Abstract
Purpose of Review
The present review focuses in the hypertension-associated changes in the microbiota and the current insights regarding the impact of probiotics on blood pressure in animal models and in human hypertensive patients.
Recent Findings
Gut dysbiosis in hypertension is characterized by (i) the gut microbioma that is less diverse and less rich with an increased Firmicutes/Bacteroidetes ratio and (ii) a decrease in acetate- and butyrate-producing bacteria and an increase in lactate-producing bacterial populations. The meta-analysis of the human studies supports that supplementation with probiotics reduces blood pressure. The mechanism of this antihypertensive effect of probiotics and its protective effect on endothelial function has not been fully elucidated.
Summary
Further investigations are needed to clarify if the effects of probiotic bacteria result from the changes in the gut microbiota and its metabolic by-products; the restoration of the gut barrier function; and the effects on endotoxemia, inflammation, and renal sympathetic nerve activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is one of the most powerful risk factors for cardiovascular events, including myocardial infarction and stroke. Hypertension is present in about one quarter of the world’s population and is responsible for about 41% of the cardiovascular disease-related deaths [1]. Treatment with any commonly used antihypertensive regimen reduces the risk of total major cardiovascular events; the larger the reductions in blood pressure (BP) are, the larger the reductions in risk are [2]. Although more than 60 distinct drugs are available to lower BP, less than half of the treated hypertensives reach the currently recommended BP targets. Furthermore, an estimated 10–30% of the general hypertensive population present resistant hypertension, defined as high BP not adequately controlled by three antihypertensive agents from different classes, one of them a diuretic [3].
BP is determined by the following three main factors: vascular smooth muscle cell (VSMC) tone, cardiac function, and renal Na+ excretion and plasma and total body volume, which control peripheral vascular resistances (PVRs), cardiac output, and intravascular volume, respectively. Even when the underlying cause of hypertension is unknown, in ~95% of patients, an increase in PVR is the key component of hypertension. In recent years, progress in basic research has led to the identification of new VSMC signaling pathways and possible therapeutic targets for the treatment of hypertension, and several promising drugs have subsequently been developed [4]. Current guidelines for the management of arterial hypertension propose lifestyle measures, including dietary approaches in all patients, including those who require drug treatment. The purpose of these lifestyle measures is to lower BP, to control other risk factors, and to reduce the number or the dose of antihypertensive drugs [3]. These preventive strategies have had limited success. Therefore, there is an urgent need for alternative strategies.
In recent years, gut microbiome dysbiosis has been associated with hypertension [5•], although the mechanisms involved in the BP control by the microbiota has not been fully elucidated. Recently, a brain-gut-bone marrow triangular interaction has been suggested as a new mechanism involved in the pathophysiology of hypertension [6•]. Therefore, manipulation of the gut microbiota may lead to the development of novel antihypertensive therapies. Several clinical trials and animal studies have been conducted evaluating the use of probiotics. Herein, we review the changes in the microbiota associated to hypertension and discuss the current insights regarding the impact of probiotics in animal models and in human hypertensive patients.
Gut Microbiota in Hypertension
The mammalian microbiome consists of unique assemblages of microorganisms (i.e., bacteria, archaea, fungi, and viruses) associated with various niches in and on the body. The gut microbiota is dominated to a large extent by Firmicutes and Bacteroidetes and to a lesser extent by Actinobacteria and Proteobacteria [7]. However, gut microbiota constantly adapts to lifestyle modifications, such as diet and even exercise. In addition, it has been commonly observed that a change in the host health status has been accompanied by a shift in the gut microbiota.
To date, there are limited studies indicating a direct association between gut microbiota and hypertension in both animal models and humans. Recently, it has been demonstrated that the normal gut microbiota may influence BP. Karbach et al. [8•] showed that BP was not different between germ-free and conventionally raised mice, which is consistent with previous observations describing no effect in BP after dramatic reduction in fecal microbial biomass induced by antibiotic treatment [9•]. Similarly, it has been described that both the Firmicutes/Bacteroidetes ratio and the abundance levels of selected genera in pre-hypertensive spontaneously hypertensive rats (SHR) were not significantly different from the age-matched Wistar-Kyoto rats (WKY) [6•]. However, the absence of gut microbiota protects mice from angiotensin II-induced hypertension, vascular dysfunction, and hypertension-induced end-organ damage, showing for the first time that commensal microbiota, an ecosystem that is acquired directly after birth, could represent an environmental factor promoting angiotensin II-induced high BP [8•]. Similarly, oral administration of antibiotics improved BP in angiotensin II-induced hypertension and in SHR [5•]. Case report of resistant hypertensive patients shows a BP reduction from 160/90 to 130/60 mmHg after a combination of antibiotic treatment [10].
A delicate balance in the gut microbiota composition is key in maintaining intestinal immunity and whole-body homeostasis. An imbalance in gut microbiota is commonly known as dysbiosis. Recently, it has been demonstrated that high BP is associated with gut microbiota dysbiosis, both in animal and human hypertension [5•, 11]. In animal studies, gut dysbiosis in hypertension was characterized by (i) the gut microbioma that is less diverse, less rich, and shows an increased Firmicutes/Bacteroidetes ratio, caused by an expansion of Firmicutes and a contraction of Bacteroidetes, and (ii) a decrease in acetate- and butyrate-producing bacteria and increase in lactate-producing bacterial populations. However, the clinical evidences of a role for dysbiosis in hypertension are limited. Yet, obese school girls with high BP show lower abundance of Bacteroidetes in their gut microbiota than normal-weight girls [12]. In addition, overweight and obese pregnant women at 16-week gestation, the abundance of butyrate-producing bacteria, and butyrate production in the gut microbiota are significantly negatively associated with BP and with plasminogen activator inhibitor-1 (PAI-1) levels [13]. In that study, the abundance of the butyrate-producing genus Odoribacter was inversely correlated with systolic BP.
A significant change in gut microbiota population between adult SHR and age-matched WKY has also been shown [14•] and is characterized by increased numbers of Gram-negative Bacteroides spp. (phylum Bacteroidetes) and Gram-positive Clostridium spp. (phylum Firmicutes) and reduced numbers of bifidobacteria (phylum Actinobacteria). Considering the different methods used to explore gut microbiota in this study (qRT-PCR for some bacteria genera) in comparison to those used by Yang et al. [5•] (16-s ribosomal DNA sequencing), comparing the data from both microbiota analyses is difficult. However, a significant depletion of Bifidobacterium sp. in SHR was noted in both studies. Bifidobacterium is commonly considered a beneficial bacterial genus that plays a critical role in the maturation and regulation of the immune system [15]. Consequently, Bifidobacterium depletion might contribute to the dysregulation of the immune system found in SHR [16]. It is important to note that in pre-hypertensive SHR, the abundance levels of selected genera were not significantly different from the age-matched WKY (Fig. 1) and the Firmicutes/Bacteroidetes ratio was also similar between both strains [6•]. These findings support the suggestion that the BP increase in the SHR is closely associated with the development of gut dysbiosis. However, in other studies, no significant changes in gut microbiota after L-NAME treatment in rats [17] or between WKY and SHR [18] were observed, which could be attributed to difference in technologies used.
This association between gut microbial dysbiosis and hypertension does not necessarily indicate a cause-effect relationship. The demonstration of the involvement of these microbial populations in pathophysiology requires more complex experimental design. We performed fecal transplantation studies between control WKY and SHR. Interestingly, fecal microbiota transplantation from adult SHR to adult WKY results in a chronic raise in BP (Fig. 2), vascular oxidative stress, and impaired endothelial function. Conversely, fecal microbiota transplantation from WKY to adult SHR induced a BP reduction and improvement of endothelial dysfunction. These results confirm previous studies suggesting a strong association between gut microbial dysbiosis and hypertension, establishing a cause-effect relationship between altered microbiota and high BP.
Tail systolic blood pressure (SBP) measured after 4 weeks of fecal microbiota transplantation from adult normotensive Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR). Groups W-W, WKY transplanted with fecal microbiota from WKY; W-S, WKY transplanted with fecal microbiota from SHR; S-S, SHR transplanted with fecal microbiota from SHR; S-W, SHR transplanted with fecal microbiota from WKY
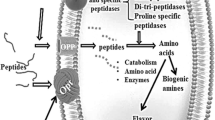
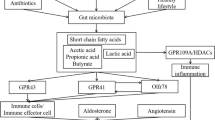
The gut microbiota can potentially influence host BP through multiple mechanisms. The modulation of the immune system by gut microbiota is an area of active investigation. One potential mechanism for this is through bacterial products that enter the circulation. Although several bacteria genera, such as Streptococcus, Escherichia, Lactobacillus, and Bifidobacterium, can synthesize neurotransmitters, the evidence for a pathophysiological role is very weak [19]. However, there is abundant evidence that gut microbiota has important influences on host cell physiology through bacterial metabolic products such as short-chain fatty acids (SCFAs) [9•] or trimethylamine-N-oxide [20] or bacterial wall components such as lipopolysaccharide (LPS) [21, 22]. Therefore, adiposity and inflammatory response are controlled by the gut microbiota [23, 24]. Furthermore, the gut flora may interact with intestinal epithelial cells and dendritic cells, which control the innate and adaptive immunities [25]. For example, SCFAs such as acetate and butyrate have been shown to have antiinflammatory effects on myeloid cells as well as intestinal epithelial cells [26]. Through histone deacetylase inhibition, butyrate regulates intestinal macrophage function [27], whereas acetate promotes T-helper 17 cell development [28]. T-helper 17 cells are modulated by various gut immune and microbial mechanisms and have been associated with the development of hypertension and vascular dysfunction [29]. The SCFA levels produced by gut microbial fermentation were associated with BP in human [30]. In fact, butyrate attenuates angiotensin II-induced hypertension in mice [31], and both, a diet rich in fiber, which substantially increases the production of SCFAs such as acetate, or acetate supplementation, prevented the development of hypertension in deoxycorticosterone acetate (DOCA) salt [32].
SCFAs modulate BP through the renal and vascular olfactory receptor (Olfr)78 and G-protein-coupled receptor (GPR)41 in mice [9•]. These receptors are mutually antagonistic and respond to SCFAs. Stimulation of Olfr78 elevates BP, whereas stimulation of GRP41 lowers BP. However, Olfr78 is less sensitive to SCFAs compared with GPR41 [33]. Therefore, we sought to determine whether there were alterations in messenger RNA (mRNA) expression of the rat orthologs of Olfr78 and GPR41, namely, Olfr59 and free fatty-acid receptor 3 (Ffar3) at the vasculature, comparing WKY and SHR models. Interestingly, we found a threefold upregulation of Olfr59 in aorta accompanied by a 70% downregulation of Ffar3 (Fig. 3) in SHR. In addition, prohypertensive angiotensin II also induces similar qualitative expressional changes in rat aortic endothelial cells (RAECs) (Fig. 3b). Because Ffar3 induces vasodilatation in response to SCFAs, and subsequently reduces BP [9•], these expressional data support the suggestion that altered SCFA receptors in aorta may play a role in elevated BP of the SHR. In contrast, other receptors for SCFA do not appear to play any role. Thus, in hypertensive DOCA-salt mice, GPR43 and Olfr78 were not expressed in the kidney or heart [32] and GPR43 mRNA was also downregulated in the gut with mineralocorticoid excess.
The gut microbiota influences the host inflammatory response [34]. Inflammation may have an important role in the development of hypertension in both humans and experimental animal models, as is suggested by several lines of evidence. One potential mechanism by which inflammation may promote hypertension is by causing endothelial dysfunction. Endothelial dysfunction may contribute to increased PVR and thus lead to the development of hypertension, which commonly manifests as impaired endothelium-dependent vasodilation caused by an imbalance between vasoconstrictors and vasodilators [35]. Inflammation can alter the rates of synthesis and degradation of vasoconstrictors and vasodilators including nitric oxide (NO). Impaired NO bioactivity is associated with hypertension, and inflammation has been shown to downregulate NO synthase (NOS) activity [35]. Bacterial LPS, through TLR4 activation, contributes to increased BP and low-grade vascular inflammation displayed by SHR [16]. TLR4 protein expression, in fact, was higher in vascular tissues from 15-week-old SHR than in age-matched Wistar controls or in 5-week-old SHR [16]. In addition, chronic L-NAME treatment also augmented TLR4 expression [36]; TLR4−/− mice demonstrated a full BP protection against L-NAME-induced hypertension [37]. Enhanced TLR4 expression thus might be linked to the development and maintenance of hypertension. LPS stimulates and increases the expression of TLR4 in the vasculature, which resulted in increased NADPH oxidase-dependent O2 − production and inflammation [22•, 38].
Therefore, a triangular connection involving a dysfunctional sympathetic-gut-bone marrow communication has been suggested in hypertension [6•]. Increased sympathetic activity to the gut results in elevated gut permeability, altered inflammatory status, and microbial dysbiosis impacting bone marrow production of proinflammatory cells [39].
Collectively, all data suggest an association between gut microbial dysbiosis and hypertension, possibly by altering bacterial metabolism and/or structural products, which are subsequent changes in T lymphocyte populations, inflammation, vascular oxidative stress, and endothelial dysfunction, impacting in central nervous system, renal, and vascular regulation of BP.
Probiotics in Hypertension
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [40]. There are increasing numbers of probiotic products available to consumers, which include yogurt, other fermented milk, and food products as well as various forms of dietary supplements. These products are usually prepared using lactic acid bacteria of the following four general species: Lactobacillus sp., Bifidobacterium sp., Enterococcus sp., and Streptococcus sp., although the probiotic bacteria type and composition vary from product to product. Common probiotic yogurts often contain one or two bacterial strains, such as Bifidobacterium lactis and/or Lactobacillus acidophilus, whereas kefir, another fermented milk product, contains many more strains. Probiotics also affect the composition and the diversity of the gut microbiota, and their potential role in different diseases is currently a hot topic for research and discussion [41, 42]. Although the use of probiotics has been primarily associated with the improvement of gastrointestinal health, recent evidence has also shown that probiotics play an important role in other diseases, including hypertension.
Few studies are currently available on the use of probiotic microorganisms at reducing cardiovascular disease. For example, some probiotics display cholesterol-lowering properties [43, 44], improve atherosclerosis [45, 46], or attenuate myocardial hypertrophy and heart failure after myocardial infarction [47].
The beneficial effects of probiotic strains on BP have been reviewed recently [48,49,50,51]. The ability of probiotics to reduce BP has been attributed to the release of bioactive peptides during the fermentation of food products, such as the angiotensin-converting enzyme inhibitory peptides. However, the bioavailability of such peptides is unclear. In addition, it has been described that some lactic acid bacteria, such as Lactobacillus johnsonii La1 (LJLa1), a probiotic strain adhesive onto intestinal epithelial cells, has hypotensive action in urethane-anesthetized rats [52]. Intraduodenal injection of LJLa1 reduced renal sympathetic nerve activity (RSNA) and BP and enhanced gastric vagal nerve activity (GVNA). Pre-treatment with thioperamide, a histaminergic H3-receptor antagonist, eliminated the effects of LJLa1 on RSNA, GVNA, and BP. Furthermore, bilateral lesions of the hypothalamic suprachiasmatic nucleus, the master circadian oscillator, abolished the suppression of RSNA and BP and the elevation of GVNA caused by LJLa1. These findings suggest that LJLa1 or its metabolites might lower BP by changing autonomic neurotransmission via the central histaminergic nerves and the suprachiasmatic nucleus in rats. By contrast, the intragastric Lactobacillus casei Shirota injection reduced efferent sympathetic nerve outflow to the adrenal gland and liver but did not alter GVNA, RSNA, and mean BP in anesthetized rats [53]. These results suggest that BP regulation induced by probiotics is strain specific. Furthermore, the molecular mechanisms involved in the antihypertensive effects are unknown.
Only few animal studies analyzed the mechanisms involved on the antihypertensive effects of chronic probiotic consumption. It is well established that consumption of probiotics inhibited the production of proinflammatory cytokines [54,55,56]. We evaluated for the first time the effects of a probiotic with immunomodulatory properties, Lactobacillus coryniformis CECT5711, originally isolated from a traditional goat cheese, in BP and vascular function in obese mice fed on a high-fat diet (HFD) [22•]. The probiotic treatment was given for 12 weeks, and it did not affect the weight evolution, although it reduced high BP, basal glycemia, and insulin resistance. L. coryniformis administration to HFD-induced obese mice induced marked changes in microbiota composition and reduced the metabolic endotoxemia as it decreased the LPS plasma levels, which was associated with a significant improvement in gut barrier disruption. Furthermore, it lowered tumor necrosis factor α (TNFα) expression in liver, improving the inflammatory status, and thus the glucose metabolism. Additionally, the probiotic reversed the endothelial dysfunction observed in obese mice. It also restored the increased vessel superoxide levels observed in obese mice, by reducing NADPH oxidase activity and increasing antioxidant enzymes. Moreover, chronic probiotic administration for 2 weeks also improved endothelial dysfunction and vascular oxidative stress induced by in vivo administration of LPS in control mice fed on a standard chow diet. These results are in agreement with the following hypothesis: HFD induces changes in gut microbiota that disrupt the gut barrier leading to a higher LPS plasma levels. Thus, LPS impacts with target metabolic organs (such as the liver), altering glucose metabolism, and vascular wall, leading to oxidative stress. Both metabolic and direct vascular effects are involved on endothelial dysfunction and hypertension. L. coryniformis administration induced changes in the gut microbiota that improved gut barrier dysfunction, reducing plasma LPS and the subsequent alterations in glucose metabolism and vascular oxidative stress. In addition, by unknown mechanisms, the probiotic impaired LPS signaling contributing to improve endothelial dysfunction.
The effects of oral supplementation with probiotics in non-obese SHR, an established model of genetic hypertension characterized by elevated BP, arterial remodeling, endothelial dysfunction, vascular inflammation, and dysregulation of the immune system, were also studied [13]. Two groups of treatments with probiotics with inmunomodulatory properties were used: (a) Lactobacillus fermentum CECT5716 or (b) L. coryniformis plus L. gasseri. Both probiotic treatments induced a change in the cecum microbiota of SHR, with higher counts of the Lactobacillus spp. cluster and lower counts of Bacteriodes spp. and Clostridium spp. These changes were associated with the improvement of vascular oxidative and inflammatory status resulting in increased NO bioavailability. Similarly, antihypertensive and vascular protective effects in SHR have been described after 8 weeks of treatment with L. casei 1011 CFU/day [57]. In addition, kefir treatment for 60 days was able to improve the endothelial function in SHR by partially restoring the reactive oxygen species (ROS)/NO imbalance and the endothelial architecture due to endothelial progenitor cell recruitment [58]. By contrast, adding probiotics to a blueberry-enriched diet does not enhance and actually may impair the antihypertensive effect of blueberry consumption. However, probiotic bacteria are not interfering with blueberry polyphenol metabolism into hippuric acid [59].
In human, a recent meta-analysis of randomized, controlled trials suggests that consuming probiotics may improve BP [60•]. In the nine trials included, probiotic consumption significantly lowered systolic BP by 3.56 mmHg and diastolic BP by 2.38 mmHg compared with the control groups. This meta-analysis suggests that consuming probiotics may improve BP by a modest degree, with a potentially greater effect when baseline BP is elevated, multiple species of probiotics are consumed, the duration of intervention is ≥8 weeks, or daily consumption dose is ≥1011 CFU.
Conclusion
Several questions would be addressed. First, if these vascular protective effects are related to changes in immune cell infiltration, reducing vascular oxidative stress, and increasing NO levels. Second, fecal microbiota transplantation studies are necessary to be known if changes in gut microbiota induced by probiotics are ultimately responsible to restore gut permeability and inflammatory status impacting in bone marrow production of proinflammatory cells, which subsequently inhibit the autonomic dysregulation in hypertension. Third, if changes induced by specific probiotic in plasma or tissue levels of SCFAs are involved in their antihypertensive effects. The answer to these questions could clarify not only the mechanisms involved on the protective effects of specific probiotic bacteria but also their potential in human hypertension treatment.
Taken into account that the pathophysiological mechanisms involved on high BP development in these patients may vary and that the mechanisms of BP-lowering effect of specific probiotics are unknown, it is worth to investigate if we can choose a specific probiotic strain to gain benefits in a particular hypertensive patient.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Wong ND, Glovaci D, Wong K, Malik S, Franklin SS, Wygant G, Iloeje U. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9:146–52.
Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16.
Tamargo J, Duarte J, Ruilope LM. New antihypertensive drugs under development. Curr Med Chem. 2015;22:305–42.
• Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40. This article described for the first time the characteristic of gut dysbiosis in animal and human with hypertension.
• Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2016a; doi:10.1161/CIRCRESAHA.116.309006. This article shows a hypothesis linking gut microbiota dysbiosis and high blood pressure. A dysfunctional sympathetic-gut communication is associated with gut pathology, dysbiosis, and inflammation, and plays a key role in hypertension.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
• Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. This article described that gut microbiota facilitate angiotensin II-induced vascular dysfunction and hypertension involving vascular Th17 immune cell infiltration and inflammation.
• Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–5. This article described how gut microbiota, through metabolic byproducts such as short chain fatty acids, regulated blood pressure involving olfactory receptors.
Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension—a case report. Int J Cardiol. 2015;201:157–8.
Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–74.
Xu P, Li M, Zhang J, Zhang T. Correlation of intestinal microbiota with overweight and obesity in Kazakh school children. BMC Microbiol. 2012;12:283.
Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, et al. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–81.
• Gómez-Guzmán M, Toral M, Romero M, Jiménez R, Galindo P, Sánchez M, et al. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:2326–36. This article described for the first time the blood pressure lowering properties of probiotics in genetic hypertension.
Grangette C. Bifidobacteria and subsets of dendritic cells: friendly players in immune regulation! Gut. 2012;61:331–2.
Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond). 2012;122:535–43.
Xu J, Ahrén IL, Prykhodko O, Olsson C, Ahrné S, Molin G. Intake of blueberry fermented by Lactobacillus plantarum affects the gut microbiota of L-NAME treated rats. Evid Based Complement Alternat Med. 2013;2013:809128.
Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15:511.
Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. BioEssays. 2011;33:574–81.
Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60.
Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–88.
• Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla MP, et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci (Lond). 2014;127:33–45. This article established a link among endotoxaemia, endothelial dysfunction and hypertension, and their regulation by probiotics.
Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–72.
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6.
Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4.
Iraporda C, Errea A, Romanin DE, Cayet D, Pereyra E, Pignataro O, et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220:1161–9.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–52.
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93.
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–7.
Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157.
Kim S, Wang G, Lobaton G, Li E, Yang T, Raizada M. OS 05–10 The microbial metabolite, butyrate attenuates angiotensin II-induced hypertension and dysbiosis. J Hypertens. 2016;34-ISH 2016 Abstract Book:e60-e61.
Marques FZ, Nelson EM, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High fibre diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in DOCA-salt hypertensive mice. Circulation. 2016; doi:10.1161/CIRCULATIONAHA.116.024545.
Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H, Kimura I. The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 2016;25:379–83.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960.
Eissler R, Schmaderer C, Rusai K, Kühne L, Sollinger D, Lahmer T, et al. Hypertension augments cardiac toll-like receptor 4 expression and activity. Hypertens Res. 2011;34:551–8.
Sollinger D, Eißler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, et al. Damage-associated molecular pattern activated toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res. 2014;101:464–72.
Liang CF, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol. 2013;33:777–84.
Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis: implications for hypertension and related therapeutics. Circ Res. 2016b;118:1327–36. Review
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–58.
Singh VP, Sharma J, Babu S, Rizwanulla SA. Role of probiotics in health and disease: a review. J Pak Med Assoc. 2013;63:253–7.
Rerksuppaphol S, Rerksuppaphol L. A randomized double-blind controlled trial of Lactobacillus acidophilus plus Bifidobacterium bifidum versus placebo in patients with hypercholesterolemia. J Clin Diagn Res. 2015;9:KC01–4.
Ishimwe N, Daliri EB, Lee BH, Fang F, Du G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol Nutr Food Res. 2015;59:94–105.
Chan YK, Brar MS, Kirjavainen PV, Chen Y, Peng J, Li D, et al. High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A-FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamnosus GG (LGG) or telmisartan in ApoE(−/−) mice. BMC Microbiol. 2016a;16:264.
Chan YK, El-Nezami H, Chen Y, Kinnunen K, Kirjavainen PV. Probiotic mixture VSL#3 reduce high fat diet induced vascular inflammation and atherosclerosis in ApoE(−/−) mice. AMB Express. 2016b;6:61.
Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–9.
Thushara RM, Gangadaran S, Solati Z, Moghadasian MH. Cardiovascular benefits of probiotics: a review of experimental and clinical studies. Food Funct. 2016;7:632–42.
Upadrasta A, Madempudi RS. Probiotics and blood pressure: current insights. Integr Blood Press Control. 2016;9:33–42.
Daliri EB, Lee BH, Oh DH. Current perspectives on antihypertensive probiotics. Probiotics Antimicrob Proteins. 2016; doi:10.1007/s12602-016-9241-y.
de Brito Alves JL, de Sousa VP, Cavalcanti Neto MP, Magnani M, Braga VA, da Costa-Silva JH, et al. New insights on the use of dietary polyphenols or probiotics for the management of arterial hypertension. Front Physiol. 2016;7:448.
Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389:109–14.
Tanida M, Imanishi K, Akashi H, Kurata Y, Chonan O, Naito E, et al. Injection of Lactobacillus casei strain Shirota affects autonomic nerve activities in a tissue-specific manner, and regulates glucose and lipid metabolism in rats. J Diabetes Investig. 2014;5:153–61.
Matsuzaki T, Takagi A, Ikemura H, Matsuguchi T, Yokokura T. Intestinal microflora: probiotics and autoimmunity. J Nutr. 2007;137:798S–802S.
Arribas B, Rodríguez-Cabezas ME, Comalada M, Bailón E, Camuesco D, Olivares M, et al. Evaluation of the preventative effects exerted by Lactobacillus fermentum in an experimental model of septic shock induced in mice. Br J Nutr. 2009;101:51–8.
Arribas B, Garrido-Mesa N, Perán L, Camuesco D, Comalada M, Bailón E, et al. The immunomodulatory properties of viable Lactobacillus salivarius ssp. salivarius CECT5713 are not restricted to the large intestine. Eur J Nutr. 2012;51:365–74.
Yap WB, Ahmad FM, Lim YC, Zainalabidin S. Lactobacillus casei strain C1 attenuates vascular changes in spontaneously hypertensive rats. Korean J Physiol Pharmacol. 2016;20:621–8.
Friques AG, Arpini CM, Kalil IC, Gava AL, Leal MA, Porto ML, et al. Chronic administration of the probiotic kefir improves the endothelial function in spontaneously hypertensive rats. J Transl Med. 2015;13:390.
Blanton C, He Z, Gottschall-Pass KT, Sweeney MI. Probiotics blunt the anti-hypertensive effect of blueberry feeding in hypertensive rats without altering hippuric acid production. PLoS One. 2015;10:e0142036.
• Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. A meta-analysis of nine trials showed that consuming probiotics decreased blood pressure.
Acknowledgments
This work was supported by Grants from Comisión Interministerial de Ciencia y Tecnología, Ministerio de Economía y competitividad (SAF2014-55523-R); Junta de Andalucía (Proyecto de excelencia P12-CTS-2722 and CTS 164) with funds from the European Union; and by the Ministerio de Economia y Competitividad, Instituto de Salud Carlos III (RIC, RD12/0042/0011, CIBER-Enfermedades Cardiovasculares), Spain. M.S. is a postdoctoral fellow of Junta de Andalucía, and M.R. is a postdoctoral fellow of University of Granada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gut Microbiome, Sympathetic Nervous System, and Hypertension
Rights and permissions
About this article
Cite this article
Robles-Vera, I., Toral, M., Romero, M. et al. Antihypertensive Effects of Probiotics. Curr Hypertens Rep 19, 26 (2017). https://doi.org/10.1007/s11906-017-0723-4
Published:
DOI: https://doi.org/10.1007/s11906-017-0723-4