Abstract
Plants defend from herbivores by activating a plethora of genetic and biochemical mechanisms aimed at reducing insect survival and plant damage. In this study, we analyzed constitutive and insect damage induced macromolecules, antioxidant enzymes and corresponding antioxidants in order to identify strength of resistance in maize in response to attack by Sesamia inferens. There were significant differences among the maize genotypes for all the test biochemicals. Further, the S. inferens damage resulted in significant increase in total proteins, total sugars, catalase, phenyl ammonia lyase, tyrosine ammonia lyase, total antioxidants, total phenol, tannins and Ferric ion reducing antioxidant power, but there was also a significant variation in increase in these biochemicals with respect to genotypes. The integrative analysis of these macromolecules, antioxidant enzymes and antioxidants revealed that the S. inferens damage in maize is characterized by higher secondary metabolite production and a strong redox response in resistant maize genotypes, mainly mediated by tannins and phenols as anti-nutritive compounds. Furthermore, the maize genotypes viz., CPM 2, CPM 9, CPM 13, CPM 15 and CML 345 were found with greater constitutive and/or pink stem borer induced defense phytochemicals like enzymatic activity and nonenzymatic antioxidant defense biochemicals, thus could be used to develop S. inferens resistant varieties of maize.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is one of the important cereal crops occupying third rank globally in area and production next to rice and wheat (Kumar et al., 2014). It is subjected to attack by about 130 insect pests during different growth phases but only a dozen are quite serious (Siddiqui & Marwaha, 1993; Dhillon et al., 2014). Stem tunneling by maize stem borers is an important constraint to achieve the potential yield of maize varieties across the world (Rodríguez et al. 2021). Among the stalk feeding insects, pink stem borer, Sesamia inferens (Walker) pose a great challenge to increase productivity of maize in India (Dhillon et al., 2014; Soujanya et al., 2020). Several management strategies including crop rotation, field sanitation, biological control agents and synthetic pesticides have been recommended for the control of S. inferens, but none of these have been found effective particularly when the larvae enter inside the stalks. Host plant resistance could be one of the most effective component for managing this pest. Resistance occurs when plant structural or chemical traits deter herbivore feeding and thus minimize the amount of herbivore damage experienced by the plant (Mitchell et al., 2016).
The biochemical constituents in host plant have a great influence on growth, development, survival and reproduction of insects (Kumar, 1997). Plant resistance to insect attack includes both constitutive and induced biochemical defense. There is adequate information on contribution of some of the constitutional biochemical compounds in host plant defense against Chilo partellus (Swinhoe) in maize and sorghum (Dhillon & Chaudhary, 2015; Dhillon & Kumar, 2017; Yele et al., 2021). However, evidence indicates that induced defenses evolved because they have lower resource allocation than constitutive resistance traits (Howe & Jander, 2008), and growth penalties could be reduced if the plant synthesizes defense compounds only in response to insect attack (Cao et al., 2019). Different kinds of stimuli like mechanical wounding, herbivore attack, application of insect regurgitation etc. results in a change in the biochemical constituents of plants and induces defense against herbivores (Soujanya et al., 2021). Biotic stress induced by herbivory can lead to the overproduction of reactive oxygen species (ROS) which are highly reactive and toxic, and cause damage to proteins, lipids, carbohydrates and DNA which ultimately results in oxidative stress. Different genotypes of plants have been categorized (resistance and susceptible) based on their response to herbivory as how much they manage to maintain ROS level within right range (Huang et al., 2019).
Plants possess very efficient enzymatic and non-enzymatic antioxidant defense systems which work in concert to control the cascades of uncontrolled oxidation and protect plant cells from oxidative damage by scavenging of ROS (Gill & Tuteja, 2010). Several antioxidant/enzymes are induced in plants in response to insect herbivory like phenyl ammonia lyase, tyrosine ammonia lyase and catalase, etc. (Zhao et al., 2009; Scott et al., 2010). The increase in activity of these enzymes could be involved in plant resistance by decreasing the nutritive value of host plants (Chen et al., 2009). The catalase enzymes have the potential to directly dismutase H2O2 into H2O and O2 and indispensable for ROS detoxification during stressed conditions. Oxidative state of plants is an important tactic that enables plants to defend against stresses and regulated by various antioxidant enzymes. However, plants regulate the levels of ROS in such a way that increase in H2O2 is sensitive to the pathogen but not to the host plant (Trivedi, 2021). Any kind of biotic or abiotic stress leads to the generation of a large amount of ROS including O2−, HO2−, and H2O2 in plant cells. These ROS are further processed by different antioxidant enzymes to counter the effect of damage (Marta et al., 2016). An efficient antioxidant system could be useful to protect the maize plants against oxidative stress caused by S. inferens, however, no concerted efforts have been made in this direction in the past. Therefore, present studies were carried out to decipher the extent of constitutive and induced biochemical defense in selected maize genotypes against pink stem borer, S. inferens for sustainable maize production.
Materials and methods
Mass rearing of Sesamia inferens
The S. inferens larvae were collected from maize crop in the experimental fields of ICAR- Indian Agricultural Research Institute (ICAR-IARI), New Delhi, and reared on maize stalk cuttings in plastic jars having lids fitted with wire-mesh (12 cm height and 8 cm diameter) under laboratory conditions at 27 ± 2 ℃, 80 ± 5% RH and 12 L:12D. The larval food was changed after every 2 days till pupation. The pupae thus obtained were collected and kept on butter paper in separate jars. On emergence, 5–7 pairs of adult moths were transferred to oviposition cages (16 cm height and 8 cm diameter) provided with butter paper at the bottom for egg laying and kept at 27 ± 2 ℃, 80 ± 5% RH and 12 L:12D conditions. The eggs were collected and butter papers changed daily. The collected eggs were kept in plastic jars with wire-mesh fitted lids (16 cm height and 4 cm diameter) having butter paper at the bottom, beneath which moisten blotting paper was kept to protect the eggs from desiccation. The color of S. inferens eggs turn to yellowish before hatching. At this stage a moist cotton swab was kept on the wire-meshed lid of egg jars to increase humidity for uniform egg hatching. These newly hatched larvae again transferred to maize stem cuttings (50 neonates per jar) with the help of camel hair brush to maintain the S. inferens culture. The neonate larvae of S. inferens obtained from this nucleus culture were used for further studies.
Plant sample collection for analysis
The experimental material consisted of ten maize genotypes including resistant and susceptible checks viz., CPM 2, CPM 4, CPM 8, CPM 9, CPM 13, CPM 15, CPM 18, CPM 19, CML 345 (resistant check), and Basi Local (susceptible check). The information on biological performance and host selection by Sesamia inferens on these genotypes have already been reported by Sau & Dhillon (2022). Each test maize genotype was sown in the plastic pots (10 L capacity) using standard crop growing practices under net-house conditions during 2018-19. There were four pots of each test maize genotype accommodating five plants each, out of which two pots were kept for S. inferens larval inoculation as infested treatment and two were kept uninoculated as healthy control. The plants in infested treatment designated pots of test genotypes were inoculated each with two 3rd instar S. inferens larvae (10 days old) in the central whorl of 20 days old plants. After 24 h of exposure, healthy and S. inferens infested maize seedlings were collected separately and processed immediately for estimation of different biochemical parameters. The damaged and counterpart healthy portion of each test plant of the maize genotypes weighing 2 g were separated, and crushed with liquid nitrogen. In each crushed sample 10 ml of 50 mM phosphate buffer of pH 7.8 was added and again macerated in pestle and mortar. The slurry was filtered by passing in muslin cloth and filtrate was collected in Micro-centrifuge tube. Transferred filtrate was centrifuged at 12,000 rpm for 20 min at 4 °C. The clear supernatant was collected and stored at -20 °C in deep freezer for further analysis. All the biochemical estimations were done in three replications in a completely randomized design.
Estimation of soluble protein, sugar, starch and lipids
Total soluble protein content was estimated through Bradford’s method (Bradford, 1976). From the processed plant samples, test buffer extract and water were taken in equal volume (1:1). In these samples 3 ml of Bradford dye was added and reaction mix was allowed to incubate at 28 °C for 30 min under dark. Absorbance was recorded against blank at 595 nm wavelength. The amount of total soluble protein was calculated with the help of BSA standard and amount expressed as mg/g of dry weight. Total sugar content was estimated through modified concentrate sulphuric acid (H2SO4) method (Dubois et al., 1956). In diluted plant extract 0.5 ml of 5% phenol (freshly prepared) was added followed by 2.5 ml of concentrate H2SO4 under dark. Absorbance was measured at 490 nm wavelength. Total sugar content was calculated against glucose standard and expressed as mg/g of dry weight. Starch in plant samples was estimated by Perchloric acid digestion method (Clegg, 1956). Test plant samples weighing 250 mg were crushed separately in liquid nitrogen and then 80% ethanol was added for elimination of soluble sugars. Ethanol extract was centrifuged for 15 min at 10,000 rpm and the residue was retained. The residue was again washed with 80% ethanol, till the discoloration of supernatant with anthrone reagent. Then 0.5 ml distilled water and 0.65 mL cold 52% Perchloric acid were added to residue. Test samples were kept for digestion at 0 °C for 20 min, and then centrifuged at 10,000 rpm for 15 min to get the supernatant. The digestion step was repeated one more time and the supernatant was mixed together and made up to 100 ml in a volumetric flask. From prepared sample, 200 µl was made up to 1 ml by addition of distilled water and 4 ml freshly prepared anthrone reagent (200 µl anthrone reagent powder 5 N mixed in 100 ml of concentrated sulphuric acid) was added. This reaction mixture was kept for 8 min in boiling water bath, and cooled rapidly to room temperature by keeping in cold water. Absorbance was recorded at 620 nm wavelength against blank. These values thus obtained for various test samples were computed using glucose standard for total starch content and expressed as mg/g of dry weight. The lipid content of the test samples was estimated through standard protocol given by Bligh & Dyer (1959) with slight modifications. The healthy and S. inferens infested maize seedlings (2 g of each) taken in a mortar and pestle and crushed with liquid nitrogen. To the crushed samples, chloroform, methanol and water were added in the ratio of 1:2:1 to the final volume of 20 ml. Then entire solution was filtered through vacuum filtration system (Milllipore). A portion of solution containing liquid was evaporated to dryness in a vacuum desiccator to determine the weight of lipid residue. The total lipid content was estimated using the formula: Lipid (mg/g) \(=\text{Weight of lipid in aliquot} \times \text{Volume of chloroform layer} / \text{Volume of aliquot}\)
Catalase, phenyl-ammonia lyase (PAL) and tyrosine-ammonia lyase (TAL) assays
The catalase activity was determined through the method given by Aebi (1984). Reaction was initiated by mixing 800 µl potassium phosphate buffers (pH 7.0) and 100 µl of enzyme extract with 100 mM H2O2 to the 1.0 mL reaction mixture. Decrease in absorbance was measured at 240 nm for 3 min. The catalase activity was expressed as the change in absorbance per min/mg of soluble protein in sample. The PAL activity was tested through the method given by Fritz et al., (1976). The 1.0 ml of reaction mixture containing 0.1 mM Tris-acetate buffer at pH 8.5, 0.833 mM of L-Phenyl-ammonia lyase was added with 50 µl enzyme extract to initiate the reaction. The decrease in absorbance was measured at 290 nm and monitored for 5 min. The TAL activity was measured through the method given by Thorpe & Beaudoin-Eagan (1985). The 1.0 ml of reaction mixture containing 0.1 mM tris-acetate buffer at pH 8.5, 0.833 mM of Tyrosine-ammonia lyase was added with 50 µl enzyme extract to initiate the reaction. The decrease in absorbance was measured at 290 nm and monitored for 5 min. The PAL and TAL activities were expressed as change in absorbance per min/mg of soluble protein in sample.
Quantification of total phenol, total antioxidant, tannins and ferric ion reducing antioxidant power (FRAP)
Total phenol was determined by Folin-Ciocalteau reagent (FCR) method given by Singleton and Rossi (1965). A 200 µl of buffer extract and 300 µl of water were taken in individual 2 ml test tubes for each sample. To these test tubes, 0.1 ml of FCR reagent were added under dark condition and mixed properly, incubated in room temperature for 15 min. Thereafter, 2.5 ml of saturated sodium carbonate was added and incubated for 30 min at room temperature. From these prepared solutions, 200 µl of each test sample were loaded in 96 well microtiter plate, and absorbance was recorded at 760 nm wavelength in ELISA Reader. The values thus obtained for each test sample were computed for total phenol content using gallic acid standard curve, and expressed as mg/g of plant tissue. Total antioxidant content in the test samples was estimated through total antioxidant reagent method using ascorbic acid as standard (Prieto et al., 1999). Plant extract and reagent was added in 1:2 ratio followed by 3 ml of freshly prepared total antioxidant reagent (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM of ammonium molybdate) under dark. Reaction mixtures were kept in hot water bath at 95 °C for 90 min. Absorbance was recorded at 695 nm wavelength. Total antioxidant activity was calculated for each test sample with the help of ascorbic acid standard and expressed as mg/g of plant tissue. Total tannins in the test samples were estimated by Folin-Ciocalteu method given by Amorim et al. (2008). 500 mg test tissues were transferred individually to 50 ml flask and 25 ml of 80% ethanol was added. After 30 min of incubation, the contents were filtered and final volume was made up by adding 80% ethanol. Then, 200 µl of ethanol extracts were loaded in 96 well microtiter plate, and absorbance was recorded at 760 nm wavelength in ELISA Reader. These values thus obtained for the test samples were computed for total tannin content using gallic acid standard curve, and expressed as mg/g of plant tissue. The FRAP content in the test samples were estimated by FRAP reagent method using ascorbic acid as standard (Benzie & Strain, 1999). A 100 µl of test samples were taken in individual 2 ml test tubes and 1.9 ml of FRAP reagent were added under dark conditions and mixed properly, incubated at room temperature for 30 min. From these reaction mixtures, 200 µl of each test sample were loaded in 96 well microtiter plate, and absorbance was recorded at 593 nm wavelength in ELISA Reader. The values thus obtained for each test samples were computed for FRAP content using ascorbic acid standard curve, and expressed as mg/g of plant tissue.
Statistical analysis
The data on various biochemical constituents in the seedlings of test maize genotypes under healthy and S. inferens damaged conditions and genotype × treatment interactions were subjected to analysis of variance using factorial design. The significance of differences were tested by F-test, and the treatment means and their interactions were compared by least significant differences (LSD) at P = 0.05 using statistical software SAS® version 9.2.
Results
Total protein
Total protein content in the healthy and S. inferens damaged seedlings of different maize genotypes varied from 0.90 to 4.0 mg/g and 1.60 to 7.85 mg/g, respectively (Table 1). There was significant variation in amount of total protein in the seedlings of different maize genotypes (F = 7.85; df = 9,18; P < 0.001) both under healthy and S. inferens damaged (F = 75.78; df = 1,18; P < 0.001) conditions. Across genotypes, total protein content increased significantly under S. inferens damaged conditions (Table 1). Among the test maize genotypes, it was significantly higher in CPM 2, CPM 8, CPM 9 and CPM 13 under damaged condition. The genotype × treatment interaction for total protein content also varied significantly in the seedlings of different maize genotypes (F = 7.18; df = 9,18; P < 0.001). Moreover, the total protein content was significantly higher in healthy seedlings of resistant maize genotypes which further increased at a greater rate in response to damage by S. inferens as compared to susceptible maize genotypes including susceptible check, Basi Local (Fig. 1A).
Total sugars
The total sugar content varied from 1.51 to 16.62 mg/g in healthy and 4.65 to 19.09 mg/g in S. inferens damaged seedlings of different maize genotypes (Table 1). There were significant differences in total sugar content in the seedlings of different maize genotypes (F = 34.14; df = 9,18; P < 0.001) under healthy and S. inferens damaged (F = 0.28; df = 1,18; P < 0.001) conditions. The genotype × treatment interactions were also significant for total sugar content in the seedlings of different maize genotypes (F = 39.68; df = 9,18; P < 0.001). Across maize genotypes, the total sugar content was significantly lower in the healthy (6.46 mg/g) than in S. inferens damaged (12.86 mg/g) maize seedlings. The total sugar content under S. inferens damaged conditions significantly increased over the healthy counterparts (0.91 to 14.84 mg/g) (Fig. 1B).
Starch
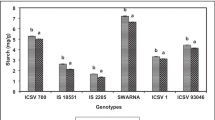
The starch content varied from 2.36 to 9.93 mg/g in healthy and 7.53 to 16.66 mg/g in the S. inferens damaged maize seedlings (Table 1). There were no significant differences in starch content in the seedlings of different maize genotypes (F = 0.88; df = 9,18; P = 0.56) both under healthy and S. inferens damaged (F = 0.14, df = 1,18 P = 0.71) conditions, and genotype × treatment interactions (F = 1.38; df = 9,18; P = 0.23). The starch content under S. inferens damaged conditions was increased in all genotypes (0.80 to 10.83 mg/g). Among the test maize genotypes, it was comparatively higher in CPM 15, CPM 9 and CML 345 under damaged conditions than other test genotypes, but differences were non-significant (Fig. 1C).
Total lipids
The total lipid content varied from 1.67 to 6.00 mg/g in the healthy and 0.47 to 3.93 mg/g in S. inferens damaged seedling of different maize genotypes (Table 1). There were significant differences in total lipid content in the seedlings of different maize genotypes (F = 13.28; df = 9,18; P < 0.001) both under healthy and S. inferens damaged (F = 107.94; df = 1,18; P < 0.001) conditions. The genotype × treatment interactions were also significant for total lipid content in the seedlings of different maize genotypes (F = 33.66; df = 9,18; P < 0.001). The total lipid content in the test maize genotypes under S. inferens damaged conditions decreased in range of -0.13 to -4.72 mg/g. Among the test genotypes, the total lipid content decreased significantly in the infested seedlings of test maize genotypes, except CPM 13 as compared to their healthy counterparts (Fig. 1D).
Catalase
The catalase activity varied from 1.5 to 16.8 U/ml of protein in healthy and 3.0 to 19.0 U/ml of protein in the Sesamia inferens damaged seedlings of different maize genotypes (Table 2). There were significant differences in catalase activity in the seedlings of different maize genotypes (F = 59.6, df = 9,18; P < 0.001) both under healthy and S. Inferens damaged (F = 28.02, df = 1,18; P < 0.001) conditions. Furthermore, the genotype × treatment interaction for catalase activity also significantly varied in the test maize seedlings (F = 9.13; df = 9,18; P < 0.001). Across genotypes, the catalase activity was significantly higher in S. Inferens damaged (8.4 U/ml) than in healthy (5.6 U/ml) maize seedlings (Table 2). Over all catalase activity was found lowest in Basi Local (Fig. 2A). Among test maize genotypes, the catalase activity was significantly higher in CPM 8 and CPM 13, CPM 19 and CPM 2, while it recorded highest in CML 345 under both healthy and S. inferens damaged conditions (Fig. 2A).
Phenyl ammonia lyase (PAL)
The PAL activity varied from 54.0 to 915.3 U/ml of protein in healthy and 524.7 to 1473.3 U/ml of protein in the S. inferens damaged seedlings of different maize genotypes (Table 2). There was significant variation in activity of PAL in the seedlings of different maize genotypes (F = 44.58, df = 9,18, P < 0.001) both under healthy and S. inferens damaged (F = 1248.7, df = 1,18, P < 0.001) conditions. The PAL activity was significantly higher in CPM 2, CPM 4, CPM 8, CPM 9, CPM 13, CPM 15, and CPM 19 than in susceptible check, Basi Local, while lower than in resistant check, CML 345, under S. inferens damaged conditions (Table 2). The genotype × treatment interactions were also significant for PAL activity in the seedlings of test maize genotypes (F = 41.09; df = 9,18; P < 0.001). Across maize genotypes, the PAL activity was significantly higher in S. inferens damaged (42.7 to 1042.0 U/ml) than in healthy maize seedlings (Fig. 2B).
Tyrosine ammonia lyase (TAL)
The TAL activity varied from 91.9 to 1017.0 U/ml of protein in healthy and 419.3 to 2370.4 /ml of protein in the S. inferens damaged seedlings of different maize genotypes (Table 2). There were significant differences in TAL activity in the seedlings of different maize genotypes (F = 195.29; df = 9,18; P < 0.001), both under healthy and S. inferens damaged conditions (F = 1.46; df = 1,18; P < 0.001), and for genotype × treatment interactions (F = 88.81; df = 9,18; P < 0.001). Across genotypes, TAL activity was significantly higher in test genotypes than in susceptible check, Basi Local, while lower than in resistant check, CML 345 under S. inferens damaged conditions (Table 2). Among test maize genotypes, the PAL activity was significantly higher in CPM 13, CPM 9, CPM 8, CPM 15, CPM 2 and CPM 19 under S. inferens damaged conditions. The TAL activity was significantly high in resistant check, CML 345 both under healthy and S. inferens damaged conditions (Fig. 2C).
Total phenol
There was significant variation in amount of total phenol in the seedlings of different maize genotypes (F = 10.36; df = 9,18; P < 0.001) both under healthy and S. inferens damaged (F = 218; df = 1,18; P < 0.001) conditions. The total phenol content varied from 0.78 to 1.46 mg/g in healthy and 1.72 to 3.49 mg/g in the S. inferens damaged seedlings (Table 3). Across maize genotypes, the total phenol content was significantly lower in healthy as compared to S. inferens damaged maize seedlings (Table 3). Furthermore, the genotype × treatment interaction was also found significant for total phenol content in the seedlings of test maize genotypes (F = 15.05; df = 9,18; P < 0.001). The total phenol content in test maize genotypes were recorded highest in CPM 13 under S. inferens damaged conditions. Overall CPM 13, CPM 9, CPM 18, CPM 15, CPM 2 and CML 345 had significantly higher total phenol than the susceptible check, Basi Local in the S. inferens damaged seedlings (Fig. 3A).
Total antioxidant
The total antioxidant content varied from to 0.14 to 2.57 mg/g in healthy and 1.49 to 3.49 mg/g in S. inferens damaged seedlings of different maize genotypes (Table 3). There were significant differences in amount of total antioxidant in the seedlings of different maize genotypes (F = 16.67, df = 9,18, P < 0.001) both under healthy and S. inferens damaged (F = 151.65, df = 1,18, P < 0.001) conditions, and for genotype × treatment interactions (F = 10.76; df = 9,18; P < 0.001). Across genotypes, average total antioxidant content was significantly higher in S. inferens damaged (2.38 mg/g) than in healthy (1.02 mg/g) maize seedlings (Table 3). Among test maize genotypes, total antioxidant content was higher in resistant check, CML 345, CPM 2, CPM 13 and CPM 19 under S. inferens damaged conditions as compared to other maize genotypes (Fig. 3B).
Tannins
There was significant variation in amounts of tannins in the seedlings of different maize genotypes (F = 10.81; df = 9,18; P < 0.001) both under healthy and S. inferens damaged conditions (F = 326.10; df = 1,18; P < 0.001), and for genotype × treatment interaction (F = 18.01; df = 9,18; P < 0.001). The tannins content varied from 0.24 to 1.02 mg/g in healthy and 0.81 to 2.38 mg/g in the S. inferens damaged seedlings (Table 3). Across genotypes, total tannins content increased significantly under S. inferens damaged conditions (0.06 to 1.94 mg/g) The tannin content in test maize genotypes CPM 13, CPM 9, CPM 2, CPM 8, CPM 15 and resistant check, CML 345 was significantly higher than Basi Local under S. inferens damaged conditions (Fig. 3C).
Ferric ion reducing antioxidant power (FRAP)
The FRAP content varied from to 0.17 to 0.48 mg/g in healthy and 0.51 to 1.28 mg/g in S. inferens damaged seedlings of different maize genotypes (Table 3). There were significant differences in FRAP content in the seedlings of different maize genotypes (F = 3.86, df = 9,18; P = 0.002) both under healthy and S. inferens damaged conditions (F = 82, df = 1,18; P < 0.001), and for genotype × treatment interactions (F = 9.37; df = 9,18; P < 0.001). Across genotypes, the FRAP content was significantly higher in S. inferens damaged (0.66 mg/g) than in healthy (0.35 mg/g) maize seedlings (Table 3). Among test maize genotypes, FRAP content was significantly higher in CPM 2 CPM 4, CPM 8, CPM 9, CPM 13, CPM 19 and CML 345 under S. inferens damaged conditions than susceptible check, Basi Local (Table 3). The highest increase in amount of FRAP in response to damage by S. inferens was recorded in CPM 2, CPM 9 CPM 13 and CML 345 as compared to other test maize genotypes including susceptible check, Basi Local (Fig. 3D).
Discussion
The constitutive nutritional components of the host plants determine the preference and performance of the herbivorous insects (Caroline & Simon, 2002). However, the host plants in response to damage by insect pests activate the plant biochemical defense system that interfere with feeding, digestion and absorption of essential nutrients (Howe & Jander, 2008; Wu & Baldwin, 2010; Smith & Clement, 2012), and decode the level of resistance against the insect pests. Present studies found that the total starch and soluble sugar contents increased in all the test maize genotypes in response to damage by S. inferens, but the comparative increase was greater in CPM 8, CPM 9 and resistant check, CML 345. Earlier studies also reported impact of plant primary metabolism, and Ostrinia nubilalis (Hubner) (Dafoe et al., 2013) and Chilo partellus (Swinhoe) (Bhoi et al., 2021) damage induced increase in nutritional components of maize genotypes. Higher protein content was also observed in all test maize genotypes after infestation by S. inferens which is corresponding to increase in activity of antioxidant enzymes could be resulting in defense activation. Based on these findings it can be hypothesized that the increase in primary metabolites of carbohydrates and proteins would render resistant plant tissues less nutritious while the susceptible ones more nutritious for the insects. As the primary metabolism is reorganized to reallocate these resources to defensive metabolic pathways or play direct role in signalling and defense in response to herbivore attack (Schwachtje & Baldwin, 2008; Rodriguez et al., 2018).
The phenyl ammonia lyase is the entry-point enzyme into the phenylpropanoid pathway responsible for the synthesis of plant phenolics and polyphenols, many of which play crucial role in plant defense under stress conditions (Asada, 1992). The phenyl ammonia lyase catalyzes conversion of phenolics to quinone through shikimic acid pathway and enhance production of quinones which cause toxicity to herbivores. Furthermore, induction of antioxidant enzymes such as catalase is preliminary reaction induced with respect to infection, followed by rest of antioxidant enzymes like tyrosine ammonia lyase (Huang et al., 2019). Present study revealed that all the genotypes under S. inferens damage conditions had stimulatory effects on catalase, phenyl ammonia lyase and tyrosine ammonia lyase activities, but there was significant variation in increase in the enzymatic activities with respect to genotypes. These findings suggest that the differential expression of enzymatic activities in the host plants in response to insect herbivory ultimately determine their level of resistance to biotic stress. The pink stem borer damage induced greater increase in phenyl ammonia lyase activity in the test maize genotypes including resistant check (CML 345) in comparison to susceptible check (Basi Local), is an indication of strong biochemical defense in these genotypes against S. inferens. Earlier studies also suggested that the increase in catalase activity in response to biotic stress act as local signal to activate defense genes and involve in increasing the cell wall resistance (Chen et al., 2009; Garcia-Lara & Bergvinson, 2014) reported a significant association between insect resistance and antioxidant activity, and overall concentration of these antioxidants decide the strength of resistance and susceptibility in a particular maize genotype. Furthermore, Rodriguez et al., (2021) found up-regulation of ROS scavenging mechanism for long-term defensive response to damage by S. nonagrioides. A meta-analysis has also shown positive correlation between plant genetic resistances and multiple herbivores in cases where both the compared species are generalist herbivores or both are specialist herbivores, however the comparison between insects of different feeding guilds showed the lowest genetic correlation for the multiple resistance (Leimu & Koricheva, 2006).
The phenols and tannins are plant secondary metabolites which reduce the nutritional value for herbivores as the digestive enzymes decrease the ability to digest plant tissues and induce plant resistance against biotic stresses (Sánchez & Contreras, 2017). Though phenols and tannins are constitutively produced in plants, induction in their concentration upon insect infestation has also been well documented for various crops and insect pests (Grayer et al., 1992; Barbehenn & Constabel, 2011; War et al., 2013; Taggar et al., 2014). Present studies found significant variation in tannin content among the test genotypes both under healthy and insect damaged conditions. Further, the phenol content was although significantly higher in all the test maize genotypes under S. inferens damage conditions, but it was highest in CPM 13 and the resistant check, CML 345 as compared to other genotypes. Earlier studies have also reported that the phenolic compounds play a role in determining resistance against S. inferens (Santiago et al., 2017; Soujanya et al., 2020), Sesamia nonagrioides (Lefebvre) (Gesteiro et al., 2021), and C. partellus (Bhoi et al., 2021) in maize. Furthermore, the FRAP activity significantly increased in response to damage by S. inferens in all the test maize genotypes, however the genotypes CPM 13, CPM 9 and CML 345 (resistant check) were recorded with higher increase as compared to other genotypes including susceptible check, Basi Local. Bhoi et al. (2021) also reported significantly higher FRAP activity in response to damage by C. partellus in resistant maize genotypes. This insect-induced differential increase in FRAP activity in resistant and susceptible genotypes could be due to varying capacity to acclimate and induce antioxidant enzymes in the form of secondary defense metabolites.
Present studies found that both primary and secondary biochemical compounds through activation of enzymatic antioxidants determine the level of resistance against S. inferens in maize. In a natural system most plant–insect interactions reach a ‘stand-off’ during herbivory where both involve a metabolic cost, wherein the balance between constitutive and induced defenses can be altered by both genotype and the environment (Gatehouse, 2002). These findings suggest that the maize genotypes having either constitutive or herbivory induced or both types of defensive biochemicals although could be useful for the development of pink stem borer resistant varieties, but it need to be ensured that it does not penalize the quality and yield potential of the host plants. Biological studies earlier found that the maize genotypes CPM 2, CPM 4, CPM 8, CPM 15 and CML 345 have greater detrimental effects on the development, survival and fecundity of S. inferens (Sau & Dhillon, 2022). However, in present studies the genotypes CPM 2, CPM 9, CPM 13, CPM 15 and CML 345 were found with greater constitutive and/or pink stem borer damage induced defense phytochemicals like enzymatic activity and nonenzymatic antioxidant defense biochemicals, thus could be used to develop S. inferens resistant varieties of maize. This information could also be useful for further studies on metabolic pathways and the enzymes involved in synthesis of defense compounds, and their gene regulation system as an advanced approach in plant defense against herbivorous insects.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126
Amorim, L. C., Nasciment, J. E., Monteiro, J. M., Sobrinho, J. S., Araujo, A. S., & Albuquerque, U. P. (2008). A simple and accurate procedure for the determination of tannin and flavonoid levels and some applications in ethnobotany and ethnopharmacology. Functional Ecosystems and Communities, 2, 88–94
Asada, K. (1992). Ascorbate peroxidase–a hydrogen peroxide scavenging enzyme in plants. Physiologia Plantarum, 85, 235–241
Barbehenn, R. V., & Constabel, P. C. (2011). Tannins in plant-herbivore interactions. Phytochemistry, 72, 1551–1565.
Benzie, I. F., & Strain, J. J. (1999). Ferric reducing /antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology, 299, 15–27
Bhoi, T. K., Trivedi, N., Kumar, H., Tanwar, A. K., & Dhillon, M. K. (2021). Biochemical defense in maize against Chilo partellus (Swinhoe) through activation of enzymatic and nonenzymatic antioxidants. Indian Journal of Experimental Biology, 59(1), 54–63
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254
Cao, A., Butrón, A., Malvar, R. A., Garrido, D. F., & Santiago, R. (2019). Effect of long-term feeding by borers on the antibiotic properties of corn stems. Journal of Economic Entomology, 112, 1439–1446
Caroline, S. A., & Simon, R. L. (2002). Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology, 47, 817–844
Chen, Y., Ni, X., & Buntin, G. D. (2009). Physiological, nutritional, and biochemical bases of corn resistance to foliage-feeding fall armyworm. Journal of Chemical Ecology, 35, 297–306
Clegg, K. M. (1956). The application of the anthrone reagent to the estimation of starch in cereals. Journal of the Science of Food and Agriculture, 7, 40–44
Dafoe, N. J., Thomas, J. D., Shirk, P. D., Legaspi, M. E., Vaughan, M. M., Huffaker, A. … Schmelz, E. A. (2013). European corn borer (Ostrinia nubilalis) induced responses enhance susceptibility in maize. PLoS One, 8, e73394
Dhillon, M. K., Kalia, V. K., & Gujar, G. T. (2014). Insect pests and their management: Current status and future need of research in quality maize. In D. P. Choudhary, S. Kumar, & S. Langyan (Eds.), Maize: Nutrition Dynamics and Novel Uses. Springer
Dhillon, M. K., & Chaudhary, D. P. (2015). Biochemical interactions for antibiosis mechanism of resistance to Chilo partellus (Swinhoe) in different maize types. Arthropod-Plant Interactions, 9(4), 373–382
Dhillon, M. K., & Kumar, S. (2017). Amino acid profiling of Sorghum bicolor vis-à-vis Chilo partellus (Swinhoe) for biochemical interactions and plant resistance. Arthropod-Plant Interactions, 11(4), 537–394550
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356
Fritz, R. R., Hodcins, D. S., & Abell, C. W. (1976). Phenylalanine ammonia lyase induction and purification from yeast and clearance in mammals. Journal of Biological Chemistry, 251, 4646–4650
Garcia-Lara, S., & Bergvinson, D. J. (2014). Phytochemical and nutraceutical changes during recurrent selection for storage pest resistance in tropical maize. Crop Science, 54, 2423–2432
Gatehouse, J. A. (2002). Plant resistance towards insect herbivores: a dynamic interaction. New Phytologist, 156, 145–169
Gesteiro, N., Butrón, A., Estévez, S., & Santiago, R. (2021). Unraveling the role of maize (Zea mays L.) cell-wall phenylpropanoids in stem-borer resistance. Phytochemistry, 185, 112683
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930
Grayer, R. J., Kimmins, F. M., Padgham, D. E., Harborne, J. B., & Ranga Rao, D. V. (1992). Condensed tannin levels and resistance in groundnuts (Arachis hypogoea (L.)) against Aphis craccivora (Koch). Phytochemistry, 31, 3795–3800
Howe, G. A., & Jander, G. (2008). Plant immunity to insect herbivores. Annual Review of Plant Biology, 59, 41–66
Huang, H., Ullah, F., Zhou, D. X., Yi, M., & Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Frontiers of Plant Science, 10, 800
Kumar, H. (1997). Resistance in maize to Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae), an overview. Crop Protection, 16, 243–250
Kumar, R., Srinivas, K., Boiroju, N. K., & Gedam, P. C. (2014). Production performance of maize in India: approaching an inflection point. International Journal of Agricultural and Statistical Sciences, 10, 241–248
Leimu, R., & Koricheva, J. (2006). A meta-analysis of genetic correlations between plant resistances to multiple enemies. The American Naturalist, 168, E15–E37
Marta, B., Szafranska, K., & Posmyk, M. M. (2016). Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Frontiers of Plant Science, 7, 575
Mitchell, C., Brennan, R. M., Graham, J., & Karley, A. J. (2016). Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Frontiers of Plant Science, 7, 1132
Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry, 269, 337–341
Rodriguez, V. M., Padilla, G., Malvar, R. A., Kallenbach, M., Santiago, R., & Butrón, A. (2018). Maize stem response to long-term attack by Sesamia nonagrioides. Frontiers of Plant Science, 9, 522
Rodriguez, V. M., Velasco, P., Cao, A., Santiago, R., Malvar, R. A., & Butrón, A. (2021). Maize resistance to stem borers can be modulated by systemic maize responses to long-term stem tunneling. Frontiers of Plant Science, 11, 627468
Sánchez, H. S., & Contreras, A. M. (2017). Chemical plant defense against herbivores. In: Herbivores (Ed. Shields, V.D.C.). IntechOpen. https://doi.org/10.5772/67346
Santiago, R., Cao, A., Butrón, A., López-Malvar, A., Rodriguez, V. M., Sandoya, G. V., & Malvar, R. A. (2017). Defensive changes in maize leaves induced by feeding of Mediterranean corn borer larvae. BMC Plant Biology, 17, 44
Sau, A. K., & Dhillon, M. K. (2022). Photosynthetic pigments in maize vis-à-vis biological performance and host selection by Sesamia inferens. Indian Journal of Agricultural Sciences, 92(3), 61–65
Schwachtje, J., & Baldwin, I. T. (2008). Why does herbivore attack reconfigure primary metabolism? Plant Physiology, 146, 845–851
Scott, M. I., Thaler, S. J., & Scott, G. F. (2010). Response of a generalist herbivore Trichoplusia ni to jasmonate-mediated induced defense in tomato. Journal of Chemical Ecology, 36, 490–499
Siddiqui, K. H., & Marwaha, K. K. (1993). The vistas of maize entomology in India. Kalyani Publishers
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic- phosphotungestic acid reagents. American Journal of Enology and Viticulture, 16, 144–158
Smith, C. M., & Clement, S. L. (2012). Molecular bases of plant resistance to arthropods. Annual Review of Entomology, 57, 309–328
Soujanya, L. P., Sekhar, J. C., Ratnavathi, C. V., Shobha, E., Karjagi, C. G., Suby, S. B., et al. (2020). Role of soluble, cell wall-bound phenolics, tannin and flavonoid contents in maize resistance to pink stem borer Sesamia inferens Walker. Maydica, 65(1), 1–12.
Soujanya, P. L., Sekhar, J. C., Ratnavathi, C. V., Karjagi, C. G., Shobha, E., Suby, S. B. … Rakshit, S. (2021). Induction of cell wall phenolic monomers as part of direct defense response in maize to pink stem borer (Sesamia inferens Walker) and non-insect interactions. Scientific Reports, 11, 14770
Taggar, G. K., Gill, R. S., Gupta, A. K., & Singh, S. (2014). Induced changes in the antioxidative compounds of black gram (Vigna mungo (L.) Hepper) genotypes due to infestation by Bemisia tabaci (Gennadius). Journal of Environmental Biology, 35, 1037–1045
Thorpe, T. A., & Beaudoin-Eagan, L. D. (1985). Tyrosine and Phenylalanine ammonia lyase Activities during shoot Initiation in tobacco callus cultures. Plant Physiology, 78, 438–441
Trivedi, N. (2021). Improved plant resistance by phytomicrobiome community towards biotic and abiotic stresses. In A. Verma, J. K. Saini, A. E. Hesham, & H. B. Singh (Eds.), Phytomicrobiome Interactions and Sustainable Agriculture (pp. 207–216). Wiley
War, A. R., Sharma, H. C., Paulraj, M. G., Hussain, B., Buhroo, A. A., War, M. Y. … Sharma, H. C. (2013). Effect of plant secondary metabolites on Helicoverpa armigera. Journal of Pest Science, 86, 399–408
Wu, J., & Baldwin, I. T. (2010). New insights into plant responses to attack from insect herbivores. Annual Review of Genetics, 44, 1–24
Yele, Y., Dhillon, M. K., Tanwar, A. K., & Kumar, S. (2021). Amino and fatty acids contributing to antibiosis against Chilo partellus (Swinhoe) in maize. Arthropod-Plant Interactions, 15(5), 721–736
Zhao, L. Y., Chen, J. L., Cheng, D. F., Sun, J. R., Liu, Y., & Tian, Z. (2009). Biochemical and molecular characterizations of Sitobion avenae–induced wheat defense responses. Crop Protection, 28, 435–442
Acknowledgements
This study is part of M.Sc. thesis of Mr. Ashok K. Sau. The authors are thankful to the ICAR-Indian Agricultural Research Institute, New Delhi for encouragement, providing necessary facilities and senior research fellowship to the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sau, A.K., Dhillon, M.K. & Trivedi, N. Activation of antioxidant defense in maize in response to attack by Sesamia inferens (Walker). Phytoparasitica 50, 1043–1058 (2022). https://doi.org/10.1007/s12600-022-00996-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-00996-2