticles
Efficacy of microencapsulation
To estimate the number of probiotic cells in the encapsulation product, the initial number of incorporated bacteria for the encapsulation procedure was counted. One gram of microencapsulated probiotic was dissolved in 99 ml of 1% w/v sterile sodium citrate at pH 7.2. After 20 min at room temperature, microspheres were dissolved entirely and bacteria were released; the number of bacteria was counted using serial dilution and pour plate method on MRS agar (Halimi et al. 2020). The ratio of bacteria in the microencapsulated product to the initial bacteria was determined as microencapsulation efficiency.
Analysis of particle size and distribution
Size and distribution of the particles were determined by photon correlation spectroscopy using a particle size analyzer (ScatterScope 1 qudix, Korea). The microcapsules were dispersed in disinfected deionized water and the consequences were examined based on the mean diameter (VMD) of the particle ± standard error, dpeak, d10, d50, and d90, based on the mean diameter formulas.
Determining the zeta potential
The zeta potential of the particles dispersions was determined using a zeta sizer (Zen 3600; Malvern instruments; UK). The encapsulated probiotics dispersion was diluted (1:100 (v/v)) with double distilled water before the assessments.
Determination of particle morphology
The phase contrast microscopy of particles was used for morphology of particles in different phases of encapsulation procedure. To determine particle ultrastructure and the shape and the surface morphology of the particles, SEM technique was used by electron microscopy (LEO 1455 VP, Germany) with the electron beam 10 kV. Accordingly, capsules were fixed and dried with ascending concentration of ethanol (25%, 50%, 75%, and 96%) and then double-sided glue on cautery (SC 7620, England) was used. Finally, particles coated with gold and palladium for 2 min and SEM images were prepared.
Preparation of simulated gastric conditions
Simulated gastric fluid conditions were performed according to the method of (Pinto et al. 2006). The pepsin (7000 Sigma Aldrich P) was mixed with 0.5% sodium chloride solution to a concentration of 3 g/l, and then its pH was reduced to 2 and 4 by sterile hydrochloric acid (0.1 M). It was sterilized by 0.45 μm (Millipore) microfilter.
Preparation of simulated intestinal conditions
Simulated intestinal fluid conditions were performed according to the method of (Charteris et al. 1998). Thus, pancreatin (1500Sigma Aldrich P) was mixed with 0.5% sodium chloride to a final concentration of 1 g / L with 4.5% bile salt solution (Oxoid, Basingstoke UK), then pH with 0.1 M sodium bicarbonate solution. The sterilization was reduced to about 7.2, the resulting solution was sterilized by a 0.45 μm microfilter.
Evaluation of bacterial viability in simulated gastric and intestinal conditions
The method recommended by Vizoso (Pinto et al. 2006) was used to compare the survival of bacteria in normal and microscopic conditions in simulated gastric and intestinal conditions. To prepare gastric juice, electrolyte solution containing 6.23 g/l sodium chloride, 2.29 g/l potassium chloride, 0.229 g/l calcium chloride and 1.2 g/l sodium bicarbonate in sterile form and its pH with hydrochloric acid 8 normal to Decreased by 2 ± 0.2. During the experiment, pepsin was added to a final concentration of 0.3%. Simulated intestinal juice was prepared using sterile electrolyte solution containing 0.239 g / l potassium chloride, 1.28 g / l sodium chloride, 6.4 g / l calcium bicarbonate and 0.5% oxal (X gal).
Then, 1 g of bacterial capsules or the equivalent of one ml of free bacterial suspension was poured into a tube containing 9 ml of gastric juice. Place in a 25°C incubator for two hours, shaking, then remove. Transfer to a tube containing 10 ml of the simulated intestinal medium and incubate for 2 h at 25°C, during which time the number of viable cells at 0-, 30-, 60-, 90-, and 120-min intervals. In samples containing probiotics, it was cultured freely using Peptone Water dilution method and counted after 24 to 48 h in the incubator. This was done with 3 replications for each sample.
Growth indices
The growth indices of experimental groups were calculated and compared based on following formula:
Daily weight gain (DWG): (FW – IW) / experimental period (d).
Specific growth rate (SGR): [(ln FW – ln IW) ×100] / experimental period (d).
Feed conversion ratio (FCR): feed consumed (g) / (FW – IW).
Protein efficiency ratio (PER): (FW – IW) / protein intake (g).
Condition factor (CF): (FW × 100) / standard length3 (cm).
Hematological parameters
Three fish from each replicate were selected and blood was collected from the caudal vein by heparinized syringe. The hematological profile was determined immediately after collection of whole-blood samples using a manual hematology method (Feldman et al. 2000). Hemoglobin (Hb) measurement was determined by the cianometahemoglobin method. Packed cell volume (PCV) was determined by micro-hematocrit apparatus. Total red blood cell was calculated by Neubauer hemocytometer after diluting in Natt–Herrick solution. The blood sample was diluted with Natt–Herrick solution to determine total white blood cell (TWBC) by using Neubauer hemocytometer chamber; then, the Total WBC was calculated.
Digestive enzyme activity
To evaluate the effect of probiotics on digestive enzymes, alpha-amylase activity was measured according to the modified Bernfeld method as described previously (Areekijseree et al. 2004), trypsin (Erlanger et al. 1961), chymotrypsin (Hummel 1959), lipase (Worthington 1991), protease, and alkaline phosphatase (Badoei-Dalfard et al. 2020) were sampled from the gastrointestinal tract of fish (3 pieces per replication) (Chang et al. 2002). The fish intestines were immediately stored in a nitrogen tank (-196°C) until enzymatic activity was measured (Kuzmina 2010).
Immunological responses of treated fish
Respiratory burst activity (NBT) Assay
To evaluate the respiratory burst of leukocytes, 100 μl of heparinized blood was placed in microplate wells and 100 μl of 0.2% nitrobltetrazolium (NBT) solution was added. The plate was incubated for 30 min at laboratory temperature and then 0.1 ml of the resulting mixture was added to a test tube containing 2 ml of dimethylformamide. After centrifugation of the sample at 3000 rpm for 10 min, the optical absorption of the supernatant was read at 620 nm (Ellis 1990).
Measurement of total protein and plasma immunoglobulin
Total immunoglobulin concentration was measured based on the method described by (Ellis 1990). In summary, total protein and plasma albumin were measured using total protein and albumin kit (Pars Azmoun Co, Iran). Serum total immunoglobulin was calculated by subtracting albumin from total plasma protein.
Serum lysozyme activity
Serum lysozyme activity was measured by turbidity method based on (Ellis 1990) with some modification. For this purpose, 10 μl of serum with 200 μl of suspension of Micrococcus lysodeikticus (0.2 mg/ml) (Sigma) in 0.05 mol of sodium phosphate buffer, pH = 6.2 was added to 96-well pellets and light absorption Samples were read after 1 and 6 minutes using an ELISA pellet reader at 530 nm. Each unit of enzyme activity was measured as the amount of enzyme that reduced uptake by 0.001 per minute per ml of serum. Different concentrations of this enzyme were compared using standard curves of egg white lysozyme concentrations. PBS was used as a blank.
Complement activity
The activity of complement system (ACH) was measured spectro-photometrically at 540 nm by the hemolysis of rabbit red blood cells (RRBC) based on the method described by (Yano et al. 1988) with some modifications. For this purpose, serum samples were first diluted in Veronal buffer and then 1% RRBC suspension was added. After previous stage, the solution was incubated for 24 h at 4°C and then centrifuged at 3500 g for 5 min. Eventually, the supernatant (150 μl) was poured into wells of an ELISA reader (Accu Reader, Taiwan) to read the optical density at 540 nm.
Bactericidal activity
The bactericidal activity was assayed dependent on the method for Budino et al. 2006 with some changes. Briefly, 50 μl of bacterial suspension (106 cfu ml-1) was added to 25 μl of serum sample diluted in 25 μl sterile PBS. The brooding procedure of this suspension was done for 6 h at room temperature. After brooding, 50 μl MTT (dimethylthiazol-diphenyl tetrazolium bromid) (Sigma, M5655) was poured to suspension and after that hatched at room temperature for 15 min. At last, by using an ELISA reader (Accu Reader, Taiwan), the viable bacteria (Formazan positive cells) were assessed spectrophotometrically at 600 nm.
Metallothionein gene expression in the liver
Gene samples were taken from the liver of three fish from each treatment in the eighth and eleventh weeks. Tissue samples were placed in liquid nitrogen immediately after sampling and transferred to -70°C freezer after one hour and kept in this condition until RNA extraction (Matsuyama et al. 2007; Mohanty and Sahoo 2010).
RNA extraction
RNA extraction was performed using RNXTM reagent and according to the instructions of the manufacturer (Sinagen Iran).
CDNA synthesis from RNA
For this purpose, the YT4500 cDNA synthesis kit was Yekta Tajhiz (Iran). The volume of PCR reactions was considered to be 20 μl. The list of primers used in this study is given in Table 1.
Performing real-time PCR reactions
For real-time PCR, the resulting cDNA sample was diluted 1: 2 with sterile distilled water. For this purpose, because the final volume of cDNA was 20 μl, 20 μl of distilled water was added to it. The following compounds were added to the 2-ml cap strips for real-time PCR reaction (Biorad, USA), respectively. Reactions were performed in a volume of 12, 25 μl. Fifty cycles were considered for each gene.
Bacterial challenge
To evaluate the effect of lead toxicity, probiotic and encapsulation of probiotics on protective immunity of rainbow trout against bacterial challenge, at the end of week 11, remained fish of each group were challenged with Streptococcus iniae which isolated, identified and its LD50 was calculated in our previous work (Hassani et al. 2021) and mortality rate was recorded for 10 days after the challenge, the recorded losses and the percentage of losses between treatments were compared by Kaplan-Meier (KM) method.
Data analysis
Statistical analysis of data was performed using SPSS software version 23. In order to statistically compare the results between groups, one-way ANOVA method was used and Duncan post hoc test was used to evaluate the significance of the mean differences at a significant level (p <0.05). Charts and tables were also drawn in Excel software space.
Results
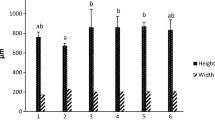
Characterization of microcapsules
The output of particle size analyzer showed the average diameter of the microcapsules in the final emulsion product were 2.87±0.51μm (Mean ±ST). Phase contrast microscope observations showed relatively similar and round capsules in final product (Fig. 1). SEM observation showed successful encapsulation of Lactococcus plantarum bacteria with alginate/chitosan and the produced capsules were largely spherical and elliptical in appearance (Figs. 2 and 3).
Zeta potential of microcapsules produced by internal emulsion using alginate and chitosan method was determined to be + 39.2 mv.
Survival rates of probiotic in simulating GI condition
In this study, the probiotic survival of Lactobacillus plantarum in two different free (uncoated) and emulsion-coated (internal) methods was evaluated for 120 min in simulated gastric and intestinal conditions (Table 2).
The survival rate of uncoated probiotic cells decreased to 61.3 and 53.2% of the initial number 1 and 2 h after exposure in the same stomach and intestine conditions, and these percentages were 90 and 82 in the encapsulated treatment (Fig. 4).
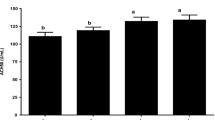
Metallothionein gene expression rate in experimental groups in weeks 8 and 11 of experiment. (G1: encapsulated probiotic-treated group, G2: probiotic-treated group, G3: alginate/chitosan-treated group, G4: untreated or control group and G5: lead-treated control group). Results are reported based on Means ± SD. Non-identical Latin letters on the standard deviation bar indicate a significant difference at the level of 0.05 in each column
Growth performance indices
Results of growth performance indices were in eight and eleventh weeks of experiment shown in Table 3. Analyzing of growth indices showed that the growth performance indices including special growth rate, protein efficiency rate, and daily weight gain increased significantly (P<0.05) in G2 (encapsulated probiotic-treated group) compare to the control group.
The mentioned indices increased in G1 (free probiotic-treated group) but not in significant extent (P>0.05). However, the lowest FCR in the eighth and eleventh weeks was observed in G2 and G1, respectively; there was no difference in FCR value among the other groups. Results showed no significant difference in condition factor (CF) among the experimental groups (P>0.05).
Hematological parameters
Among the blood indices in the 8th week of the experiment, a significant increase in RBC, WBC, hemoglobin, and hematocrit was observed in the G2 compared to other groups. At this sampling point, a relative increase in the above indices was also observed just in the probiotic-treated groups. After lead poisoning period, WBC and hematocrit increased in the ninth week in both G1 and G2. Meanwhile after lead administration, a decrease in the above indicators was observed in the group of lead poisoning (positive control) in the eleventh week.
MCV, MCH, and MCHC hematopoietic indices did not show significant differences between treatments and different stages of sampling (Tables 4, 5, 6).
The investigated enzymes in the eighth and ninth weeks in the G2 and G1 treatments showed the highest activity compared to the other treatments, which had a significant difference compared to the other treatments. In the first, 10th and 11th weeks, no significant difference was observed between the digestive enzyme activity of the research treatments.
Metallothionein gene expression rate
Although no significant difference was observed in metallothionein gene expression rate of liver samples in week 8 of experiment, a significant upregulation of this gene was seen in G1 and G2 compare to control groups (p<0.05).
Bacterial challenge
The results of cumulative mortality of experimental groups after bacterial challenge at the end of week 11 with the pathogenic Streptococcus iniae is shown in Fig. 5. As showed the highest mortality rate was in lead-treated control group (100%) and control group (80%) respectively, while probiotic-treated groups (G1 and G2) showed the lowest mortality rate (30% and 50%), respectively. This study showed that encapsulated L. plantarum increased rainbow trout resistance to Streptococcus iniae infection.
Dissection
In this study, not only the positive effect of uncoated and encapsulated L. plantarum on growth and health indices but also, the beneficial effects of microencapsulated L. plantarum in reducing of lead toxicity in rainbow trout were reported.
Heavy metal (HM) exposure remains a global occupational and environmental problem that creates a hazard to general health. Even low-level exposure to toxic metals contributes to the pathogenesis of various metabolic and immunological diseases, whereas, in this process, the gut microbiota serves as a major target and mediator of HM bioavailability and toxicity.
Probiotic-based protective strategies against HM-induced gut dysbiosis are now well established from studies that suggest the relationship between gut microbiota and HM exposure is mutual and bidirectional. In the present study, in order to increase the survival of L. plantarum in the stomach and intestine of rainbow trout exposed to lead, this bacterium was coated with sodium alginate and chitosan by internal emulsion method. The results of the characteristics of encapsulated probiotics showed suitable encapsulation of probiotic cells with biological polymers. In addition to the diameter of the capsules, the type of protected microorganism also affects the viability of the bacteria. In current work the survival rate of L. plantarum under gastric and intestinal simulation conditions was investigated and as expected, microencapsulation of L. plantarum significantly increased bacterial survival.
Results indicated that the microcapsules produced by internal emulsification techniques exhibited relatively homogeneous and rational particle-size distribution. Observation by phase contrast light microscope and SEM showed that the capsules are largely spherical and elliptical shape. Particle’ size was 2.87±0.51μm which is suitable size, proper distribution, and homogeny for encapsulated probiotics. Mohammadian et al. (2022) report a similar size and distribution for encapsulated Lactocucus bulgaricus with alginate/chitosan. The average particle size of microcapsules is an important feature for stability, efficacy and viability. Microcapsules with a greater size provide more protection against environmental stress, but have a negative impact on quorum sensing characteristics of bacteria. The microcapsules of small size may not provide adequate protection for probiotics (Yudiati et al. 2020; Liu et al. 2019). In accordance with our work, (Dezfuly et al. 2020) reported similar size, and distribution of encapsulated Yersinia ruckeri oral vaccine using alginate/chitosan via similar method.
Zeta potential values obtained by microcapsules were +39.2 mv. This high zeta potential lead to better stability and it is important to prevent agglomeration. Similarly, Halimi et al. 2020 reported zeta potential around +36 mv for microspheres of alginate/chitosan encapsulated Streptococcus iniae oral vaccine developed via similar method. Zeta potential is an important and useful indicator of electrical condition of particle surfaces which can be used to predict and control the stability of colloidal suspensions or emulsions. High level of zeta potential in colloidal particles lead to the rise of electrostatic repulsion force and the physical stability of the system and as a result physical consistency increases (Burgos and Moreno 2009). Zeta potential above +30 or below -30 mv is usually considered to be more balanced.
The survival rates of microencapsulated L. plantarum after 60- and 120-min incubation in GI simulated situation were significantly higher than free bacteria. Preservation of cell viability is an important factor in selecting the encapsulation method for probiotics. The small number of lost bacteria indicates the proper accuracy used in the method of encapsulation. In the study of (Song et al. 2013), which coated the yeast cells with the emulsion method, the microenvironment efficiency of the internal emulsion method was reported to be 80%.
Encapsulated L. plantarum effects on growth indices of poisoned fish
Results of this study showed that the use of encapsulated L. plantarum not only positively affected most of growth indices of rainbow trout even after lead poisoning, but also reduce the adverse effects of lead toxicity on intestinal enzymes. The highest SGR, BWG, and FCR were seen in encapsulated probiotic-treated group (G1) and non-coated probiotic groups (G2) respectively which significantly (P<0.05) improved compare to control groups. Meanwhile, the activity of intestinal enzymes including of lipase, trypsin and chymotrypsin and protease were significantly (P<0.05) higher in G1 and G2 at weeks 8, 9, and 11 of experiment. This event probably can refer to the higher concentration and efficacy of L. plantarum in the gastrointestinal tract of encapsulated probiotic-treated group compare to the other groups. In accordance with results of the present study, (Veisi et al. 2023) reported the protective effects of Lactobacillus casei against Cadmium toxicity in common carp. Meanwhile (Mohammadian et al. 2020) showed positive effect of microencapsulation on protective effects of Lactococcus bulgaricus and L. delbrueckii against Lead toxicity in rainbow trout. Similarly (Boonanuntanasarn et al. 2019) reported significant positive effects of encapsulation of Saccharomyces cerevisiae on growth indices in Striped Catfish (Pangasianodon hypophthalmus). On the contrary to our results (He et al. 2020) showed that dietary microcapsulated probiotic with chitosan had suppressive effect on the growth performance parameters of silver carp.
HM exposure alters the microbiome composition and metabolomic profiles of the gut microbiota, conversely, the gut microbiota has strong HM-metabolizing capabilities to limit HM absorption while increasing HM excretion from the fish body. For this reason, the application of probiotics is considered as a promising approach to alleviating HM-induced toxicity, which raises great prospects to be the next-generation therapeutics for HM intoxication (Emenike et al. 2022). Various mechanisms have been presented so far as modes of probiotic resistance against HM intoxication, 1—they directly affected the absorption and metabolism of HMs within the intestine; 2—they have intense abilities to bioaccumulate, bind, or transform HMs via various enzymatic reactions; 3—they are considered as strong antioxidants with immune regulatory capability (Musawi et al. 2019). Based on these three strategies, it can be concluded that encapsulated L. plantarum effectively survived in GI situation and showed stronger and more efficient protective effects against HM toxicity compare to other groups. Moreover, the negative charge on L. plantarum particularly in encapsulated form and the cationic charges of HMs, result in the chelation of HMs and decreasing its bioavailability. on the other hand, the metabolites such as extracellular proteins, organic acids, indole, bacteriocins, hydrogen peroxide, and nitrous oxide produced by probiotics induce goblet cells to secrete mucus, increase the productivity of antimicrobial peptides, and enhance the expression of tight junction (TJ) proteins to protect the gut epithelial barrier.
Encapsulated L. plantarum effects on intestinal enzymes of poisoned fish
Intestinal enzymes activity of fish is an important indicator for evaluating the health status, environmental quality and the presence of toxic compounds (Assan et al. 2022). In this study, the results of the use of probiotics in rainbow trout for eight weeks showed that the amount of lipase enzymes, chymotrypsin in fish fed with microencapsulated probiotics and, the levels of protease and alkaline phosphatase increased in fish fed just with probiotic, but only the increase of alkaline phosphatase and protease was insignificant (p<0.05). The reason may be due to the increasing of enzyme-secreting cells, the secretion of a wide range of probiotic bacterial enzymes (exoenzymes) or the increase in the activity of digestive enzymes (endoenzymes). The amount of trypsin enzyme also relatively increased in fish fed with microcoated probiotic compared to the control group. L. plantarum play a vital role in improving the activity of digestive enzymes such as amylase, protease and lipase due to better digestion of starch, fat, and protein (Abumourad et al. 2013). (Mohammadian et al. 2022) reported a significant increase in the activity of amylase, protease and lipase digestive enzymes in rainbow trout as a result of consuming commercial probiotics and prebiotics for 12 weeks. ALP has been introduced as a good biomarker in toxicological studies. In the present study, significant increase in the amount of ALP in the first week after being exposed to lead in microencapsulated probiotic treatment showed compared to the other treatments and controls (p ˂0.05). Since the membranes of intestinal microvilli of fish have the ability to make ALP enzyme, this increase in activity probably indicates the further development of the membranes microvilli of enterocytes in these groups. It can be stated that probably the encapsulated probiotics in the present study have been enhanced the development of brush-like microvillis of rainbow trout enterocytes.
Effect of Lactobacillus plantarum on Hematoimmunological parameters
In this study, the amount of RBC, hemoglobin, hematocrit, MCH, and MCV Significantly increased in encapsulated and non-capsulated probiotic-fed treatments, and also in probiotic-containing treatments that were exposed to lead. The increasing number of these cells indicates the immunogenic effects and microbial defense by probiotics. In similar works has been shown that food supplementation with alginate along with Pediococcus acidilactici in Asian sea bass (Ashouri et al. 2018) and Bacillus bulgaricus in rainbow trout (Mohammadian et al. 2022) and Bacillus plantarom in Common carp (Mohammadian et al. 2020) led to improvement of hematological parameters. The positive change in hematological parameters might be attributed to increased hematoopoietic stimulation and improvement of health statues in probiotic-treated fish. Furthermore, the glycopeptide cell wall of probiotic enhances the fish immune response by activating lymphocytes (Hotel 2001). Under stressful conditions, immature erythrocytes are released from the spleen, and as metabolism increases, oxygen delivery increases to important organs, followed by erythrocytes, hemoglobin concentration, and hematocrit levels being increased (NavinChandran 2014). Also, the high number of blood RBCs in the encapsulated probiotic-treated group can indicate the health and strengthening of the blood circulatory system of the rainbow trout and the higher oxygenation of the gills in stressful conditions such as oxygen reduction and exposure to heavy metals (Li et al. 2022). In fact, one of the reasons for higher RBC level is the accumulation of RBC in the gills of fish exposed to the stress condition caused by the pollutant, which causes a decrease in their number in the blood (Narain and Srivastava 1989).
In the present study, the total number of WBC increased with the start of feeding the experimental diets, so that in the eighth week, the fish receiving microencapsulated L. plantarum showed a significant increase compared to the control group and the lead control group (p<0.05). After the addition of lead to the experimental diets, the number of WBCs of probiotic treatments in three weeks of sampling increased compared to other treatments, showing that the normal probiotic treatment had the highest number of WBCs (p<0.05).
Encapsulated L .plantarum effects on immune response of poisoned fish
Based on the results of the present study, it can be stated that the use of encapsulated L.plantarum significantly increases the serum lysozyme and complement activity in most of sampling points (p<0.05). After adding lead to the experimental diets, the serum lysozyme levels in the ninth and tenth weeks were significantly reduced in the probiotic treatment, respectively. In the study of (Mohammadian et al. 2016), the probiotic positive effects of some lactic acid bacteria on Tor grypus were evaluated. In similar study, Lactococcus lactis showed the highest antibacterial activity against Aeromonas hydrophila in huso huso (Soltani et al. 2016). Such antibacterial activity against Aeromonas hydrophila may be due to the production of organic acids, hydrogen peroxide, carbon dioxide, acetic acid, bacteriocins, diacetyl, acetaldehyde, ethanol, and low molecular weight antimicrobial compounds of some bacteria. Be lactic acid (Lee et al. 2019).
After the addition of lead, G2 showed the highest amount of NBT reduction (p<0.05). Increased respiratory burst strength in leukocytes is caused by immune cytokines, which are immune mediators. The results of the present study showed that in the eighth week, total protein and serum immunoglobulin increased in the G1 and 2 compared to the control group. Changes in serum immunoglobulin levels have been reported in many studies following the use of immune stimulants, probiotics, and prebiotics (Nayak 2010; Kandati et al. 2023).
Based on the results, it can be concluded that the addition of regular and finely microencapsulated probiotic Lactobacillus plantarum in the diet of rainbow trout can improve some of the immune responses of this fish even after exposure to lead.
Effect of Lactobacillus plantarum on metallothionein gene expression
As mentioned in the results, before the addition of lead a significant increase on the expression of metallothionein (MT) gene was seen compared to other treatments (p <0.05), while the use of chitosan and alginate in the diet of rainbow trout (G3) had no effect on gene expression. In the last sampling point after the addition of lead, a significant increase was observed in all probiotic-treated groups. Due to the importance of the detoxification of MTs, these proteins can be considered as biomarkers of toxic metal pollution in the environment. On the other hand, the comparison between the number of heavy metals and MT measured is not only useful from the environmental toxicology point of view, but also from a biochemical point of view, because it provides a better understanding of the functions of MT in an organism (Sarkar et al. 2006).
The biological functions of metallothioneins include the storage, transport or separation of essential metals and the neutralization of excess amounts of heavy metals in the cell, and they can also be considered as biomarkers for inorganic contaminants (Vardy et al. 2014). Exposure to heavy metals increases MT in liver, kidney, and gill tissues in a dose- and time-dependent manner. Heavy metals are reabsorbed through the active transfer process in renal tubular cells, which are rich in MT protein. The liver appears to be the first organ of detoxification. When the liver is saturated, the metallothionein-heavy metal complex is delivered to the kidneys. MT is then observed in the kidney as a response to reabsorption of this complex and synthesis of renal MT to store heavy metals (Chowdhury et al. 2005). Wangsongsak et al reported the expression of MT mRNA at very low concentrations (0.012 mg / L) in the liver and kidneys of Puntius gonionotus after exposure to different concentrations of Pb. They also found that MT mRNA expression in the liver was time-dependent and in the kidney was concentration-dependent.
Bacterial challenge
In the present study, challenge with Streptococcus iniae at the end of experiment showed the lowest mortality (30% and 50%) in G1 and G2 respectively which was significantly lower than control group (80%) and lead-treated group (100%). This study showed that microcoated Lactobacillus plantarum increased their resistance to Streptococcus iniae infection by increasing the health statue and immune responses. This finding is consistent with the study of (Abumourad et al. 2013). The results of their study showed an improvement in survival rate in Nile tilapia treated with Lactolacillus plantarum after challenge with Pseudomonas fluorescence. (Aly et al. 2008) also reported the positive effect of Bacillus pumilus on the growth, survival, and resistance of the pathogen Aeromonas hydrophila.
Conclusion
Based on the results of this experiment, it can be concluded that using microencapsulated L. plantarum with alginate/chitosan in the diet of rainbow trout cause:
-
1.
reducing the adverse side effects of lead toxicity
-
2.
improving the hemato-immunological parameters of rainbow trout.
-
3.
increasing resistance against gastrointestinal conditions of rainbow trout and its intestinal enzyme activity.
-
4.
higher metallothionein gene expression in liver of treated fish
-
5.
increasing the growth performance indices of rainbow trout even in lead-treated fish
-
6.
reducing the mortality rate after challenge with S. iniae even in lead-treated fish
Therefore, the use of microencapsulation of probiotics with alginate/chitosan can be used for reducing lead toxicity as well as increasing the health status, growth, and immune response of rainbow trout, so, this method is recommendable in lead-polluted ecosystems.
Data availability
The datasets generated during and/or analyzed during the current study are available in below Google drive link: https://drive.google.com/drive/folders/1QqAEBepqYu-S 99FsAmdYaSF8Y0zGV34?usp=share_link
References
Abumourad IM, Abbas WT, Awaad ES, Authman MM, El-Shafei K, Sharaf OM, Ibrahim GA, Sadek ZI, El-Sayed HS (2013) Evaluation of Lactobacillus plantarum as a probiotic in aquaculture: emphasis on growth performance and innate immunity. J Appl Sci Res 9(1):572–582. https://www.researchgate.net/publication/257958772
Alves LC, Wood CM (2006) The chronic effects of dietary lead in freshwater juvenile rainbow trout (Oncorhynchus mykiss) fed elevated calcium diets. Aquat Toxicol 78(3):217–232. https://doi.org/10.1016/j.aquatox.2006.03.005
Aly SM, Ahmed YA, Ghareeb AA, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25(1-2):128–136. https://doi.org/10.1016/j.fsi.2008.03.013
Antunes AE, Liserre AM, Coelho AL, Menezes CR, Moreno I, Yotsuyanagi K, Azambuja NC (2013) Acerola nectar with added microencapsulated probiotic. LWT-Food Sci Technol 54:125–131. https://doi.org/10.1016/j.lwt.2013.04.018
Areekijseree M, Engkagul A, Kovitvadhi U, Thongpan A, Mingmuang M, Pakkong P, Rungruangsak-Torrissen K (2004) Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 234(1-4):575–587. https://doi.org/10.1016/j.aquaculture.2003.12.008
Ashouri G, Soofiani NM, Hoseinifar SH, Jalali SA, Morshedi V, Van Doan H, Mozanzadeh MT (2018) Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5M on growth performance, haematological and innate immune responses of Asian sea bass (Lates calcalifer) juveniles. Fish Shellfish Immunol 79:34–41. https://doi.org/10.1016/j.fsi.2018.05.009
Assan D, Kuebutornye FK, Hlordzi V, Chen H, Mraz J, Mustapha UF, Abarike ED (2022) Effects of probiotics on digestive enzymes of fish (finfish and shellfish); status and prospects: a mini review. Comparative Biochem Physiol Part B: Biochem Mol Biol 257:110653. https://doi.org/10.1016/j.cbpb.2021.110653
Badoei-Dalfard A, Khankari S, Karami Z (2020) One-pot synthesis and biochemical characterization of protease metal organic framework (protease@ MOF) and its application on the hydrolysis of fish protein-waste. Coll Surf B: Biointerf 196:111318. https://doi.org/10.1016/j.colsurfb.2020.111318
Bhattacharya S (2019) Probiotics against alleviation of lead toxicity: recent advances. Interdis Toxicol 12:89. https://doi.org/10.2478/intox-2019-0010
Boonanuntanasarn S, Ditthab K, Jangprai A, Nakharuthai C (2019) Effects of microencapsulated Saccharomyces cerevisiae on growth, hematological indices, blood chemical, and immune parameters and intestinal morphology in striped catfish, Pangasianodon hypophthalmus. Probiot Antimicrob Proteins 11:427–437. https://doi.org/10.1007/s12602-018-9404-0
Budiño B, Cal RM, Piazzon MC, Lamas J (2006) The activity of several components of the innate immune system in diploid and triploid turbot. Comparative Biochem Physiol Part A: Mol Integr Physiol 145(1):108–113. https://doi.org/10.1016/j.cbpa.2006.05.007
Burgos-Montes O, Moreno R (2009) Stability of concentrated suspensions of Al2O3–SiO2 measured by multiple light scattering. J Eur Ceram Soc 29(4):603–610. https://doi.org/10.1016/j.jeurceramsoc.2008.07.044
Chang PH, Lin CW, Lee YC (2002) Lactococcus garvieae infection of cultured rainbow trout, Oncorhynchus mykiss in Taiwan and associated biophysical characteristics and histopathology. Bull Eur Assoc Fish Pathol 22:319–327
Charteris C, Kelly K, Morelli M, Collins C (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84(5):759–768. https://doi.org/10.1046/j.1365-2672.1998.00407.x
Chen R, Tu H, Chen T (2022) Potential Application of Living Microorganisms in the Detoxification of Heavy Metals. Foods 11(13):1905. https://doi.org/10.3390/foods11131905
Chowdhury MJ, Baldisserotto B, Wood CM (2005) Tissue-specific cadmium and metallothionein levels in rainbow trout chronically acclimated to waterborne or dietary cadmium. Arch Environ Contaminat Toxicol 48:381–90
Das S, Mandal S, Dhara H, Chatterjee PN (2022) Dietary amelioration of lead toxicity in fish-A review. Indian J Anim Health 61(2):173–189. https://doi.org/10.36062/ijah.2022.spl.03422
Dezfuly ZT, Alishahi M, Ghorbanpoor M, Tabandeh MR, Mesbah M (2020) Immunogenicity and protective efficacy of Yersinia ruckeri lipopolysaccharide (LPS), encapsulated by alginate-chitosan micro/nanoparticles in rainbow trout (Oncorhyncus mykiss). Fish Shellfish Immunol 1(104):25–35
El-Greisy ZA, El-Gamal AH (2015) Experimental studies on the effect of cadmium chloride, zinc acetate, their mixture and the mitigation with vitamin C supplementation on hatchability, size and quality of newly hatched larvae of common carp, Cyprinus carpio. The. Egypt J Aquat Res 41(2):219–226. https://doi.org/10.1016/j.ejar.2015.03.007
Emenike EC, Iwuozor KO, Anidiobi SU (2022) Heavy metal pollution in aquaculture: sources, impacts and mitigation techniques. Biol Trace Element Res 200:4476–4492. https://doi.org/10.1007/s12011-021-03037-x
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95(2):271–278. https://doi.org/10.1016/0003-9861(61)90145-X
Halimi M, Alishahi M, Abbaspour MR, Ghorbanpoor M, Tabandeh MR (2020) High efficacy and economical procedure of oral vaccination against Lactococcus garvieae/Streptococcus iniae in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 99:505–513. https://doi.org/10.1016/j.fsi.2020.02.033
Hassani F, Peyghan R, Alishahi M, Ghorbanpour M, Ahangarzadeh M (2021) Molecular and biochemical investigation of the role of streptococcus iniae in mortality of Lates calcarifer cages culturing in the Persian Gulf. Exp Animal Biol 9(3):19–27
He N, Wang S, Lv Z, Zhao W, Li S (2020) Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct 11(11):9947–9959. https://doi.org/10.1039/D0FO01871F
Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH (2008) Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem 111(1):50–55. https://doi.org/10.1016/j.foodchem.2008.03.036
Hooshyar Y, Abedian Kenari A, Paknejad H, Gandomi H (2020) Effects of Lactobacillus rhamnosus ATCC 7469 on different parameters related to health status of rainbow trout (Oncorhynchus mykiss) and the protection against Yersinia ruckeri. Probiot Antimicrob Proteins 12(4):1370–1384
Hotel AC, Cordoba A (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 5(1):1
Hummel BC (1959) A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Canad J Biochem Physiol 37(12):1393–1399. https://doi.org/10.1139/o59-157
Kandati K, Reddy AS, Nannepaga JS, Viswanath B (2023) The Influence of Probiotics in Reducing Cisplatin-Induced Toxicity in Zebrafish (Danio rerio). Curr Microbiol 80(4):109. https://doi.org/10.1007/s00284-023-03203-5
Kirillova AV, Danilushkina AA, Irisov DS, Bruslik NL, Fakhrullin RF, Zakharov YA, Bukhmin VS, Yarullina DR (2017) Assessment of resistance and bioremediation ability of Lactobacillus strains to lead and cadmium. Int J Microbiol 4:2017. https://dspace.kpfu.ru/xmlui/handle/net/144112
Kuzmina VV, Gavrovskaya LK, Ryzhova OV, Taurine (2010) Effect on exotrophia and metabolism in mammals and fish. J Evol Biochem Physiol 46:19–27. https://doi.org/10.1134/S0022093010010020
Lee B, Paek E, Mitlin D, Lee SW (2019) Sodium metal anodes: emerging solutions to dendrite growth. Chem Rev 119(8):5416–5460. https://doi.org/10.1021/acs.chemrev.8b00642
Li Z, Junaid M, Chen G, Wang J (2022) Interactions and associated resistance development mechanisms between microplastics, antibiotics and heavy metals in the aquaculture environment. Rev Aquacult 14(2):1028–1045. https://doi.org/10.1111/raq.12639
Liu M, Kakade A, Liu P, Wang P, Tang Y, Li X (2019) Hg2+-binding peptide decreases mercury ion accumulation in fish through a cell surface display system. Sci Total Environ 659:540–547. https://doi.org/10.1016/j.scitotenv.2018.12.406
Matsuyama T, Fujiwara A, Nakayasu C, Kamaishi T, Oseko N, Hirono I, Aoki T (2007) Gene expression of leucocytes in vaccinated Japanese flounder (Paralichthys olivaceus) during the course of experimental infection with Edwardsiella tarda. Fish Shellfish Immunol 22(6):598–607. https://doi.org/10.1016/j.fsi.2006.08.006
Michida H, Tamalampudi S, Pandiella SS, Webb C, Fukuda H, Kondo A (2006) Effect of cereal extracts and cereal fiber on viability of Lactobacillus plantarum under gastrointestinal tract conditions. Biochem Eng J 28(1):73–78. https://doi.org/10.1016/j.bej.2005.09.004
Mohammadian T, Alishahi M, Tabandeh MR, Ghorbanpoor M, Gharibi D, Tollabi M, Rohanizade S (2016) Probiotic effects of Lactobacillus plantarum and L. delbrueckii ssp. bulguricus on some immune-related parameters in Barbus grypus. Aquacult Int 24(1):225–242. https://doi.org/10.1007/s10499-015-9921-8
Mohammadian T, Dezfuly ZT, Motlagh RG, Jangaran-Nejad A, Hosseini SS, Khaj H, Alijani N (2020) Effect of encapsulated lactobacillus bulgaricus on innate immune system and hematological parameters in rainbow trout (Oncorhynchus mykiss), post-administration of Pb. Probiot Antimicrob Proteins 12(2):375–388
Mohammadian T, Ghanei-Motlagh R, Jalali M, Nasirpour M, Mohtashamipour H, Osroush E, Nejad AJ (2022) Protective effects of non-encapsulated and microencapsulated Lactobacillus delbrueckii subsp. bulgaricus in rainbow trout (Oncorhynchus mykiss) exposed to lead (Pb) via diet. Ann Animal Sci 22:325–348. https://doi.org/10.2478/aoas-2021-0026
Mohanty BR, Sahoo PK (2010) Immune responses and expression profiles of some immune-related genes in Indian major carp, Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol 1(28):613–621. https://doi.org/10.1016/j.fsi.2009.12.025
Musawi AM, Johari WL, Ikhsan NF, Ahmad SA, Yasid NA, Shukor MY (2019) The Growth Potential and Bioaccumulation Ability of Probiotics under the Exposure of Different Heavy Metals. Pertanika Journal of Tropical Agricultural. Pertanika J Trop Agri 42:1511-3701 e-ISSN: 2231-8542
Narain AS, Srivastava PN (1989) Anemia in the freshwater teleost, Heteropneustes fossilis, under the stress of environmental pollution. Bull Environ Contam Toxicol 43(4):627–634
NavinChandran M, Iyapparaj P, Moovendhan S, Ramasubburayan R, Prakash S, Immanuel G, Palavesam A (2014) Influence of probiotic bacterium Bacillus cereus isolated from the gut of wild shrimp Penaeus monodon in turn as a potent growth promoter and immune enhancer in P. monodon. Fish Shellfish Immunol 36(1):38–45. https://doi.org/10.1016/j.fsi.2013.10.004
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Nezamdoost-Sani N, Khaledabad MA, Amiri S, Khaneghah AM (2023) Alginate and derivatives hydrogels in encapsulation of probiotic bacteria: An updated review. Food Biosci 27:102433. https://doi.org/10.1016/j.fbio.2023.102433
Pinto MG, Franz CM, Schillinger U, Holzapfel WH (2006) Lactobacillus spp. with in-vitro probiotic properties from human faeces and traditional fermented products. International journal of food microbiology 109(3):205–214. https://doi.org/10.1016/j.ijfoodmicro.2006.01.029
Rahman A, Khan KM, Al-Khaledi G, Khan I, Al-Shemary T (2012) Over activation of hippocampal serine/threonine protein phosphatases PP1 and PP2A is involved in lead-induced deficits in learning and memory in young rats. Neurotoxicology 33(3):370–383. https://doi.org/10.1016/j.neuro.2012.02.014
Sarkar A, Ray D, Shrivastava AN, Sarker S (2006) Molecular biomarkers: their significance and application in marine pollution monitoring. Ecotoxicology 15:333–340
Shi G, Zhai M, Mao G, Li M, Hu D (2014) Chemical compositions and antifungi activities of pyroligneous acids from walnut shell of two species. Acta Botanica Boreali-Occidentalia Sinica 34(10):2109–2117
Soltani M, Shenavar Masouleh A, Ahmadi M, Pourkazemi M, Taherimirghaed A (2016) Antibacterial activity, antibiotic susceptibility and probiotic use of lactic acid bacteria (LAB) in Persian sturgeon (Acipenser persicus). Sustain Aquacult Health Manag J 2(1):54–65. http://ijaah.ir/article-1-100-en.html
Song H, Yu W, Gao M, Liu X, Ma X (2013) Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr Polym 96(1):181–189. https://doi.org/10.1016/j.carbpol.2013.03.068
Vardy DW, Santore R, Ryan A, Giesy JP, Hecker M (2014) Acute toxicity of copper, lead, cadmium, and zinc to early life stages of white sturgeon (Acipenser transmontanus) in laboratory and Columbia River water. Environ Sci Pollut Res 21(13):8176–8187. https://doi.org/10.1007/s11356-014-2754-6
Veisi RS, Taghdir M, Abbaszadeh S, Hedayati A (2023) Dietary Effects of Probiotic Lactobacillus casei on Some Immunity Indices of Common Carp (Cyprinus carpio) Exposed to Cadmium. Biol Trace Elem Res 24:1–9
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30(3):404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
Wangsongsak A, Utarnpongsa S, Kruatrachue M, Ponglikitmongkol M, Pokethitiyook P, Sumranwanich T (2007) Alterations of organ histopathology and metallolhionein mRNA expression in silver barb, Puntius gonionotus during subchronic cadmium exposure. J Environ Sci 19(11):1341–1348. https://doi.org/10.1016/S1001-0742(07)60219-8
Worthington DG, Westoby M, Bell JD (1991) Fish larvae settling in seagrass: effects of leaf density and an epiphytic alga. Aust J Ecol 16(3):289–293. https://doi.org/10.1111/j.1442-9993.1991.tb01056.x
Yano T, Hatayama Y, Matsuyama H, Nakao M (1988) Titration of the alternative complement pathway activity of representative cultured fishes. Nippon Suisan Gakkaishi (Japanese Edition) 54(6):1049–1054. https://doi.org/10.2331/suisan.54.1049
Yudiati E, Subagiyo S, Djarod MS (2020) Preliminary study of polysaccharide and oligosaccharide alginate (AOS) as prebiotic of probiotic bacteria. Jurnal Kelautan Tropis 23(2):234–238. https://doi.org/10.14710/jkt.v23i2.7674
Feldman BV, Zinkl JG, Jain NC, Schalm OW (2000) Schalm's veterinary hematology/editors, Bernard V. Feldman, Joseph G. Zinkl, Nemi C. Jain. Lippincott Williams & Wilkins.
Acknowledgements
This work financially supported by research council of Shahid Chamran University of Ahvaz and Centre of Excellence for Warm Water Fish Health and disease, Shahid Chamran University of Ahvaz, Ahvaz, Iran
Funding
This work was supported by Research council, Shahid Chamran University of Ahvaz and Centre of Excellence for Warm Water Fish Health and disease, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
Author information
Authors and Affiliations
Contributions
The authors of the manuscript certify that they have participated sufficiently in the work as mentioned below:
Maryam Ahmadmoradi (1st author) As a Ph.D. student was directly involved with the experiment, laboratory tests, data analyzing, and writing the article. Mojtaba Alishahi (2nd author) designed and planned the experiment and handled laboratory tests and encapsulation procedures, Siavash Soltanian (third author) performed molecular and cellular parts of the work. Ali Shahriari (4th author ), contributes to the work by assaying the intestinal enzyme activity and hematological indices and analyzing the results, and writing the manuscript. Azadeh Yektaseresht (5th author) performed bacteriological and immunological parts of the experiment.
Corresponding author
Ethics declarations
Ethics approval
In vivo phase of this experiment has been conducted as the guidelines of the Institutional Animal Ethics Committee, Faculty of Veterinary, Shahid Chamran University, Iran (Approved NO: EE/1401.2.24.78971/SCU.ac.ir).
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmadmoradi, M., Alishahi, M., Soltanian, S. et al. Effects of encapsulation of Lactobacillus plantarum on probiotic potential and reducing lead toxicity in rainbow trout (Oncorhynchus mykiss Walbaum). Aquacult Int 32, 337–359 (2024). https://doi.org/10.1007/s10499-023-01164-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01164-x