Abstract
Hypertension is a major risk factor for cardiovascular diseases. Optimizing blood pressure results in an overall health outcome. Over the years, the gut microbiota has been found to play a significant role in host metabolic processes, immunity, and physiology. Dietary strategies have therefore become a target for restoring disturbed gut microbiota to treat metabolic diseases. Probiotics and their fermented products have been shown in many studies to lower blood pressure by suppressing nitrogen oxide production in microphages, reducing reactive oxygen species, and enhancing dietary calcium absorption. Other studies have shown that hypertension could be caused by many factors including hypercholesterolemia, chronic inflammation, and inconsistent modulation of the renin-angiotensin system. This review discusses the antihypertensive roles of probiotics and their fermented products via the reduction of serum cholesterol levels, anti-inflammation, and inhibition of angiotensin-converting enzyme. The ability of recombinant probiotics to reduce high blood pressure has also been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
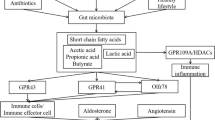
Hypertension (high blood pressure) is an important disease characterized by a sustained systolic blood pressure (BP) value of ≥140 mmHg and a diastolic pressure of ≥90 mmHg (140/90) in young persons. Meanwhile, BP increases with age and hence only elderly people ≥60 years with BPs above 150/90 mmHg may require treatment [1]. Many obese persons have high BP. In obesity, the increased visceral adiposity may physically compress the kidneys leading to impaired renal-pressure natriuresis and high BP [2]. Therefore, control of BP in obesity requires that the body mass index is first reduced [3, 4]. If left untreated, hypertension can lead to insufficient blood supply to vital organs, which can cause myocardial infarction, stroke, and eventually death. Common treatment regimens are aimed at reducing BP which eventually reduces the associated risks [5]. Current guidelines for managing arterial BP involve proper life style measures such as exercise and diet (reduced salt intake and low fat diets rich in vegetables) [6, 7]. Hypertension may be primary or secondary. The causes of primary hypertension, which accounts for about 95% of all hypertensive cases, remain elusive [8]. However, secondary hypertension may be as a result of pregnancy, diseases such as Cushing’s syndrome, kidney malfunction as well as a side effect of various drugs. Several risk factors that increase the risks of primary hypertension include hypercholesterolemia, inflammation, sleep apnea, and obesity [9]. A number of pathways such as the fluid and electrolyte balance pathway, the renin-angiotensin system (RAS), the kinin-kallikrein system, the neutral endopeptidase system, and the endothelin-converting enzyme system are known to control human BP [10]. Of the physiological mechanisms of hypertension, the renin-angiotensin system has attracted much scientific attention. The RAS is maintained by two proteases, renin, and angiotensin-converting enzyme (ACE). Renin (EC 3.4.23.15) hydrolyzes the Leu10-Val11 peptide bond of angiotensinogen (a 55-kDa protein produced in the liver) to produce angiotensin I (Ang I), an inactive decapeptide. ACE (EC 3.4.15.1), a transmembrane metallopeptidase, then cleaves a dipeptide from the C-terminal of angiotensin I to produce angiotensin II (Ang II) [11]. Ang II then binds to angiotensin type 1 (AT1) receptors to cause vasoconstriction in vascular smooth muscle cells (VSMC) or to angiotensin type 2 receptors (AT2) in endothelial and VSMC to cause vasodilation by triggering the release of nitric oxide (NO), a vasodilator [10]. In disease conditions, the activity of renin and/or ACE may increase to cause an increase in BP. Also, pathologic conditions may upregulate AT1 to reduce NO production leading to elevated BP [12]. Alternatively, ACE inactivates bradykinin (a nanopeptide vasodilator) by cleaving a dipeptide from the C-terminal [13]. Active bradykinin binds to its receptors (B1 and B2) to induce NO generation. Over the years, several studies have demonstrated the role of gut microbiota in the maintenance of physiological homeostasis such as BP [14]. Different studies have shown that imbalances in the richness, the reciprocal abundance, and the presence and/or localization of normal gut bacteria species are associated with hypertension. The changes result in a decrease in acetate- and butyrate-producing bacteria and a marked increase in Firmicutes/Bacteroidetes ratio [14, 15]. This has raised scientific interest about the use of dietary intervention to correct gut microbiota disturbances and to control high BP. Probiotics are known to exert health effects when administered in adequate quantities. Studies on the ability of probiotic bacteria alone (e.g., probiotic capsules) [6, 16, 17] and the ability of probiotics in combination with their fermented products to control high BP [18] have shown positive effects. Recent studies have discovered new functional properties of probiotics that affect BP. Probiotic Lactobacillus rhamnosus GG, Lactobacillus helveticus, Lactobacillus gasseri, Lactobacillus reuteri, and Bifidobacterium have been found to induce NO production in microphages when the bacteria are present in adequate quantities [19, 20]. They therefore enhance vasodilation and could reduce high BP. Other probiotics such as VSL#3 (a cocktail of Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. Bulgaricus, Bifidobacterium longum, Lactobacillus Bifidobacterium breve, Bifidobacterium infantis, and Streptococcus salivarius subsp. Thermophilus) and L. breves have been found to reduce the levels of polyamines in tissues [21, 22]. Reduction of polyamines in the vasculature is known to reduce BP. Another mechanism by which probiotic bacteria may decrease BP is by their antioxidant abilities. Lactobacillus fermentum E-3 and E-18, L. gasseri, and S. thermophilus produce superoxide dismutase [23–25] while S. thermophilus 821, B. longum 15708, L. plantarum KCTC 3099, L. helveticus CD6, and L. rhamnosus GG have strong metal chelating abilities [26, 27]. These properties may enable probiotics to regulate vascular contraction and relaxation. Added to these, it has been reported that probiotics enhance dietary calcium ion absorption in the gut. They produce short chain fatty acids and lactic acid which reduce gut pH and increase dietary calcium solubility and absorption [28]. Other probiotics such as L. breves enhance dietary transepithelial calcium transport by increasing TRPV6 (a membrane calcium channel) expression in the gut [29]. In hypertensive patients, dietary calcium absorption leads to a calcium-induced suppression of renin and inhibition of extracellular calcium uptake resulting in a lowered BP [30].
Since controlling the risk factors associated with primary hypertension is critical in preventing and/managing the disease, the following section discusses our current knowledge on the roles of probiotics and their fermented products in controlling cholesterol levels, inflammation, and the renin-angiotensin system in the effort to reduce hypertension. The potential of recombinant antihypertensive probiotics to reduce BP has also been discussed.
Probiotics, Hypercholesterolemia, and Hypertension
There is a large pool of evidence to show that high BP and high total cholesterol are linked [31–33]. In fact, a positive cross-sectional relation has been found between dietary cholesterol intake and systolic BP (SBP) as well as diastolic BP (DBP) [31]. Hypertension is more prevalent in hypercholesterolemic subjects relative to normolipid subjects. Also, high BP has been found to be induced when the total blood cholesterol level exceeds 6.4 mmol/l [8]. Excess blood cholesterol is deposited on the arterial walls making them hardened and narrowed with cholesterol plaque [34]. The heart therefore strains to pump blood through the narrowed arteries and BP becomes abnormally high. In a study to investigate the ability of serum cholesterol to independently affect BP levels, Ferrara et al. [35] examined 73 patients with sustained newly discovered and never-treated hypertension. After grouping the patients according to their serum cholesterol levels, they observed that BP at rest and during 24-h monitoring was similar in the three groups but increased with increasing serum cholesterol during sympathetic stimulation. Also, intima-media layer of the carotid arteries was significantly thickened in the groups with higher cholesterol levels relative to those of lower cholesterol levels. These results indicate that cholesterol levels alone can influence BP.
Over the years, metabolic disease has been shown to have a direct link with a shift in the balance of the microbiota [36–38]. Many studies have demonstrated the cholesterol-lowering effects of probiotic consumptions in humans [39–42] which may consequently lower the risk of high BP. In a randomized controlled trial, administration of L. reuteri NCIMB 30242 capsules to 127 volunteers decreased LDL-cholesterol by 11.64% (P < 0.001), total cholesterol by 9.14%, (P < 0.001), non-HDL-cholesterol by 11.30% (P < 0.001), and apoB-100 by 8.41% (P = 0.002) relative to placebo [43]. Also in a double double-blind, placebo-controlled, randomized, parallel-arm, multi-center study, a yogurt formulation of L. reuteri NCIMB 30242 reduced LDL-cholesterol by 8.92% (P = 0.016), total cholesterol by 4.81% (P = 0.031), and non-HDL-cholesterol by 6.01% (P = 0.029) over placebo [44]. Though the actual mechanism by which probiotics reduce cholesterol is not clear, a few hypotheses have been proposed. Probiotics from the genera Lactobacillus, Lactococcus, and Bifidobacterium have been found to express bile salt hydroxylases (BSH) which may reduce serum cholesterol levels in humans [45, 46]. BSH hydrolyzes conjugated bile acids to liberate free primary bile acids which are excreted in feces [47]. Probiotics with active BSH may therefore reduce cholesterol levels by increasing free bile salt production from cholesterol in their colonized area thus reducing cholesterol-associated problems. However, since overproduction of bile salts can lead to bile acid malabsorption, gastrointestinal problems, and gallstones [48], extensive in vivo studies are required to ascertain the safety of probiotic BSH cholesterol reduction. Other probiotics such as L. acidophilus, L. bulgaricus, and L. casei ATCC 393 possess both intracellular and extracellular cholesterol reductase with which they reduce cholesterol into coprostanol [49]. Probiotics also metabolize prebiotics (nondigestible food ingredients that selectively stimulate the growth and/or activity of one or a limited number of resident colonic bacterial species) to produce short chain fatty acids (SCFAs) which play an important role in cholesterol reduction. For instance, Marcil et al. [50] reported that butyrate may inhibit cholesterol biosynthesis by inhibiting DL-3-hydroxy-3-methyl-glutaryl-CoA reductase activity. Ooi et al. [51] in a randomized, double-blind, placebo-controlled, and parallel-designed study observed that 12 weeks consumption of L. gasseri CHO-220 and inulin reduced total cholesterol by 7.84% and low-density lipoprotein cholesterol by 9.27% in 32 hypercholesterolemic men and women. The probiotics and the SCFAs produced from inulin may have played vital roles in cholesterol reduction in the patients. Several other hypotheses such as binding of cholesterol to probiotic cellular surface and subsequent incorporation into their cell membrane to influence their membrane fluidity [52] and coprecipitation of cholesterol with deconjugated bile have been proposed as possible mechanisms by which probiotics reduce cholesterol and prevent cholesterol-associated diseases such as high BP [45]. Since reduction in total and low-density lipoprotein cholesterol reduce BP [53, 54], the administration of cholesterol-lowering probiotics may be effective in protecting against or reducing high BP. However, it is important that the mechanism by which these probiotics reduce cholesterol levels in humans be established in light of the host-microbial crosstalk and other biochemical networks that underlie BP.
Probiotics, Inflammation, and Hypertension
Several studies have suggested the role of inflammation in the pathophysiology of hypertension in both human and experimental animal models [55–57]. Even though the relationship between inflammatory cytokines and hypertension is inconstant among different ethnic groups [58, 59], there is still reason to believe that inflammation and hypertension may be linked. Sessol et al. [60] followed a study involving 20,525 females (≥45 years) for a median of 7.8 years and observed that 5365 of the participants developed hypertension and these were those who had high levels of C-reactive protein (CRP), an inflammatory cytokine. It was also observed that CRP was significantly linked with a high risk of developing hypertension even in individuals with very low levels of baseline BP (<140/<90 mmHg) and no traditional CVD risk factors. Also, Niskanen et al. [61] after following 379 middle-aged men with no evidence of diabetes or hypertension at baseline for 11 years reported that subjects with CRP levels ≥3 mg/l were 3.6 times more likely to develop hypertension than men with levels ≤1.0 mg/l (P ˂0.001). Many other markers of vascular inflammation and thrombosis such as IL-6, TNF-α, endothelin-1, and ICAM-1 have been shown to have a positive correlation with hypertension [62]. A potential mechanism by which inflammation may promote hypertension is by causing endothelial dysfunction. Endothelial dysfunction can lead to an increase in systemic vascular resistance and reduce nitric oxide availability [63] resulting in increased BP. In another study to find any association between inflammation and hypertension, Guzik et al. [64] found that mice lacking T and B cells (RAG-1 deficient mice) do not develop hypertension after Ang II and desoxycorticosterone acetate salt infusion. However, adoptive transfer of T cells but not B cells restored the hypertensive effect in the mice. By mRNA analysis, T and natural killer (NK) cells have been shown to express renin, the renin receptor, angiotensinogen, and ACE. T cells also express AT1 and AT2 receptors [65] and hence, a direct relationship may exist between inflammation and high BP. Trott et al. [66] have suggested how inflammation and high BP may be related. They hypothesized that the RAS, oxidative stress, salt, and other hypertensive stimuli may cause protein modification and the modified proteins may serve as neoantigens. The neoantigens cause the activation of T cells and T cell-derived signals to promote the entry of inflammatory cells into blood vessels and the kidneys resulting in the release of cytokines. Together with water and salt retention in the kidney, T cells promote vasoconstriction in the blood vessels and this can elevate BP. Many different studies have however reported a relationship between gut dysbiosis and inflammation [67, 68]. An increase in the levels of Veillonellaceae, Enterobacteriaceae, Pasteurellacaea, and Fusobacteriaceae and a decrease in Erysipelotrichales, Bacteroidales, and Clostridiales levels have been shown to be strongly linked with inflammation [69, 70]. Therefore, many studies have conducted to show the potential of probiotics to mitigate the condition. Probiotics such as B. infantis 35624 are known for their ability to induce T regulatory (TREG) cells [71]. TREG cells maintain tolerance to self-antigens and their depletion can result in inflammation and autoimmune diseases [72, 73]. Probiotic B. infantis promotes an increased production of CD25+Foxp3+ lymphocytes in murine models which protect against lipopolysaccharide or pathogen-induced NFκB activation [74]. B. infantis also stimulates human dendritic cells and selectively enhances the upregulation of Foxp3 expression in naïve lymphocytes [74]. It has also been reported that B. infantis induced the production of high levels of Foxp3+ TREG cells and interleukin-10 (IL-10) within peripheral blood of human volunteers who consumed B. infantis [75] and hence may reduce inflammations. A combination of L . casei, Bifidobacterium breve, and galactooligosaccharides [76] and B. longum (alone) [77] have also been shown to reduced serum CRP levels and improve the overall clinical appearance of patients with chronic inflammation. Administration of L. acidophilus ATCC 4356 [78], L. helveticus NS8 [79], and L. rhamnosus (LGG) [80] has also been reported to increase the production of IL-10 while inhibiting the production of proinflammatory cytokines in murine models, and this may play a role in inhibiting the onset of hypertension. Recently, Gomez-Guzman et al. [6] observed that administration of probiotics (L. fermentum CECT5716 (LC40) or Lactobacillus coryniformis CECT5711 (K8) plus L. gasseri CECT5714 (LC9) (1:1)) for 5 weeks reduced vascular reactive oxygen species levels in spontaneous hypertensive rats (SHR) by reducing NADPH oxidase activity. The probiotic treatment also significantly improved endothelial relaxation induced by acetylcholine in SHR rats and resulted in a reduction in SBP (13.4 ± 1.9 and 14.7 ± 1.9%) by LC40 and K8/LC9, respectively, with no significant changes in the heart rate. They also observed significant increase in the levels of Lactobacillus sp. and reduced the numbers of Bacteroides and Clostridium sp. relative to the control group indicating the ability of the probiotics to promote an increase in the levels of certain beneficial bacteria required for lowering BP. Though many probiotics are known to have immunomodulatory effects, the effects may be strain specific [81]. Therefore, it is important that probiotics that trigger specific immune response involved in BP regulation be identified and developed for managing hypertension.
Probiotics, RAS, and Hypertension
The health benefits and clinical effects associated with probiotic fermented foods have been known for ages. Most studies on the ability of probiotics to reducing BP have been elucidated through fermentation of food products in order to release bioactive peptides, such as the ACE inhibitory peptides that play a crucial role in inhibiting the RAS (Table 1). Probiotic fermented foods tend to be more effective in significantly reducing SBP or DBP compared to probiotics alone [17]. This is probably because some of the biopeptides released through fermentation are also active against high BP [18, 83, 84] and hence the combined effect is higher than that observed from probiotics alone. Nevertheless, even a small reduction in high BP can have significant health implications and cardiovascular consequences [85]. The fermentation method exploits the proteolytic systems of probiotic bacteria to hydrolyze food proteins and to release bioactive peptides (Fig. 1). Food-derived antihypertensive peptides may be safe [86] and have no side effects as those caused by synthetic drugs. Synthetic antihypertensive drugs are known to cause dysgeusia, dizziness, headache, angioedema, and cough [87, 88].
The LAB proteolytic systems. (A) Extracellular components: PrtP (a cellular envelope proteinase) requires PrtM, (proteinase maturation protein) for maturation; Opp (an oligopeptide permease) transports oligopeptides into the cell; DtpT (an ion linked transporter for di- and tripeptides), and Opt (an ABC transporter for peptides). (B) Intracellular peptidases: general (PepN, PepC) and specific (PepX, PepQ) peptidases and amino acid catabolic enzymes (carboxylase, aminotransferases, etc.)
Lactic Acid Bacteria Proteolytic Systems
Lactic acid bacteria (LAB) possess cell-envelope proteinase (CEP) with which they initiate milk protein hydrolysis into oligopeptides [89]. CEPs are serine proteases and belong to the subtilisin family. They are anchored to the cell wall via sortase A (SrtA). The type of CEP in LAB may be strain and specie dependent. However, the most abundant CEP in LAB is prtH3 and is present in over 80% of LAB strains followed by prtH and prtH4 [90]. The CEP genes in lactobacilli are genome encoded while those in lactococci are either genome or plasmid encoded. CEPs are synthesized as preproteins of about 2000 residues with several functional domains: a prepro (PP) domain, A, B, helix (H), S domains, a catalytic serine protease domain (PR), and a cell wall spacer domain (W) [91, 92]. CEP activation requires the maturation of PrtM (PrtM1 and PrtM2). PrtM1 is required for PrtH activation while PrtM2 is involved in the activation of other CEPs [90].
Peptides produced by CEP hydrolysis are transported into LAB cells for further hydrolysis (Fig. 1). Dipeptides are transported by Opp transport systems, tripeptides, and tetrapeptides (containing hydrophobic branched-chain amino acids) by Dpp transport systems while oligopeptides (hydrophilic and charged) are transported by the DtpT transport system [93]. Only one peptide transporter (DtpT) has however been identified in L. reuteri [94]. Various peptidases in LAB cells hydrolyze the absorbed peptides to release essential amino acids.
Four main LAB endopeptidases have been characterized namely PepO, PepF, PepG, and PepE [93]. PepO is a monomeric metalloprotease that can hydrolyze peptides from 5 up to 35 amino acid residues. Three paralogous genes encode PepO namely pepO, pepO2, and pepO3. PepF hydrolyzes peptides containing 7~17 amino acids and has a broad specificity. It is encoded by pepF, pepF1, and pepF2. Two paralogous genes have been reported for PepE in lactobacilli (pepE and pepE2). PepG and PepE are however absent in lactococci and streptococci [90]. Four LAB exopeptidases have been identified based on their specificities. They are aminopeptidases, dipeptides, tripeptidases, and proline-specific proteases. There are three classes of aminopeptidases based on their specificities (broad specificity, specific aminopeptidases for acidic or basic amino acids, and those specific for hydrophobic or aromatic residues). PepC and PepN are aminopeptidases with broad specificity and are present in all genomes. PepC, a member of the C1 family of cysteine peptidases, is specific for basic, acidic, hydrophobic/uncharged, and aromatic residues. Some studies have shown that antihypertensive peptides containing aromatic amino acids at the C-terminus and those with hydrophobic side chains have enhanced effects [95]; therefore, overexpressing the pepC gene in lactic acid bacteria could yield large amounts of ACE inhibitory peptides when used to ferment high protein foods. PepN on the other hand preferentially hydrolyzes basic residues followed by hydrophobic or uncharged residues [93].
The aminopeptidase PepS preferentially hydrolyzes aromatic residues and has been identified in Pediococcus pentosaceus, S. thermophilus, Leuconostoc mesenteroides, L. casei, and Lactobacillus sakei. PepA (glutamyl aminopeptidases) prefers to hydrolyze Glu and Asp residues. PepA has been identified in Lactobacillus, Streptococci, and Lactococcus but absent in Pediococcus and Oenococcus strains [89]. LAB tripeptidases usually have broad specificities for tripeptides but preferentially hydrolyze those containing hydrophobic amino acids. They however do not cleave tripeptides with proline residues. The tripeptidase PepT is found in all LAB strains and the pepT gene may occur as two paralogous genes in some strains such as L. acidophilus, L. gasseri, Lactobacillus johnsonii, and Lactobacillus sanfranciscensis [96]. Good quantities of isoleucyl-prolyl-proline (IPP) and valine-prolyl-proline (VPP) (the most popular anti-ACE peptides) may therefore be obtained in food fermented with LAB with overexpressed pepT genes or by treating the substrates with PepT tripeptidases. IPP and VPP have been shown to be resistant to gastrointestinal digestion and significantly reduce BP in both animal and humans [97]. The rigid structure of proline has been described to lock the carboxyl group into a conformation favorable for interaction with the positively charged residue at the active site of ACE to cause inhibition [98]. Therefore, overexpression of PepT in LAB could also yield other short peptides with proline at their C-terminus with anti-ACE activities. LAB also possess dipeptidases that cleave only dipeptides into amino acids as shown in Fig. 1. These peptidases may be highly specific or have broad specificity. Peptidases in the PepD and PepV families hydrolyze a large variety of dipeptides. PepD genes are heterogeneously distributed in LAB genomes. The PepL dipeptidase however is highly specific for Leu and Ala residues and has only been identified in L. delbrueckii [94].
Proline-specific peptidases hydrolyze proline residues from the N-terminal of peptides. PepR is a broad spectrum prolinase with a broad specificity for dipeptides including Met-Ala, Leu-Leu, and Leu-Gly-Gly [99] while the proline iminopeptidase (PepI) preferentially cleaves proline residues at the N-terminal of tripeptides [100]. PepP cleaves N-terminal amino acids that are directly linked to proline residues in oligopeptides. One pepP gene is ubiquitous in LAB genomes except in L. sakei and P. pentosaceus. PepQ is also present in all LAB strains as a copy per genome except for L. delbrueckii subsp. Bulgaricus which have two pepQ paralogs. While one paralogue is located in a separate cluster, the other is clustered with other orthologues. The proline-specific endopeptidase PepX is also ubiquitous in all LAB as one gene per genome [101, 102]. However, LAB strains from dairy environments may have two PepX peptidase homologues [94].
Although probiotics and their fermented foods reduce high BP in SHRs, results in human studies are controversial. A meta-analysis of the effects of lactotripeptides IPP and VPP showed that the lactotripeptides could significantly reduce BP in Asians (Japanese) but only slightly in Caucasians [97, 103, 104]. In these studies, however, most of the individual studies were conducted on small populations making it difficult to statistically detect small effects [104]. Studies using larger populations are required to overcome such barriers. No study has yet reported the ability of live probiotics alone to reduce high BP in humans though such studies are required to establish the direct effects of probiotics in mitigating the condition.
Recombinant Probiotics with Antihypertensive Effects
ACE inhibitory peptides released after probiotic fermentation are usually difficult to purify from the digested mixture. Also, capitalizing on the proteolytic ability of probiotics alone does not guarantee high quantities of ACE inhibitory peptides since the bacteria are living organisms and hence the type and quantity of the enzymes produced are difficult to control. This therefore makes the production of ACE inhibitory peptides by this method hardly reproducible. For these reasons, several studies have focused on producing recombinant probiotics that express ACE inhibitory peptides. Rao et al. [105] designed and expressed the antihypertensive peptide multimer AHPM by cloning the peptide sequence into the plasmid pGEX-3X and expressing it in Escherichia coli BL21. The recombinant AHPM fused with glutathione S-transferase (GST) and the proteins were expressed as inclusion bodies forming 35% of the total intracellular protein. A large quantity (399 mg/l) of the pure soluble GST-AHPM was obtained. They then cloned the ACE inhibitory peptide multimer AHPM-2 into pET32a and expressed it in E. coli. The expressed fusion Trx-AHPM-2 obtained after purification was subjected to simulated gastrointestinal digestion and the hydrolysate showed a strong ACE inhibitory activity (IC50 = 4.5 ± 0.3 μg/ml) [106]. Huang et al. [107] also used the plasmid pET-30a (+) bearing the anti-ACE peptide IYPR for protein expression in E. coli strains DH5а and BL21 (DE3). The recombinant protein accounted for 31% of the cellular protein with an IC50 value of 61 mg/l. The peptides reduced SBP significantly in SHRs after a single oral administration. These studies prove the possibility of producing large quantities of ACE inhibitory peptides for use as nutraceuticals using recombinant technology. Live recombinant probiotics have also been applied in BP lowering studies. In a quest to study the in vivo effect of recombinant antihypertensive probiotics, Yang et al. [16] transformed L. plantarum NC8 with pSIP409 plasmid-bearing ACE inhibitory peptides YFP and TFP originally obtained by chymotryptic hydrolysis of yellowfin sole (Limanda aspera) frame protein [108]. Rats fed with the recombinant probiotic strains had significantly reduced SBP relative to those who consumed L. plantarum (wild type) and PBS controls. The antihypertensive function of recombinant probiotic was maintained for at least 10 days (the SBP of the RLP-treated rats was 181.517 ± 2.312 mmHg, that of the L. plantarum treated rats was 195.876 ± 2.109 mmHg, and that of the PBS control rats was 197.376 ± 4.982 mmHg on the 24th day (P < 0.05)). Although recombinant probiotics could be effective in lowering BP, it is challenging to clone short peptides (especially di- and tripeptides). More studies are required to ascertain the possible safety concerns that may arise from consuming recombinant probiotics before they can be used in human studies.
Conclusion
The evidence that high BP is associated with gut dysbiosis makes it important to establish the ability of probiotics to reduce high BP in humans. However, though many probiotics and their fermented foods reduce high BP in SHRs, the results are conflicting in humans. Yet, the evidence that probiotics and their fermented products effectively reduce inflammation and hypercholesterolemia and affect the RAS (all of which have links with high BP) could support their application in improving cardiovascular health. Enhancing the proteolytic ability of probiotics by genetic engineering will be essential in increasing the levels of anti-ACE peptides in fermented foods. Recombinant probiotics could be a cheap and dependable source of antihypertensive peptides since the use of wild probiotics is tedious and may not be reproducible. Taken together, dietary interventions to correct gut dysbiosis and/or the consumption of fermented foods containing antihypertensive peptides could be novel nutritional therapeutic strategies for hypertension. As our knowledge about the hypotensive effects of probiotics grows, the mechanism by which they work is worth exploring. Also, a better understanding of the gut microbiota-host crosstalk and other networks underlying the control of BP will be critical in promoting the use of antihypertensive probiotics and their products.
References
James P, Oparil S, Carter BL, Cushman WC et al (2014) Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5):507–520
Hall JE, do Carmo JM, da Silva AA et al (2015) Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116(6):991–1006
Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E et al (1993) Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med, 1993 153(7):849–858
Stevens VJ, Obarzanek E, Cook NR et al (2001) Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 134(1):1–11
Turnbull F, Blood Pressure Lowering Treatment Trialists’ Collaboration (2003) Blood pressure lowering treatment trialists, effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 362(9395):1527–1535
Gomez-Guzman M, Toral M, Romero M et al (2015) Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59(11):2326–2336
Aburto NJ, Hanson S, Gutierrez H et al (2013) Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 346:f1378
Lye HS, Kuan CY, Ewe JA et al (2009) The improvement of hypertension by probiotics: effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci 10(9):3755–3775
Nelson RH (2013) Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 40(1):195–211
Majumder K, Wu J (2015) Molecular targets of antihypertensive peptides: understanding the mechanisms of action based on the pathophysiology of hypertension. Int J Mol Sci 16(1):256–283
Guang C, Phillips RD, Jiang B et al (2012) Three key proteases—angiotensin-I-converting enzyme (ACE), ACE2 and renin—within and beyond the renin-angiotensin system. Arch Cardiovasc Dis 105(6–7):373–385
Skidgel RA, Stanisavljevic S, Erdos EG (2006) Kinin- and angiotensin-converting enzyme (ACE) inhibitor-mediated nitric oxide production in endothelial cells. Biol Chem 387(2):159–165
Kuoppala A, Lindstedt KA, Saarinen J et al (2000) Inactivation of bradykinin by angiotensin-converting enzyme and by carboxypeptidase N in human plasma. Am J Physiol Heart Circ Physiol 278(4):H1069–H1074
Yang T, Santisteban MM, Rodriguez V et al (2015) Gut dysbiosis is linked to hypertension. Hypertension 65(6):1331–1340
Honour JW (2015) Historical perspective: gut dysbiosis and hypertension. Physiol Genomics 47(10):443–446
Yang G, Jiang Y, Yang W et al (2015) Effective treatment of hypertension by recombinant Lactobacillus plantarum expressing angiotensin converting enzyme inhibitory peptide. Microb Cell Factories 14:202
Khalesi S, Sun J, Buys N et al (2014) Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64(4):897–903
Aluko RE (2015) Antihypertensive peptides from food proteins. Annu Rev Food Sci Technol 6:235–262
Korhonen R, Korpela R, Saxelin M et al (2001) Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation 25(4):223–232
Tejada-Simon MV, Ustunol Z, Pestka JJ (1999) Ex vivo effects of lactobacilli, streptococci, and bifidobacteria ingestion on cytokine and nitric oxide production in a murine model. J Food Prot 62(2):162–169
Linsalata M, Russo F, Berloco P et al. (2005) Effects of probiotic bacteria (VSL#3) on the polyamine biosynthesis and cell proliferation of normal colonic mucosa of rats. In Vivo 19(6): 989–995.
Linsalata M, Russo F, Berloco P et al (2004) The influence of Lactobacillus breves on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter 9(2):165–172
Kullisaar T, Zilmer M, Mikelsaar M et al (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72(3):215–224
Carroll IM, Andrus JM, Bruno-Bárcena JM et al (2007) Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 293(4):G729–G738
Andrus JM, Bowen SW, Klaenhammer TR et al (2003) Molecular characterization and functional analysis of the manganese-containing superoxide dismutase gene (sodA) from Streptococcus thermophilus AO54. Arch Biochem Biophys 420(1):103–113
Xing J, Wang G, Zhang Q et al (2015) Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: a comparison with traditional methods. PLoS One 10(3):e0119058
Ahire JJ, Mokashe NU, Patil HJ et al (2013) Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J Food Sci Technol 50(1):26–34
Parvaneh K, Jamaluddin R, Karimi G et al (2014) Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal 2014:595962
Vinderola G, Matar C, Perdigon G (2007) Milk fermentation products of L. helveticus R389 activate calcineurin as a signal to promote gut mucosal immunity. BMC Immunol 8:19
Resnick LM (1999) The role of dietary calcium in hypertension: a hierarchical overview. Am J Hypertens 12(1 Pt 1):99–112
Sakurai M, Stamler J, Miura K et al (2011) Relationship of dietary cholesterol to blood pressure: the INTERMAP study. J Hypertens 29(2):222–228
Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA (1996) Relationship to blood pressure of combinations of dietary macronutrients. Findings of the Multiple Risk Factor Intervention Trial (MRFIT). Circulation 94(10):2417–2423
Stamler J, Liu K, Ruth KJ et al (2002) Eight-year blood pressure change in middle-aged men: relationship to multiple nutrients. Hypertension 39(5):1000–1006
Hashimoto M, Eto M, Akishita M, Kozaki K, Ako J, Iijima K, Kim S, Toba K, Yoshizumi M, Ouchi Y (1999) Correlation between flow-mediated vasodilatation of the brachial artery and intima-media thickness in the carotid artery in men. Arterioscler Thromb Vasc Biol 19(11):2795–2800
Ferrara LA, Guida L, Iannuzzi R et al (2002) Serum cholesterol affects blood pressure regulation. J Hum Hypertens 16(5):337–343
Prakash S, Rodes L, Coussa-Charley M et al (2011) Gut microbiota: next frontier in understanding human health and development of biotherapeutics. Biologics 5:71–86
Larsen N, Vogensen FK, van den Berg FW et al (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5(2):e9085
Ley RE, Bäckhed F, Turnbaugh P et al (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102(31):11070–11075
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A et al (2011) Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci 94(7):3288–3294
Anderson JW, Gilliland SE (1999) Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J Am Coll Nutr 18(1):43–50
Agerbaek M, Gerdes LU, Richelsen B (1995) Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur J Clin Nutr 49(5):346–352
Bertolami MC, Faludi AA, Batlouni M (1999) Evaluation of the effects of a new fermented milk product (Gaio) on primary hypercholesterolemia. Eur J Clin Nutr 53(2):97–101
Jones ML, Martoni CJ, Prakash S (2012) Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 66(11):1234–1241
Jones ML, Martoni CJ, Parent M et al (2012) Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 107(10):1505–1513
Ishimwe N, Daliri EB, Lee BH et al (2015) The perspective on cholesterol-lowering mechanisms of probiotics. Mol Nutr Food Res 59(1):94–105
Lye H, Rahmat-Ali G, Liong M (2010) Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J 20:6
Huang Y, Wang X, Wang J, Wu F, Sui Y, Yang L, Wang Z (2013) Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J Dairy Sci 96(5):2746–2753
Chiang JY (2013) Bile acid metabolism and signaling. Compr Physiol 3(3):1191–1212
Lye HS, Rusul G, Liong MT (2010) Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J Dairy Sci 93(4):1383–1392
Marcil V, Delvin E, Garofalo C, Levy E (2003) Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer trotein in caco-2 cells. J Nutr 133:4
Ooi LG, Ahmad R, Yuen KH et al (2010) Lactobacillus gasseri [corrected] CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J Dairy Sci 93(11):5048–5058
Remagni MC, Paladino M, Locci F et al (2013) Cholesterol removal capability of lactic acid bacteria and related cell membrane fatty acid modifications. Folia Microbiol (Praha) 58(6):443–449
Ferrier KE, Muhlmann MH, Baguet JP et al (2002) Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol 39(6):1020–1025
Buchwald H, Boen JR, Williams SE, Nguyen PA, Matts JP (2003) Blood pressure, weight, and cholesterol. J Am Coll Cardiol 41:1
Gurantz D, Cowling RT, Varki N, Frikovsky E, Moore CD, Greenberg BH (2005) IL-1β and TNF-α upregulate angiotensin II type 1 (AT 1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J Mol Cell Cardiol 38(3):505–515
Hoch NE, Guzik TJ, Chen W et al (2009) Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296(2):R208–R216
Marvar PJ, Vinh A, Thabet S et al (2012) T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry 71(9):774–782
Bautista LE, Vera LM, Arenas IA, Gamarra G (2005) Independent association between inflammatory markers (C-reactiveprotein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 19(19):5
Lakoski SG, Cushman M, Palmas W, Blumenthal R, D’Agostino RB Jr (1869-1874) Herrington DM (2005) the relationship between blood pressure and C-reactive protein in the multi-ethnic study of atherosclerosis (MESA). J Am Coll Cardiol 46:5
Sesso HD, Buring JE, Rifai N et al (2003) C-reactive protein and the risk of developing hypertension. JAMA 290(22):2945–2951
Niskanen L, Laaksonen D, Nyyssönen K, Punnonen K, Valkonen VP, Fuentes R, Tuomainen TP, Salonen R, Salonen JT (2004) Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension 44(6):6
Ghanem FA, Movahed A (2007) Inflammation in high blood pressure: a clinician perspective. J Am Soc Hypertens 1(2):113–119
Yu B, Shahid M, Egorina EM, Sovershaev MA et al (2010) Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology 112(3):586–594
Guzik TJ, Hoch NE, Brown KA et al (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204(10):2449–2460
Jurewicz M, McDermott DH, Sechler JM et al (2007) Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol 18(4):1093–1102
Trott DW, Harrison DG (2014) The immune system in hypertension. Adv Physiol Educ 38(1):20–24
Jiang W, Wu N, Wang X et al (2015) Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 5:8096
Fallucca F, Porrata C, Fallucca S et al (2014) Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab Res Rev 30(Suppl 1):48–54
Shaw KA, Bertha M, Hofmekler T et al (2016) Dysbiosis, inflammation, and response to treatment: a longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med 8(1):75
Gevers D, Kugathasan S, Denson LA et al (2014) The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15(3):382–392
Konieczna P, Akdis CA, Quigley EM et al (2012) Portrait of an immunoregulatory Bifidobacterium. Gut Microbes 3(3):261–266
Corthay A, Miyara M, Costantino CM (2009) How do regulatory T cells work? Scand J Immunol 70(4):490–500
Sakaguchi S, Miyara M, Costantino CM et al (2010) FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10(7):490–500
O'Mahony C, Scully P, O'Mahony D et al (2008) Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog 4(8):e1000112
Konieczna P, Groeger D, Ziegler M et al (2012) Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 61(3):354–366
Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 244:8
Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT (2005) Synbiotic therapy (Bifidobacterium longum/synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54:8
Chen L, Liu W, Li Y et al (2013) Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int Immunopharmacol 17(1):108–115
Rong J, Zheng H, Liu M et al (2015) Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol 15:196
Mirpuri J, Sotnikov I, Myers L et al (2012) Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS One 7(12):e51955
Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Järvenpää S, Kautiainen H, Julkunen I, Vapaatalo H, Korpela R (2008) Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol 14(13):7
Koyama M, Naramoto K, Nakajima T et al (2013) Purification and identification of antihypertensive peptides from fermented buckwheat sprouts. J Agric Food Chem 61(12):3013–3021
Ruiz-Gimenez P, Ibáñez A, Salom JB et al (2010) Antihypertensive properties of lactoferricin B-derived peptides. J Agric Food Chem 58(11):6721–6727
García-Tejedor A, Sánchez-Rivera L, Castelló-Ruiz M, Recio I, Salom JB, Manzanares P (2014) Novel antihypertensive lactoferrin-derived peptides produced by Kluyveromyces marxianus: gastrointestinal stability profile and in vivo angiotensin I-converting enzyme (ACE) inhibition. J Agric Food Chem 62(7):1609–1616
Cook NR, Cohen J, Hebert PR et al (1995) Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 155(7):701–709
Chakrabarti S, Jahandideh F, Wu J (2014) Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int 2014:608979
Gannon TH, Eby TL (1990) Angioedema from angiotensin converting enzyme inhibitors: a cause of upper airway obstruction. Laryngoscope 100(11):1156–1160
Israili ZH, Hall WD (1992) Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 117(3):234–242
Liu M, Bayjanov JR, Renckens B et al (2010) The proteolytic system of lactic acid bacteria revisited: a genomic comparison. BMC Genomics 11:36
Broadbent JR, Cai H, Larsen RL et al (2011) Genetic diversity in proteolytic enzymes and amino acid metabolism among Lactobacillus helveticus strains. J Dairy Sci 94(9):4313–4328
Pastar I, Tonic I, Golic N et al (2003) Identification and genetic characterization of a novel proteinase, PrtR, from the human isolate Lactobacillus rhamnosus BGT10. Appl Environ Microbiol 69(10):5802–5811
Fernandez-Espla MD, Rul F (1999) PepS from Streptococcus thermophilus. A new member of the aminopeptidase T family of thermophilic bacteria. Eur J Biochem 263(2):502–510
Savijoki K, Ingmer H, Varmanen P (2006) Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol 71(4):394–406
Mozzi F, Raya RlR, Vignolo, G.M (2010) Biotechnology of lactic acid bacteria : novel applications., Ames, Iowa: Wiley-Blackwell. xii, 393
Wu J, Aluko RE, Nakai S (2006) Structural requirements of angiotensin I-converting enzyme inhibitory peptides: quantitative structure-activity relationship study of di- and tripeptides. J Agric Food Chem 54(3):732–738
Vermeulen N, Pavlovic M, Ehrmann MA et al (2005) Functional characterization of the proteolytic system of Lactobacillus sanfranciscensis DSM 20451T during growth in sourdough. Appl Environ Microbiol 71(10):6260–6266
Fekete AA, Givens DI, Lovegrove JA (2015) Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 7(1):659–681
Manzanares P, Salom JB, García-Tejedor A et al (2015) Unraveling the mechanisms of action of lactoferrin-derived antihypertensive peptides: ACE inhibition and beyond. Food Funct 6(8):2440–2452
Varmanen P, Rantanen T, Palva A et al (1998) Cloning and characterization of a prolinase gene (pepR) from Lactobacillus rhamnosus. Appl Environ Microbiol 64(5):1831–1836
Navidghasemizad S, Takala TM, Alatossava T et al (2013) Proline iminopeptidase PepI overexpressing Lactobacillus casei as an adjunct starter in Edam cheese. Bioengineered 4(6):408–412
Sanz Y, Toldra F (2001) Purification and characterization of an X-prolyl-dipeptidyl peptidase from Lactobacillus sakei. Appl Environ Microbiol 67(4):1815–1820
Habibi-Najafi MB, Lee BH (1994) Purification and characterization of X-prolyl dipeptidyl peptidase from Lactobacillus casei subsp. casei LLG. Appl Microbiol Biotechnol 42(2–3):280–286
Cicero AF, Gerocarni B, Laghi L et al (2011) Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials. J Hum Hypertens 25(7):425–436
Cicero AF, Aubin F, Azais-Braesco V et al (2013) Do the lactotripeptides isoleucine-proline-proline and valine-proline-proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am J Hypertens 26(3):442–449
Rao S, Su Y, Li J et al (2009) Design and expression of recombinant antihypertensive peptide multimer gene in Escherichia coli BL21. J Microbiol Biotechnol 19(12):1620–1627
Rao S, Xu Z, Su Y et al (2011) Cloning, soluble expression, and production of recombinant antihypertensive peptide multimer (AHPM-2) in Escherichia coli for bioactivity identification. Protein Pept Lett 18(7):699–706
Huang L, Ma H, Li Y, Li S (2012) Antihypertensive activity of recombinant peptide IYPR expressed in Escherichia coli as inclusion bodies. Protein Expr Purif 83(1):15–20
Jung WK, Mendis E, Je JY, Park PJ, Son BW, Kim CK, Choi YK, Kim SK (2006) Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Foodchem 94(1):6
Seppo L, Jauhianen T, Poussa T, Korpela R (2003) A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 77:5
Chen Y, Liu W, Xue J et al (2014) Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J Dairy Sci 97(11):6680–6692
Dong JY, Szeto IM, Makinen K et al (2013) Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr 110(7):1188–1194
Tuomilehto J, Lindström J, Hyyrynen J et al (2004) Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. J Hum Hypertens 18(11):795–802
Aihara K, Kajimoto O, Hirata H et al (2005) Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr 24(4):257–265
Mizushima S, Ohshige K, Watanabe J et al (2004) Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am J Hypertens 17(8):701–706
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H (2003) Blood-pressure-lowering effect of a novel fermented milk containing big gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 57:490–495
Hariri M, Salehi R, Feizi A et al (2015) The effect of probiotic soy milk and soy milk on anthropometric measures and blood pressure in patients with type II diabetes mellitus: a randomized double-blind clinical trial. ARYA Atheroscler 11(Suppl 1):74–80
Hata Y, Yamamoto M, Ohni M et al (1996) A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr 64(5):767–771
Nakamura Y, Yamamoto M, Ohni M et al (1995) Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci 78(6):1253–12537
Jauhiainen T, Vapaatalo H, Poussa T et al (2005) Lactobacillus helveticus fermented milk lowers blood pressure in hypertensive subjects in 24-h ambulatory blood pressure measurement. Am J Hypertens 18(12 Pt 1):1600–1605
Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A (2000) Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci 83(2):10
Sharafedtinov KK, Plotnikova OA, Alexeeva RI et al (2013) Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients--a randomized double-blind placebo-controlled pilot study. Nutr J 12:138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Daliri, E.BM., Lee, B.H. & Oh, D.H. Current Perspectives on Antihypertensive Probiotics. Probiotics & Antimicro. Prot. 9, 91–101 (2017). https://doi.org/10.1007/s12602-016-9241-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9241-y