Abstract
There is a complex interplay of biochemical interactions which determine the host plant reaction to herbivory. We studied the Chilo partellus-induced biochemical plant defense system in sorghum. Present studies found that the total protein, starch and total sugar contents were significantly lower, while antioxidant enzymes like ascorbate oxidase (AO), catalase (CAT), ascorbate peroxidase (APX), phenyl ammonia lyase (PAL), tyrosine ammonia lyase (TAL), and nonenzymatic antioxidants such as total phenols, total antioxidants and ferric ion reducing antioxidant power (FRAP) higher in the seedlings of all the test sorghum genotypes as compared to susceptible genotype, Swarna both under healthy and C. partellus damaged conditions. Further, the C. partellus damage resulted in significant increase in amounts of all the test constitutional, enzymatic and nonenzymatic biochemicals in the test genotypes, however their percent increase was highly variable across genotypes. These studies demonstrate that both constitutive and C. partellus damage induced enzymatic and nonenzymatic antioxidants like APX, AO, PAL, TAL, CAT, total phenols and FRAP were significantly greater in IS 2205, IS 18,551, ICSV 1, ICSV 700 and ICSV 93,046 than in susceptible check (Swarna) indicating their role in plant defense. Furthermore, these genotypes can be used in sorghum breeding for resistance against C. partellus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorghum, Sorghum bicolor (L.) Moench is one of the most important cereal crops feeding millions of people in the semi-arid tropics. It is damaged by more than 150 insect species including several borer species during different crop growth stages right from sowing till harvesting (Sharma, 1993). Among the borers, spotted stem borer, Chilo partellus (Swinhoe) is the most devastating pest of sorghum in Asia and Africa (Sharma et al., 2003; Huang et al., 2013). All the three mechanisms of plant resistance to insects are functional for C. partellus resistance in sorghum (Sharma et al., 2007). The insect herbivory stimulates the plants to produce many antinutritional compounds, toxic proteins and secondary metabolites which interfere in biological and biochemical functions of the insects (Smith & Clement, 2012).
In response to biotic stress, reactive oxygen species (ROS) acts as secondary messenger to signal defense reaction in the host plants (Asada, 2006). To prevent the oxidation, plant also develop ROS scavenging mechanism (Howe & Schilmiller, 2002). In this process, several naturally occurring plant cell antioxidants/enzymes like catalase (CAT), ascorbate peroxidase (APX), ascorbate oxidase (AO), phenylalanine ammonia lyase (PAL) and tyrosine ammonia lyase (TAL); and plant defense compounds such as phenolics, flavonoids and tannins play important role in detoxification of ROS and maintaining redox balance (Gill & Tuteja, 2010; Huang et al., 2019).
Some of the constitutive biochemicals have been found to impart defense against C. partellus in maize and sorghum (Dhillon & Chaudhary, 2015; Dhillon & Kumar, 2017). It has also been shown that the oxidative state of the host plant produces ROS and toxic secondary metabolites, and is an important component for resistance against insects (Howe & Jander, 2008; Wu & Baldwin, 2010; He et al., 2011). An efficient antioxidant system could be useful to protect the sorghum plants against oxidative stress caused by C. partellus. However, the information regarding the relative levels of insect resistance and enzymatic and nonenzymatic anti-oxidative responses in sorghum under the insect pests attack is limited. Therefore, present studies were designed to elucidate the C. partellus damage-induced regulation system of certain biochemical constituents and effect on the antioxidative potential of diverse sorghum genotypes. The findings will also be useful for better understanding the metabolic pathways leading to synthesis of C. partellus defense compounds and their deployment in sorghum improvement program.
Materials and methods
Plant materials and insect culture
The experimental plant material consisted of six sorghum genotypes viz., ICSV 1, ICSV 700, ICSV 93,046, IS 18,551 and IS 2205 (resistant check), and Swarna (susceptible check). These genotypes except Swarna have been found to impart deleterious effects on the developmental biology of C. partellus (Kumar, 2018). The C. partellus culture maintained on artificial diet (Sharma et al., 1992) under laboratory conditions was used for inoculation on the test sorghum genotypes at ICAR-Indian Agricultural Research Institute, New Delhi.
Collection of sorghum seedlings for biochemical analysis
The seedlings of test sorghum genotypes were raised on the potting mixture (2 red soil: 1farm yard manure) in the pots (12 L capacity). Ten seeds were sown in each pot. There were four pots for each sorghum genotype (two pots for infestation with C. partellus larvae and two pots un-infested as control). The pots were covered with nylon net to protect the seedlings from natural insect pest infestation. Fifteen-day old seedlings of each test genotype were inoculated with two third instar C. partellus larvae in the designated pots. At 3 days after exposure, the C. partellus infested and healthy seedlings were collected separately from the designated pots. The central whorls of test seedling samples were separately collected. One-gram frozen tissue of each test sample was immediately processed for making the sample extracts by following the protocol given by Hildebrand et al. (1986). The prepared sample extracts were frozen at -20ºC, and used for various biochemical estimations. There were three replications for each treatment in a completely randomized design.
Constitutional biochemical content analysis
All the healthy and C. partellus damaged sorghum samples were analyzed for total protein, starch and total sugars. Total proteins were estimated by the method given by Bradford (1976), starch by the method given by Clegg (1956), and total sugars by concentrate sulphuric acid method given by Dubois et al. (1956). The values obtained for these biochemical constituents were expressed in mg/g of plant tissue.
Enzymatic antioxidant analysis
All the healthy and C. partellus damaged test samples were analyzed for the activity of various antioxidant enzymes like APX (Ali et al., 2005), CAT (Aebi, 1984), PAL (Fritz et al., 1976), TAL (Thorpe & Beaudoin-Eagan, 1985) and AO (Diallinas et al., 1997) by following the methods given by various workers. The activities of these test antioxidant enzymes were expressed in respect to the change in absorbance/min/mg of protein.
Nonenzymatic antioxidant analysis
All the healthy and C. partellus damaged sorghum samples were analyzed for total phenols, total antioxidants and ferric ion reducing antioxidant power (FRAP). Total phenols were estimated using the method given by Singleton and Rossi (1965), total antioxidants by Prieto et al. (1999) and FRAP by Benzie and Strain (1999). The values obtained for these biochemical constituents were expressed in mg/g of plant tissue.
Statistical analysis
The data on various biochemical constituents in the healthy and C. partellus damaged seedlings of test genotypes were subjected to analysis of variance (ANOVA) using factorial design. The significance of differences between the genotypes, treatments and genotype × treatment interactions were judged by F-test, and the differences among them were compared by least significant differences (LSD) at P = 0.05 using the statistical software SAS® version 9.2.
Results
Constitutional biochemical contents
Total protein
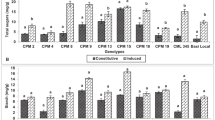
Total protein content varied from 2.49 to 5.10 mg/g in the healthy and 2.70 to 5.78 mg/g in C. partellus damaged seedlings of test genotypes (Table 1). The total protein content significantly varied in the seedlings of test genotypes (F = 28009.85; df = 5,22; P < 0.001) and under healthy and damaged conditions (F = 3798.09; df = 1,22; P < 0.001). It was significantly lower in the healthy and higher in C. partellus damaged seedlings across test genotypes (Table 1). The genotype × treatment interaction was also significant for the total protein content (F = 281.92; df = 5,22; P < 0.001). Both under healthy and C. partellus damaged conditions, the total protein was significantly lower in resistant check (IS 2205) followed by the test genotypes IS 18,551 and ICSV 700 in comparison to susceptible check (Swarna) (Fig. 1A).
Starch
There was significant variation in starch content in the seedlings of test genotypes (F = 18971.5, df = 5,22; P < 0.001), under healthy and damaged conditions (F = 930.83, df = 1,22; P < 0.001), and for genotype × treatment interaction (F = 25.69; df = 5,22; P < 0.001). It varied from 1.37 to 6.64 mg/g in healthy and 1.67 to 7.21 mg/g in C. partellus damaged sorghum seedlings (Table 1). Across genotypes, starch content was significantly lower in the healthy than in C. partellus damaged sorghum seedlings (Table 1). Both under healthy and C. partellus damaged conditions, the starch content was significantly lower in resistant check (IS 2205) followed by IS 18,551 and ICSV 1 in comparison to susceptible check (Swarna) (Fig. 1B).
Total sugars
The total sugar content varied from 2.05 to 12.88 mg/g in healthy and 3.41 to 14.66 mg/g in C. partellus damaged seedlings of test genotypes (Table 1). Total sugar content significantly varied among the test genotypes (F = 18606.48; df = 5,22; P < 0.001) both under healthy and damaged conditions (F = 3246.87; df = 1,22; P < 0.001), and for genotype × treatment interactions (F = 38.72; df = 5,22; P < 0.001). Total sugar content was significantly lower in the healthy and higher in C. partellus damaged seedlings across test genotypes (Table 1). Both under healthy and C. partellus damaged conditions, the total sugar content was significantly lower in IS 2205 (resistant check) and higher in Swarna (susceptible check) as compared to other sorghum genotypes (Fig. 1C).
Enzymatic antioxidants
Ascorbate peroxidase (APX)
The APX activity varied from 4.07 to 26.58 and 5.67 to 34.88 ΔOD/min/mg of protein in the healthy and C. partellus damaged seedlings of test genotypes, respectively (Table 2). There was significant variation for APX activity in the test genotypes (F = 990.4; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 804.05; df = 1,22; P < 0.001) conditions, and for genotype × treatment interactions (F = 469.82; df = 5,22; P < 0.001). The APX activity was significantly greater in C. partellus damaged than in healthy seedlings across test genotypes (Table 2). Both under healthy and C. partellus damaged conditions, the APX activity was significantly higher in IS 2205 and IS 18,551, and lower in Swarna (susceptible check) as compared to other test genotypes (Fig. 2A).
Catalase (CAT)
The CAT activity varied from 0.55 to 10.00 ΔOD/min/mg of protein in healthy and 0.68 to 12.49 ΔOD/min/mg of protein in the C. partellus damaged seedlings of test genotypes (Table 2). There were significant differences in CAT activity in the sorghum genotypes (F = 939.92; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 713.59; df = 1,22; P < 0.001) conditions, and for genotype × treatment interactions (F = 471.24; df = 5,22; P < 0.001). The CAT activity was significantly greater in C. partellus damaged than in healthy seedlings across test genotypes (Table 2). Both under healthy and C. partellus damaged conditions, the CAT activity was significantly greater in IS 2205 (resistant check), ICSV 1, ICSV 93,046 and IS 18,551 than in Swarna (susceptible check) and ICSV 700 (Fig. 2B).
Phenyl ammonia lyase (PAL)
The PAL activity varied from 0.56 to 8.54 and 1.54 to 10.92 ΔOD/min/mg of protein in the healthy and C. partellus damaged seedlings of the test genotypes, respectively (Table 2). There was significant variation in PAL activity in the seedlings of test genotypes (F = 6151.08, df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 5130.4; df = 1,22; P < 0.001) conditions, and for genotype × treatment interactions (F = 1048.3; df = 5,22; P < 0.001). The PAL activity was significantly greater in C. partellus damaged than in healthy seedlings across test genotypes (Table 2). The PAL activity was significantly greater in IS 2205 (resistant check), IS 18,551, ICSV 700 and ICSV 93,046 than in Swarna (susceptible check) both under healthy and C. partellus damaged conditions (Fig. 2C).
Tyrosine ammonia lyase (TAL)
The TAL activity varied from 0.68 to 6.46 and 2.56 to 8.23 ΔOD /min/mg of protein in the healthy and C. partellus damaged seedlings of test genotypes, respectively (Table 2). There was significant variation in TAL activity in the seedlings of test genotypes (F = 994.22; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 1784.45; df = 1,22; P < 0.001) conditions, and for genotype × treatment interactions (F = 556.11; df = 5,22; P < 0.001). The TAL activity was significantly greater in C. partellus damaged than in healthy seedlings across test genotypes (Table 2). The TAL activity was significantly greater in IS 2205, IS 18,551, ICSV 93,046 and ICSV 1 than in Swarna (susceptible check) under healthy as well as C. partellus damaged conditions (Fig. 2D).
Ascorbate oxidase (AO)
The AO activity varied from 4.07 to 26.58 and 5.67 to 34.88 ΔOD/min/mg of protein in the healthy and C. partellus damaged seedlings of test genotypes, respectively (Table 2). There were significant differences in AO activity in the seedlings of test genotypes (F = 990.4; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 804.05; df = 1,22; P < 0.001) conditions, and for genotype × treatment interactions (F = 469.82; df = 5,22; P < 0.001). The AO activity was significantly greater in C. partellus damaged than in healthy seedlings across test genotypes (Table 2). Both under healthy and C. partellus damaged conditions, the AO activity was significantly greater in IS 2205 (resistant check), ICSV 1, ICSV 93,046 and IS 18,551 as compared to susceptible check (Swarna) (Fig. 2E).
Non-enzymatic antioxidants
Total phenols
Total phenol content varied from 0.20 to 0.58 mg/g and 0.27 to 0.61 mg/g in the healthy and C. partellus damaged seedlings, respectively (Table 3). There was significant variation in total phenols in the seedlings of different sorghum genotypes (F = 26462.42; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 3230.58; df = 1,22; P < 0.001) conditions, and for the genotype × treatment interactions (F = 99.68; df = 5,22; P < 0.001). Total phenol content was significantly higher in the C. partellus damaged than in healthy seedlings of the test genotypes (Table 3). Both under healthy and C. partellus damaged conditions, the total phenol content was significantly higher in IS 2205 (resistant check), ICSV 1, ICSV 700, ICSV 93,046 and IS 18,551 as compared to Swarna (susceptible check) (Fig. 3A).
Total antioxidants
Total antioxidants varied from to 3.99 to 12.64 mg/g in healthy and 4.65 to 15.15 mg/g in C. partellus damaged seedlings of test genotypes (Table 3). There were significant differences in total antioxidants in the seedlings of test genotypes (F = 35462.76; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 7709.14; df = 1,22; P < 0.001) conditions, and for the genotype × treatment interactions (F = 393.32; df = 5,22; P < 0.001). Further, the total antioxidants were significantly higher in C. partellus damaged than in healthy seedlings of the test genotypes (Table 3). Both under healthy and C. partellus damaged conditions, the total antioxidants were significantly higher in IS 2205 (resistant check), ICSV 1, ICSV 700, ICSV 93,046 and IS 18,551 than in Swarna (susceptible check) (Fig. 3B).
Ferric ion reducing antioxidant power (FRAP)
The FRAP content varied from to 0.42 to 1.49 and 0.52 to 1.60 mg/g in healthy and C. partellus damaged seedlings of test genotypes, respectively (Table 3). There was significant variation in FRAP content in the seedlings of test genotypes (F = 18055.27; df = 5,22; P < 0.001) both under healthy and C. partellus damaged (F = 2064.2; df = 1,22; P < 0.001) conditions, and for genotype × treatment interactions (F = 25.34; df = 5,22; P < 0.001). The FRAP content was significantly higher in C. partellus damaged than in healthy seedlings of the test genotypes (Table 3). Both under healthy and C. partellus damaged conditions, the FRAP content was significantly higher in IS 2205 (resistant check), IS 18,551, ICSV 93,046 and ICSV 700 than in Swarna (susceptible check) and ICSV 1 (Fig. 3C).
Discussion
Both constitutive and herbivory induced host plant biochemicals determine the biological performance and resistance against insect pests (Howe & Jander, 2008; Wu & Baldwin, 2010; Smith & Clement, 2012; Dhillon & Chaudhary, 2015; Dhillon & Kumar, 2017; Bhoi et al., 2021). The present studies revealed significant increase in total protein, starch and total sugars in all the test sorghum seedlings due to damage by C. partellus, however the percent increase in these biochemical constituents was highly variable across the test genotypes (Fig. 4). Furthermore, the total protein, starch and total sugar contents were significantly greater in both C. partellus damaged and healthy seedlings of Swarna (susceptible check) and lower in IS 2205 (resistant check) and IS 18,551 (except total sugars) in comparison to other sorghum genotypes. The rise in total protein content due to C. partellus damage may be attributed to synthesis of antinutritional proteins, while increase in starch and total sugars to help withstand the plants from herbivory stress. Similar findings have also been reported due to C. partellus damage in maize (Bhoi et al., 2021). Furthermore, the greater amounts of total protein and total sugar contents in sorghum seedlings have been reported to impart susceptibility to C. partellus (Kabre & Ghorpade, 1999; Dhillon & Chaudhary, 2018). Similarly, the sugar content in the Bauhinia brevipes Vogel leaves has also been reported to be influenced by insect herbivory (Cornelissen and Fernandes 2001). The stress induced accumulation of ROS is related to increase in soluble sugars and is thought to be a stress-adaptive response (Roitsch, 1999).
The increased oxidative state could be responsible for herbivory resistance in the host through generation of ROS and their consequent removal by antioxidant system of the plant (Felton et al., 1992). The production of ROS is an early response to biotic stress, cues to induce plant defense against the stress (Maffei et al., 2007). Antioxidant enzymes including catalase, peroxidase, and superoxide dismutase show increased activity in response to ROS production (Libik-Konieczny et al., 2011). In the present studies, the activity of APX, CAT, PAL, TAL and AO were significantly higher in IS 2205 (resistant check) and lower in Swarna (susceptible check) in comparison to other test sorghum genotypes both under healthy and C. partellus damaged conditions. Earlier studies also reported greater activity of peroxidase, polyphenol oxidase, PAL and TAL in resistant as compared to susceptible seedlings of sorghum (Vashisth et al., 2022). Moreover, the damage by C. partellus resulted in significant increase in APX, CAT, PAL, TAL and AO activity, wherein highest percent increase in these enzymatic antioxidants was recorded in IS 18,551 (Fig. 4). Earlier studies have also found that the herbivory result in increased activity of CAT and PAL in the host plants (Bi & Felton, 1995; Sharma et al., 2016). Increased CAT activity due to biotic stress serves as a local signal to activate protective genes and boost host resistance (Chen et al., 2009). Since APX is part of peroxide detoxification process, its increased activity has been shown to assist in protection of host plants against biotic stress (Tománková et al., 2006).
The enzyme PAL generate a number of phenylpropanoids and phenolics, several of which are essential for plant defense system (Asada, 1992). Increased PAL activity due to herbivory allows phenolics to be oxidised to quinones through the shikimic acid pathway, causing herbivore toxicity (Zhao et al., 2009; Rani & Jyothsna, 2010). In the present studies, the C. partellus damage resulted in greater and/or on par percent increase in levels of APX, CAT, PAL, TAL and AO in susceptible check (Swarna) in comparison to test genotypes, indicating the role of these antioxidant enzymes in shielding plants from biotic stresses. The greater percent increase in most of these antioxidant enzymes due to C. partellus damage have also been reported in the susceptible maize genotypes (Bhoi et al., 2021). Similarly, Sau et al. (2022) also reported greater amounts of constitutive and induced defense biochemicals in borer-resistant maize genotypes.
Phenols play an important role in the reduction of ROS, which then initiate several reactions that stimulate defence enzymes (Maffei et al., 2007). In the present studies, the total phenols, antioxidants and FRAP were significantly lower in Swarna (susceptible check) than in other test sorghum genotypes under healthy and as well as borer-infestation conditions. However, the damage by C. partellus resulted in significantly greater increase in total phenols and FRAP in Swarna (susceptible check), whereas lower increase in the resistant genotypes IS 2205 and IS 18,551 as compared to other genotypes (Fig. 4). These findings suggest that the greater constitutive contents of nonenzymatic antioxidants like total phenols and FRAP could be playing an important role in C. partellus defense in sorghum. Vashisth et al. (2022) also found greater amounts of phenols in resistant than in susceptible sorghum genotypes.
The present studies demonstrated that both constitutive and insect damage induced enzymatic and nonenzymatic antioxidants like APX, AO, PAL, TAL, CAT, total phenols and FRAP were significantly greater in IS 2205, IS 18,551, ICSV 1, ICSV 700 and ICSV 93,046 (except in a few cases) in comparison to susceptible check (Swarna), suggesting that these biochemical constituents play important role in plant defense, and thus can be deployed in C. partellus-resistance breeding program.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aebi, H. (1984). Catalase in vitro Methods in Enzymology, 105, 121–126.
Ali, M. B., Hahn, E. J., & Paek, K. Y. (2005). Effects of light intensities on antioxidant enzymes and malondildehyde content during short-term acclimatization on micro propagated Phalaenopsis plantlet Environmental and Experimental Botany, 54, 109–120.
Asada, K. (1992). Ascorbate peroxidase–a hydrogen peroxide scavenging enzyme in plants. Physiologia Plantarum, 85, 235–241.
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, 141(2), 391–396.
Benzie, I. F., & Strain, J. J. (1999). Ferric reducing /antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology, 299, 15–27.
Bhoi, T. K., Trivedi, N., Kumar, H., Tanwar, A. K., & Dhillon, M. K. (2021). Biochemical defense in maize against Chilo partellus (Swinhoe) through activation of enzymatic and nonenzymatic antioxidants. Indian Journal of Experimental Biology, 59, 54–63.
Bi, J. L., & Felton, G. W. (1995). Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites and reactive oxygen species as components of induced resistance. Journal of Chemical Ecology, 21, 1511–1530.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Chen, Y., Ni, X., & Buntin, G. D. (2009). Physiological, nutritional, and biochemical bases of corn resistance to foliage-feeding fall armyworm. Journal of Chemical Ecology, 35, 297–306.
Clegg, K. M. (1956). The application of the anthrone reagent to the estimation of starch in cereals. Journal of the Science of Food and Agriculture, 7, 40–44.
Cornelissen, T. G., & Fernandes, G. W. (2001). Patterns of attack by herbivores on the tropical shrub Bauhinia brevipes (Leguminosae): vigour or chance? European Journal of Entomology, 98, 37–40.
Dhillon, M. K., & Chaudhary, D. P. (2015). Biochemical interactions for antibiosis mechanism of resistance to Chilo partellus (Swinhoe) in different maize types. Arthropod-Plant Interactions, 9(4), 373–382.
Dhillon, M. K., & Chaudhary, D. P. (2018). Physicochemical mechanisms of resistance in sorghum to Chilo partellus (Swinhoe). Indian Journal of Experimental Biology, 59, 29–38.
Dhillon, M. K., & Kumar, S. (2017). Amino acid profiling of Sorghum bicolor vis-à-vis Chilo partellus (Swinhoe) for biochemical interactions and plant resistance. Arthropod-Plant Interactions, 11(4), 537–550.
Diallinas, G., Pateraki, I., Sanmartin, M., Scossa, A., & Stilianou, E. (1997). Melon ascorbate oxidase: cloning of a multigene family, induction during fruit development and repression by wounding. Plant Molecular Biology, 34, 759–770.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Felton, G. W., Donato, K. K., Broadway, R. M., & Duffey, S. S. (1992). Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore. Journal of Insect Physiology, 38, 277–285.
Fritz, R. R., Hodcins, D. S., & Abell, C. W. (1976). Phenylalanine ammonia lyase induction and purification from yeast and clearance in mammals. Journal of Biological Chemistry, 251, 4646–4650.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
He, J., Chen, F., Chen, S., Lv, G., Deng, Y., Fang, Z., Guan, Z., & He, C. (2011). Chrysanthemum leaf epidermal infestation. Journal of Plant Physiology, 168(7), 687–693.
Hildebrand, D. F., Rodriquez, J. G., Brown, G. C., Luu, K. T., & Volden, C. S. (1986). Peroxidative responses of leaves in two soybean genotypes injured by two spotted spider mite (Acari: Tetranychidae). Applied Entomology and Zoology, 20, 348–349.
Howe, G. A., & Jander, G. (2008). Plant immunity to insect herbivores. Annual Review of Plant Biology, 59, 41–66.
Howe, G. A., & Schilmiller, A. L. (2002). Oxylipin metabolism in response to stress. Current Opinion in Plant Biology, 5, 230–236.
Huang, H., Ullah, F., Zhou, D. X., Yi, M., & Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science, 10, 800.
Huang, Y., Sharma, H. C., & Dhillon, M. K. (2013). Bridging conventional and molecular genetics of sorghum insect resistance. In A. K. Paterson (Ed.), Genomics of the Saccharinae, Plant Genetics and Genomics: crops and models (Vol. 11, pp. 367–389). Springer.
Kabre, G. B., & Ghorpade, S. A. (1999). Susceptibility to maize stem borer, Chilo partellus (Swinhoe) in relation to sugars, proteins and free amino acids content of maize germplasm and F1 hybrids. Journal of Insect Science, 12, 37–40.
Kumar, H. (2018). Behavioral, biological and biochemical studies in sorghum for resistance to Chilo partellus (Swinhoe). M.Sc. Thesis. Division of Entomology, ICAR-Indian Agricultural Research Institute, New Delhi, India, 56 pp.
Libik-Konieczny, M., Surówka, E., Kuźniak, E., Nosek, M., & Miszalski, Z. (2011). Effects of Botrytis cinerea and Pseudomonas syringae infection on the antioxidant profile of Mesembryanthemum crystallinum C3/CAM intermediate plant. Journal of Plant Physiology, 168(10), 1052–1059.
Maffei, M. E., Mithöfer, A., & Boland, W. (2007). Before gene expression: early events in plant–insect interaction. Trends in Plant Science, 12(7), 310–316.
Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry, 269, 337–341.
Rani, P. U., & Jyothsna, Y. (2010). Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physioliae Plantarum, 32, 695.
Roitsch, T. (1999). Source-sink regulation by sugar and stress. Current Opinion in Plant Biology, 2, 198–206.
Sau, A. K., Dhillon, M. K., & Trivedi, N. (2022). Activation of antioxidant defense in maize in response to attack by Sesamia inferens (Walker). Phytoparasitica. https://doi.org/10.1007/s12600-022-00996-2
Sharma, H. C. (1993). Host plant resistance to insects in sorghum and its role in integrated pest management. Crop Protection, 12, 11–34.
Sharma, H. C., Dhillon, M. K., Pampapathy, G., & Reddy, B. V. S. (2007). Inheritance of resistance to spotted stem borer, Chilo partellus in sorghum, Sorghum bicolor Euphytica, 156, 117–128.
Sharma, H. C., Leuschner, K., Nwanze, K. F., & Taneja, S. L. (1992). Techniques to screen sorghum for resistance to insect pests. Information Bulletin No. 32. International Crops Research Institute for the Semi-Arid Tropics, Patancheru 502 324, Andhra Pradesh, India, 48 pp.
Sharma, H. C., Taneja, S. L., Kameswara Rao, N., & Prasada Rao, K. E. (2003). Evaluation of sorghum germplasm for resistance to insect pests. Information Bulletin No. 63. International Crops Research Institute for the Semi-Arid Tropics, Patancheru 502 324, Andhra Pradesh, India, 177 pp.
Sharma, H. C., War, A. R., Pathania, M., Sharma, S. P., Akbar, S. M., & Munghate, R. S. (2016). Elevated CO2 influences host plant defense response in chickpea against Helicoverpa armigera Arthropod-Plant Interactions, 10(2), 171–181.
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic- phosphotungestic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Smith, C. M., & Clement, S. L. (2012). Molecular bases of plant resistance to arthropods. Annual Review of Entomology, 57, 309–328.
Thorpe, T. A., & Beaudoin-Eagan, L. D. (1985). Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiology, 78, 438–441.
Tománková, K., Luhová, L., Petřivalský, M., Peč, P., & Lebeda, A. (2006). Biochemical aspects of reactive oxygen species formation in the interaction between Lycopersicon spp. and Oidium neolycopersici Physiological and Molecular Plant Pathology, 68(1–3), 22–32.
Vashisth, S., Jaba, J., Sharma, S. P., & Sharma, H. C. (2022). Biochemical mechanisms of induced resistance to Chilo partellus in sorghum. International Journal of Pest Management. https://doi.org/10.1080/09670874.2022.2036863
Wu, J., & Baldwin, I. T. (2010). New insights into plant responses to attack from insect herbivores. Annual Review of Genetics, 44, 1–24.
Zhao, L. Y., Chen, J. L., Cheng, D. F., Sun, J. R., Liu, Y., & Tian, Z. (2009). Biochemical and molecular characterizations of Sitobion avenae–induced wheat defense responses. Crop Protection, 28, 435–442.
Acknowledgements
The necessary facilities and senior research fellowship to the first author by ICAR-Indian Agricultural Research Institute, New Delhi are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Mukesh K. Dhillon and Hemant Kumar conceptualized and designed the study. Experimental set up, data collection and analysis were performed by Hemant Kumar and Tanmaya K. Bhoi. The manuscript was written by Mukesh K. Dhillon and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, H., Dhillon, M.K. & Bhoi, T.K. Stress-induced defense in sorghum in response to attack by the spotted stem borer, Chilo partellus (Swinhoe). Phytoparasitica 51, 49–61 (2023). https://doi.org/10.1007/s12600-022-01037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-01037-8