Abstract
The aim of this study was to isolate, from pulque, Lactobacillus spp. capable of survival in simulated gastrointestinal stress conditions. Nine Gram-positive rods were isolated; however, only one strain (J57) shared identity with Lactobacillus and was registered as Lactobacillus casei J57 (GenBank accession: JN182264). The other strains were identified as Bacillus spp. The most significant observation during the test of tolerance to simulated gastrointestinal conditions (acidity, gastric juice and bile salts) was that L. casei J57 showed a rapid decrease (p ≤ 0.05) in the viable population at 0 h. Bile salts were the stress condition that most affected its survival, from which deoxycholic acid and the mix of bile salts (oxgall) were the most toxic. L. casei J57 showed bile salt hydrolase activity over primary and secondary bile salts as follows: 44.91, 671.72, 45.27 and 61.57 U/mg to glycocholate, taurocholate, glycodeoxycholate and taurodeoxycholate. In contrast, the control strain (L. casei Shirota) only showed activity over tauroconjugates. These results suggest that L. casei J57 shows potential for probiotic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A fermented beverage known as “pulque” is produced by the fermentation of agave sap using its indigenous microorganisms [1, 2]. The agave sap is extracted from several species of maguey cactus such as Agave atrovensis and A. Americana [1, 2]. Slightly fermented agave sap is greatly appreciated in different regions of Mexico because it is popular belief that it provides health benefits in children, pregnant women and the elderly [1]. The preparation of this beverage involves three types of fermentation: acid, alcoholic and viscous. Therefore, the diversity of associated microorganisms is complex and a mixed culture of bacteria and yeasts such as Zymomonas mobilis [3], Lactobacillus spp., Saccharomyces cerevisiae, Leuconostoc mesenteroides, Leuconostoc kimchii [2], Candida lusitaneae [4], Kluyveromyces marxianus [5], Microbacterium arborescens, Flavobacterium johnsoniae, Acetobacter pomorium, Gluconobacter oxydans and Hafnia alvei [6]. These microorganisms have been studied using molecular techniques [6], and it is known that some of them show potential biotechnological applications, such as those including the production of different compounds including lactic acid or ethanol, hydrolytic enzymes, and exopolysaccharides, and they also show applications in the health field [3, 6, 7]. In particular, the population of lactic acid bacteria (LAB) in pulque ranged from 6 × 107 to 2 × 1011 CFU/mL [1]. LAB have been identified as the main bacteria present in the early hours of fermentation of this beverage. LAB are a group of Gram-positive facultatively anaerobic bacteria, which excrete lactic acid as the main product of fermentation [6]. They are important microorganisms in some parts of the human and animal body (e.g., intestine, nasopharyngeal and vaginal mucosa) and also are found in milk, plants and fermented food such as sourdough, sauerkraut, boza [8] and pulque [2] among others. Such food and beverages have been and still are an important vehicle of living bacteria sources for the human body. Indeed, LAB strains from animal and human intestinal microbiota have been adopted as “probiotic” food supplements [9]. Lactobacillus is responsible for most of bile salt hydrolase activity detected in intestinal contents [10]. Bile salt hydrolases (BSHs) (EC 3.5.1.24) catalyze the hydrolysis of conjugated bile salts in the amide bond on the C-24 position of the steroid, which results in the production of a free amino acid (taurine or glycine) and an unconjugated bile acid molecule [11]. In recent years, the ability of some Lactobacillus to produce BSH has become the focus of attention on account of its influence on cholesterol metabolism, and hence, BSH activity can be explored as a functional probiotic biomarker for the selection of probiotic adjunct to manage hypercholesterolemia [10].
In spite of the fact that Lactobacillus in pulque has been identified by 16S rDNA analysis, it has not been isolated from this beverage and the behavior of these bacteria under gastrointestinal stress conditions where they face low pH; the presence of gastric juices and bile salts is still unknown. In order to offer health benefits, bacteria with probiotic potential have to be resistant to these stress conditions to reach the intestinal tract alive. The aim of the present study was to isolate strains of Lactobacilli from fermented agave sap (pulque) using a stress-inducing procedure to assess their BSH activity.
Materials and Methods
Agave Sap and Fermentation
Samples of freshly collected agave sap and overnight fermented agave sap were obtained from three different Mexican regions: Huitzilac in the State of Morelos (19°02′N, 99°160′W) with an altitude of 2550 m, Jocotitlan in the State of Mexico (19°44′N, 99°45′W) with an altitude of 3900 m and Singuilucan in the State of Hidalgo (19°59′N, 98°57′W) with an altitude of 2525 m. The samples were placed in sterile tubes and transported to the laboratory under a refrigerated condition of 4 °C. The fermentation of each sample was carried out in a 250-mL sterile glass container by addition of fermented agave sap and fresh agave sap at a ratio of 1:3 v/v (pulque) at 36 °C. The pH was determined after 3 h of fermentation.
Isolation Criteria, Bacterial Strains, Microbiological Media and Growth Conditions
In order to isolate bacteria capable of survival in simulated gastrointestinal stress conditions, 1 mL of pulque was added to a stress-inducing medium which consisted of Lactobacilli MRS Broth (pH 1.5, HCl 0.1 N) (Difco™, USA) and oxgall (0.5 % w/v) (Difco™, USA) and incubated for 2 h in a rotatory chamber (200 rpm, 37 °C) (Gallenkamp, UK). Afterward, 10 µL of the medium was plated onto Lactobacilli MRS Agar (Difco™, USA) and incubated for 12 h in an aerobic atmosphere at 37 °C. The resulting isolates and L. casei Shirota were observed under a light microscope. Rod-shaped microorganisms (bacilli) that were Gram positive were selected. In order to prepare enough experimental stock, the isolated microorganisms were plated onto Lactobacilli MRS Agar and incubated at 37 °C for 24 h; then, the biomass was transferred to Lactobacilli MRS Broth and incubated at 37 °C for 24 h. The broth containing the biomass was centrifuged at 30,000×g for 30 min at 4 °C under aseptic conditions. The cell pellet was suspended in 10 mL of Lactobacilli MRS Broth with 50 % glycerol and stored at −20 °C until use.
Pure culture of the probiotic bacteria Lactobacillus casei Shirota was isolated from Yakult® (Yakult Mexico), through growing it on Lactobacilli MRS Agar and incubating it for 24 h at 37 °C. In order to prepare enough experimental stock, biomass was transferred to Lactobacilli MRS Broth and incubated using the same conditions. Subsequently, the broth containing the biomass was centrifuged at 30,000×g for 30 min at 4 °C under sterile conditions. The cell pellet was suspended in 10 mL of Lactobacilli MRS Broth with 50 % glycerol and stored at −20 °C until use. Lactobacillus casei Shirota was used as a control for biochemical characteristics, antibiotic resistance, tolerance to simulated gastric juice and bile salts, and 16S rRNA sequencing [11]. This microorganism was grown in Mac Conkey Agar and incubated for 24 h at 37 °C. In order to prepare enough experimental stock, biomass was transferred to Mac Conkey Broth and the procedure mentioned above was followed.
Catalase Test
It was carried out in triplicate by placing a drop of hydrogen peroxide (J.T. Baker, USA) over a colony of microorganisms placed over a microscopic slide. If bubbles or froth were formed, the test was considered as positive [12]. E. coli ATCC 160211 was used as a positive control and L. casei Shirota as negative.
Hemolysis Test
The blood agar screening method of Ruiz-Moyano et al. [13], with slight modifications, was used. In brief, the strains were incubated overnight on Lactobacilli MRS Agar before the test. One colony of each strain was plated onto blood agar and incubated for 48 h at 37 °C in a brooder stove (Felisa®, México). For each strain, the test was carried out in triplicate. E. coli ATCC 160211 was used as a positive control and L. casei Shirota as negative.
Carbohydrate Fermentation Pattern
The fermentation profile of isolated bacteria and L. casei Shirota was characterized by API 50 CHL test (bioMe´rieux, Marcy L’Etoile, France) [14]. The fermentation profile obtained was evaluated with apiweb™ software. Lactobacillus casei Shirota was used as positive control and E. coli ATCC 160211 a negative.
Genotypic Identification
DNA extraction was performed using Easy-DNA™ kit for genomic DNA isolation (Invitrogen, Cergy-Pontoise, France) according to the supplier’s instructions. PCR amplification of the isolated strains was carried out using primers LAC1 (5′-AGCAGTAGGGAATCTTCCA-3′) and LAC2 (5′-ATTTCACCGCTACACATG-3′) derived for the amplification of 340 bp of the 16S rDNA gene of Lactobacillus [15]. Amplifications were performed with a thermal cycler T gradient (Promega) using the conditions reported by Walter et al. [15]. PCR-amplified products were purified with QIAquick PCR purification kit 50 (QIAGEN, USA) and analyzed by 2 % agarose gel electrophoresis, ethidium bromide staining under UV light. DNA sequences were determined by using the genetic analyzer ABI PRISM® 3100 (Applied Biosystems, USA). Comparisons and sequence alignments were made by using BioEdit DNA sequences analyzer (http://www.mbio.ncsu.edu/BioEdit/BioEdit.htmlt) [16] and the basic local alignment search tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Tolerance to Acid, Simulated Gastric Juice and Bile Salts
Simulated gastrointestinal stress conditions were acidity, gastric juice and bile salts. The tolerance of the isolated microorganism to these conditions was measured at different times (0, 1, 2 and 4 h). Acid tolerance was studied by inoculating an aliquot of approximately 108 CFU/mL into sterile acid water [17]. This water was adjusted with HCl (0.1 M) (J.T. Baker, USA) to 1.5 pH by using a pH meter (Orion 410A+, Thermo Scientific, USA). Gastric juice tolerance was determined according to Vinderola and Reinheimer [18] with slight modifications, using a solution containing pepsin (0.3 % w/v) (SIGMA, USA) and NaCl (0.5 % w/v) (J.T. Baker, USA) adjusted to pH 2.0 using HCl (0.1 M) (J.T. Baker, Mexico). Bile salt tolerance was tested by using bovine bile (oxgall, Difco™), primary bile acids conjugated either taurine (taurocholic, TC) or glycine (glycocholic acid, GC) (SIGMA, USA) and a secondary bile acid (deoxycholic acid, DC) (SIGMA, USA). Bile salt tolerance of each salt was studied using Lactobacilli MRS Broth supplemented with 0.3 % w/v of bile salt [6]. In all tolerance tests, decimal dilutions in peptone and water solution (0.5 % w/v) were individually stirred for 30 s. Afterward, at each time of exposure, a sample of 100 µL was taken, plated onto Lactobacilli MRS Agar and incubated in an aerobic atmosphere for 48 h at 37 °C. Tolerance was determined by comparing the plate counts at different times with the initial one at time zero. The initial count (approximately 108 CFU/mL) with no treatment was considered 100 % survival. Zero time (0 h) with treatment only means the time in which bacteria were poured to the stressful condition and the sample was taken. The results were normalized by using Eq. (1) and expressed relative survival of strains on Lactobacilli MRS Agar. All of the experiments were carried out in triplicate.
Antibiotic Resistance Test
BIO-RAD multidisc (Gram-positive II bacteria) containing cephalothin (30 µg), cefotaxime (30 µg), levofloxacin (5 µg), cefuroxime (30 µg), dicloxacillin (1 µg), erythromycin (15 µg), gentamicin (10 µg), cefepime (30 µg), penicillin (30 µg), tetracycline (30 µg), ampicillin (10 µg) and trimethoprim-sulfamethoxazole (25 µg) was employed for antibiotic resistance tests. The multidisc was placed onto Muller Hinton agar plates previously inoculated with L. casei J57 or L. casei Shirota (Yakult®). Plates were incubated for 72 h at 37 °C in an anaerobic chamber (Forma anaerobic system Model 1025, Thermo Scientific, USA) with an atmosphere of 10 % CO2, 5 % H2 and 85 % N2 [19]. The test was carried out in triplicate, and the results were expressed according to the instructions given by the supplier of the test kit. Before the test, we used E. coli ATCC 160211 to measure the reproducibility of the technique (data not shown).
Bile Salt Hydrolase Test
Bile salt hydrolase (BSH) assay was carried out in spent broth as described by González-Vázquez et al. [11] using overnight cultures of L. casei J57 or L. casei Shirota (Yakult®). The activity of each strain was evaluated in triplicate. One unit of BSH activity was defined as the amount of enzyme which liberated 1 mmol of amino acids per 1 mL of substrate per minute per mg of protein, determined by the Bradford method (Bio-Rad, Mexico) and according to the supplier’s recommendations. Each assay was carried out in triplicate.
Results
Isolation and Identification (Phenotypic and Genotypic) of Bacteria from Agave Sap
The pH after 3-h fermentation was 4.6 ± 0.06, 4.15 ± 0.05 and 3.7 ± 0.06 in the pulque of Singuilucan, Jocotitlán and Huitzilac regions, respectively. The stress-inducing medium allowed us to isolate 76 microorganisms from pulque of the three Mexican regions tested. However, only nine isolates were Gram-positive rod-shaped bacteria. Therefore, they were chosen for further investigation. Four of these nine bacteria were isolated from Singuilucan (S67, S37, S38 and S51), one from Jocotitlan (J57) and four from Huitzilac (H18, H19, H64 and H60). The strain J57 was catalase negative and non-hemolytic, and the other eight strains were catalase positive and hemolytic. The API 50 CHL test determined that L. casei Shirota and L. casei J57 had the ability to ferment the sugars galactose, lactose and sorbose and the sugar alcohol sorbitol. Other sugars that were fermented by both organisms were d-ribose, d-glucose, d-fructose, d-mannose, d-mannitol, d-sorbitol, d-cellobiose, d-maltose, d-lactose, d-trehalose, genentiobiose, d-turanose, d-tagatose, n-acetyl glucosamine, amygdalin, arbutin, esculin ferric citrate, salicin and potassium gluconate. They only differed in the fermentation of d-adonitol. Therefore, the strain J57 was identified as L. casei subsp. paracasei (99.7 %). The other isolated strains did not exhibit identity with the genus reported in the API database, since this system is designed to identify bacteria only belonging to the Lactobacillus genus. Therefore, these last strains were discarded for the rest of the tests. The BLAST analysis was used for genotypic identification. The 16S rRNA sequence of the strain shared 99 % identity with that of L. casei (GenBank accession: JN182264).
Antibiotic Resistance
In the antibiotic tests, the strain L. casei J57 showed resistance to cefepime and sensitive to the other antibiotics tested. In contrast, L. casei Shirota showed resistance to dicloxacillin and gentamicin and sensitive to the other antibiotics tested.
Tolerances
Acidity
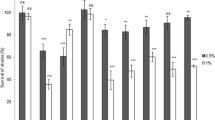
Regarding acids, simulated gastric juice and bile salt tolerance, the most significant observation was a significant decrease (p ≤ 0.05) in a viable count from the initial population (0 h). The relative survival percentage of L. casei J57 and the L. casei Shirota at pH 1.5 over 4 h is shown in Fig. 1a. Under such conditions, L. casei J57 was able to survive for over 4 h. In contrast, the L. casei Shirota survived for 1 h.
Tolerances to the different simulated gastrointestinal stress conditions tested: a acidity, b gastric juice, c taurocholic acid, d glycocholic acid, e deoxycholic acid and f oxgall of L. casei J57 (circle) and L. casei Shirota (Yakult ®) (filled circle). Values are the means of data generated from triplicate samples examined in three independent experiments
Simulated Gastric Juice
The relative survival percentage under gastric juice of L. casei J57 and L. casei Shirota is shown in Fig. 1b. L. casei J57 survived for 4 h under the experimental conditions. Conversely, L. casei Shirota did not show viability after 1 h.
Bile Salts
Lactobacillus casei J57 and the L. casei Shirota survived for 4 h of exposure to TC, GC, DC and oxgall (Fig. 1c–f, respectively). It should be noted that oxgall had a higher negative effect on the percentage of relative survival than the other bile salts tested.
Bile Salt Hydrolase Activity
Figure 2 shows the BSH activity of L. casei J57 using different bile salts (GC: 44.91; GDC: 45.27; TC: 671.72; TDC: 61.57 U/mg of protein) and the BSH activity of L. casei Shirota, which did not show any activity in glycine conjugates. However, its activity over tauroconjugates (TC: 1046.15; TDC: 264.69 U/mg of protein) was higher than the ones showed by L. casei J57. In both cases, hydrolase activity toward taurocholic acid was higher than the activity shown to other bile salts.
Bile salt hydrolase activity in the spent broth of L. casei J57 and L. casei Shirota (Yakult ®) grown in MRS broth supplemented with 0.5 % of glycocholic acid (GC), glycodeoxycholic acid (GDC), taurocholate (TC) and taurodeoxycholate (TDC). Values are the means of data generated from triplicate samples examined in three independent experiments
Discussion
In order to determine whether a bacterium has the ability to survive under gastrointestinal tract conditions, it is important to evaluate its ability to survive under such stress [9]. Therefore, the results obtained in the present work suggest that the use of a stress-inducing medium, which simulates similar conditions to those found in the gastrointestinal tract, allowed microorganisms that tolerate these conditions to be isolated. Particularly, this could be associated with the BSH activity shown by L. casei J57, since it was isolated from a medium that contained bile salts, and cross-resistance mechanisms could be present.
Many LAB have been found in several fermented native foods [8]. In agave sap, some Lactobacillus have been identified as L. acidophilus, L. kefir, L. acetotolerans, L. hilgardii, L. plantarum, L. acidophilus, L. hilgardii, L. paracollinoides and Lactobacillus spp. Y10c7 [6]. Particularly, L. casei is a ubiquitous microorganism, which is found in fermented dairy products, fresh vegetables and human sources [8]. Nevertheless, this is the first time that L. casei has been isolated and identified from pulque, and the first time that its BSH activity has been determined. The carbohydrate fermentation pattern between L. casei Shirota and L. casei J57 was similar as we expected; however, they differed in the fermentation of one carbohydrate, which indicate that probably they are different subspecies.
Regarding the tolerances tested, acidity is an environmental condition that is commonly found in the human gastrointestinal tract [20]; gastric pH increases during food intake from 1.5 to 3.0 or 5.0 [21]. In the case of beneficial bacteria, good acid tolerance is desirable, since it is usually related by cross-resistance to some other stress factor of the intestinal environment [22]. In this study, during the acid tolerance test, it was not possible to obtain colony-forming units of the control at the end of the experiment (Fig. 1a) because this condition (pH 1.5) was extremely stressful to this microorganism. Another important issue to consider is that after a meal, food ingredients can exert a protective effect over Lactobacillus strains [23]. In spite of these results, L. casei J57 (Fig. 1a) showed viability until the end of the experiment, which indicates that this microorganism could survive this stress in the gastrointestinal tract.
Related to the tolerance to gastric juice, it was found that L. casei Shirota, which showed 30–40 % of tolerance to acidity, showed a similar behavior when it was under gastric juice; in contrast, L. casei J57 showed a higher tolerance to gastric juice (50–55 %) (Fig. 1b) and survived for 4 h under this stress. These results could indicate that a cross-resistance mechanism is present, since most pH stress protection systems in bacteria include a mechanism for sustaining cytoplasmic pH, and many pH stress-inducible systems offer cross-protection to other stresses such as increasing salt tolerances [22]. In addition, Todorov et al. [24] have suggested that resistance to low pH and elevated concentrations of bile salts is important for the growth and survival of bacteria in the intestinal tract.
Another important finding with regard to bile salts was that glycine conjugates have a stronger effect over strain survival (Fig. 1d) than taurine conjugates (Fig. 1c). De Smet et al. [25] reported that glycine conjugates are far more toxic than taurine conjugates due to their different pKa (3.9 and 1.0, respectively). On the other hand, oxgall had a larger effect than the previously tested salts over the viability of L. casei J57 and L. casei Shirota (Fig. 1f), suggesting a toxic synergistic effect when the strains are in contact with the mixture of bile salts, since oxgall is manufactured from large quantities of fresh bile by the rapid evaporation of the water content and is made up of fatty acids, bile acids, inorganic salts, sulfates, bile pigments, cholesterol, mucin, lecithin, glucuronic acids, porphyrins and urea [26, 27].
In the present work, the antibiotic resistance profile of L. casei J57 matched the profile reported for other Lactobacillus [28]. However, it showed resistance to cefepime (a fourth-generation cephalosporin). We did not find any previous studies that tested cefepime susceptibility [29], and most of the studies have included only a limited number of Lactobacillus strains or antibiotics. This is probably because Lactobacillus has been considered as generally recognized as safe (GRAS), and therefore, their antimicrobial susceptibility has received only little attention [29]. Further studies are needed in support of the resistance of Lactobacillus to fourth-generation cephalosporin. At this stage, cefepime is contraindicated in patients with known allergies to cephalosporin and or penicillin antibiotic and is usually reserved to treat moderate to severe nosocomial pneumonia infections [30]. Additionally, the strain J57 was identified as Lactobacillus with non-hemolytic activity, which is particularly important since the presence of this activity can contribute to the virulence of some pathogenic bacteria [31, 32]. However, this effect depends on different factors, such as whether the host is immunocompromised [33].
Regarding BSH activity (Fig. 2), it was only determined in the case of strain L. casei J57 since this activity has been related to Lactobacillus species with a possible probiotic application, due to the association of BSH activity with serum cholesterol-lowering effects [9]. L. casei J57 showed BSH activity over primary and secondary bile salts conjugated to glycine or taurine (Fig. 1). However, lower activity was shown than for L. casei (control) regarding either primary or secondary tauroconjugates. In spite of the activity found by a quantitative method, some authors [34] have reported the lack of BSH activity by using a plate assay over tauroconjugates by several L. casei strains, including the one isolated from a popular probiotic drink (Yakult®). Conjugated bile salts are periodically released into the intestinal environment. According to Begley et al. [35], the ratio of glycine conjugates to tauroconjugates in human bile is usually 3:1, which shows the importance of BSH activity over glycine conjugates from Lactobacillus with probiotic features. It is known that bile salts are toxic to bacteria [25]. Therefore, bacteria in the intestine may express BSH activity to protect them from such toxicity. These results may explain why L. casei Shirota in the present work showed tolerance to different glycine conjugated bile salts but did not show hydrolase activity toward such salts.
We conclude that the use of a stress-inducing procedure allows the isolation of lactic acid bacterial strains capable of showing tolerance to gastrointestinal stress and BSH activity, which were higher than those of the commercial probiotic used in our study. This activity is considered as a probiotic characteristic, which could impact on health.
References
Valadez-Blanco R, Bravo-Villa G, Santos-Sánchez N, Velasco-Almendarez S, Montville T (2012) The artisanal production of pulque, a traditional beverage of the Mexican highlands. Probiotics Antimicrob Proteins 4(2):140–144. doi:10.1007/s12602-012-9096-9
Escalante A, Giles-Gomez M, Hernandez G, Cordova-Aguilar MS, Lopez-Munguia A, Gosset G, Bolivar F (2008) Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int J Food Microbiol 124(2):126–134. doi:10.1016/j.ijfoodmicro.2008.03.003
Correa-Ascencio M, Robertson IG, Cabrera-Cortés O, Cabrera-Castro R, Evershed RP (2014) Pulque production from fermented agave sap as a dietary supplement in Prehispanic Mesoamerica. Proc Natl Acad Sci 111(39):14223–14228
Lira A, Alvarado-Resendiz M, Simental S, Martini J, Reyes-Santamaria M, Guemes-Vera N (2014) Use of Lactobacillus from pulque in sourdough. Adv Microbiol 4:969–977. doi:10.4236/aim.2014.414108
Estrada-Godina AR, Cruz-Guerrero AE, Lappe P, Ulloa M, García-Garibay M, Gómez-Ruiz L (2001) Isolation and identification of killer yeasts from agave sap (aguamiel) and pulque. World J Microbiol Biotechnol 17(6):557–560. doi:10.1023/A:1012210106203
Escalante A, Rodriguez ME, Martinez A, Lopez-Munguia A, Bolivar F, Gosset G (2004) Characterization of bacterial diversity in pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol Lett 235(2):273–279. doi:10.1016/j.femsle.2004.04.045
Chellapandian M, Larios C, Sanchez-Gonzalez M, Lopez-Munguia A (1998) Production and properties of a dextransucrase from Leuconostoc mesenteroides IBT-PQ isolated from ‘pulque’, a traditional Aztec alcoholic beverage. J Ind Microbiol Biotechnol 21(1–2):51–56. doi:10.1038/sj.jim.2900560
Rivera-Espinoza Y, Gallardo-Navarro Y (2010) Non-dairy probiotic products. Food Microbiol 27:1–11
Patel AK, Singhania RR, Pandey A, Chincholkar SB (2009) Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol 162(1):166–180. doi:10.1007/s12010-009-8738-1
Kumar R, Grover S, Batish V (2012) Bile salt hydrolase (Bsh) activity screening of lactobacilli: in vitro selection of indigenous lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiotics Antimicrob Proteins 4(3):162–172. doi:10.1007/s12602-012-9101-3
González-Vázquez R, Gutiérrez-López GF, Arellano-Cárdenas S, López-Villegas EO, Téllez-Medina DI, Rivera-Espinoza Y (2014) Morphometric parameters, zeta potential and growth rate of Lactobacillus casei Shirota by effect of different bile salts. Rev Mex Ing Quim 13:189–199
MacFaddin JF (1980) Biochemical tests for identification of medical bacteria. Lippincott Williams and Wilkins, Philadelphia
Ruiz-Moyano S, Martin A, Benito MJ, Casquete R, Serradilla MJ, Cordoba Mde G (2009) Safety and functional aspects of pre-selected lactobacilli for probiotic use in Iberian dry-fermented sausages. Meat Sci 83(3):460–467. doi:10.1016/j.meatsci.2009.06.027
Annuk H, Shchepetova J, Kullisaar T, Songisepp E, Zilmer M, Mikelsaar M (2003) Characterization of intestinal lactobacilli as putative probiotic candidates. J Appl Microbiol 94(3):403–412
Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP (2001) Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67(6):2578–2585. doi:10.1128/aem.67.6.2578-2585.2001
Thomas AH (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Mishra V, Prasad DN (2005) Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int J Food Microbiol 103(1):109–115. doi:10.1016/j.ijfoodmicro.2004.10.047
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int 36(9–10):895–904. doi:10.1016/S0963-9969(03)00098-X
Melgar-Lalanne G, Rivera-Espinoza Y, Farrera-Rebollo R, Hernández-Sánchez H (2014) Survival under stress of halotolerant lactobacilli with probiotic properties. Rev Mex Ing Quim 13:323–335
Mayorga-Reyes L, Bustamante-Camilo P, Gutiérrez-Nava A, Barranco-Florido E, Azaola-Espinosa A (2009) Crecimiento, sobrevivencia y adaptación de Bifidobacterium infantis a condiciones ácidas. Rev Mex Ing Quim 8:259–264
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67(3):429–453
Beales N (2004) Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: a review. Compr Rev Food Sci Food Saf 3(1):1–20. doi:10.1111/j.1541-4337.2004.tb00057.x
Morelli L (2000) In vitro selection of probiotic lactobacilli: a critical appraisal. Curr Issues Intest Microbiol 1(2):59–67
Todorov SD, Botes M, Guigas C, Schillinger U, Wiid I, Wachsman MB, Holzapfel WH, Dicks LM (2008) Boza, a natural source of probiotic lactic acid bacteria. J Appl Microbiol 104(2):465–477. doi:10.1111/j.1365-2672.2007.03558.x
De Smet I, Van Hoorde L, Vande Woestyne M, Christiaens H, Verstraete W (1995) Significance of bile salt hydrolytic activities of lactobacilli. J Appl Bacteriol 79(3):292–301
Isenberg HD (1992) Clinical microbiology procedures handbook, vol 1. American Society for Microbiology, Washington DC
Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (1995) Manual of clinical microbiology. American Society for Microbiology, Washington DC
Mourad K, Nour-Eddine K (2006) In vitro preselection criteria for probiotic Lactobacillus plantarum strains of fermented olives origin. Int J Probiotics Prebiotics 1:27–32
Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Jarvinen A (2006) Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis 42(5):e35–e44. doi:10.1086/500214
Yahav D, Paul M, Fraser A, Sarid N, Leibovici L (2007) Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 7(5):338–348. doi:10.1016/s1473-3099(07)70109-3
Chang CI, Liu WY, Shyu CZ (2000) Use of prawn blood agar hemolysis to screen for bacteria pathogenic to cultured tiger prawns Penaeus monodon. Dis Aquat Organ 43(2):153–157. doi:10.3354/dao043153
Sritharan M (2006) Iron and bacterial virulence. Indian J Med Microbiol 24(3):163–164
Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, Valtonen V (2003) Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 36(6):775–780. doi:10.1086/368080
Dashkevicz MP, Feighner SD (1989) Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol 55(1):11–16
Begley M, Gahan CG, Hill C (2005) The interaction between bacteria and bile. FEMS Microbiol Rev 29(4):625–651. doi:10.1016/j.femsre.2004.09.003
Acknowledgments
This study was supported by SIP-IPN Projects and ICYTDF PICSO10-32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
González-Vázquez, R., Azaola-Espinosa, A., Mayorga-Reyes, L. et al. Isolation, Identification and Partial Characterization of a Lactobacillus casei Strain with Bile Salt Hydrolase Activity from Pulque. Probiotics & Antimicro. Prot. 7, 242–248 (2015). https://doi.org/10.1007/s12602-015-9202-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-015-9202-x